Abstract
Infectious diseases that affect wildlife and livestock are challenging to manage, and can lead to large scale die offs, economic losses, and threats to human health. The management of infectious diseases in wildlife and livestock is made easier with knowledge of disease risk across space and identifying stakeholders associated with high risk landscapes. This study focuses on anthrax, caused by the bacterium Bacillus anthracis, risk to wildlife and livestock in Montana. There is a history of anthrax in Montana, but the spatial extent of disease risk and subsequent wildlife species at risk are not known. Our objective was to predict the potential geographic distribution of anthrax risk across Montana, identify wildlife species at risk and their distributions, and define stakeholders. We used an ecological niche model to predict the potential distribution of anthrax risk. We overlaid susceptible wildlife species distributions and land ownership delineations on our risk map. We found that there was an extensive region across Montana predicted as potential anthrax risk. These potentially risky landscapes overlapped the ranges of all 6 ungulate species considered in the analysis and livestock grazing allotments, and this overlap was on public and private land for all species. Our findings suggest that there is the potential for a multi species anthrax outbreak on multiple landscapes across Montana. Our potential anthrax risk map can be used to prioritize landscapes for surveillance and for implementing livestock vaccination programs.
Keywords: Bacillus anthracis, anthrax, ecological niche model, Montana, wildlife infectious diseases
INTRODUCTION
Wildlife play an increasing role in the emergence of zoonotic diseases, which affect humans, livestock, and wildlife (Miller et al. 2013; Gortazar et al. 2015). Approximately 52% of recent emerging infectious diseases worldwide have originated in wildlife populations, and this trend is significantly increasing (Jones et al. 2008). Managing zoonoses in wildlife populations is associated with multiple challenges in the United States. For example, wildlife are managed by state agencies, but spend time on private land where access and resources are controlled by the landowner (Watson 2012). Wildlife on private lands have created challenges for the management of several zoonoses including brucellosis in Montana (Cross et al. 2010) and bovine tuberculosis in Michigan (O’Brien et al. 2006; Carstensen et al. 2011). Disease spillover from wildlife to livestock on public and private land has been reported for multiple diseases (Cheville et al. 1998; O’Brien et al. 2006; Brook and McLachlan 2009; Proffitt et al. 2011; Sells et al. 2015). Wildlife-livestock spillover can result from direct contact (Sells et al. 2015) or multiple hosts interacting with the same infectious material on the landscape (Hugh-Jones and Blackburn 2009; Proffitt et al. 2011). The management and prevention of these spillover events is complicated, as they tend to affect multiple stakeholders. Predicting the spatial extent of disease risk plays an important role in addressing these challenges.
Successful zoonotic disease management requires knowledge of disease risk across space and time. Ecological niche models (ENMs) have been used to predict the geography of environments supporting pathogen persistence and outbreaks (Blackburn 2010). Such approaches determine non-random relationships between locations describing disease related events (including outbreaks, vector collections, or host records) and environmental covariates. These results can be projected onto the landscape within a geographic information system (GIS) to predict the potential geographic distribution of the pathogen or disease (Blackburn 2010). For example, ENM approaches have been used to predict the spatial distribution of disease vectors (Costa et al. 2002) and pathogens (Nakazawa et al. 2010). Likewise, ENMs can be used to predict the potential distribution of environmentally maintained pathogens, such as Bacillus anthracis, which causes anthrax (Blackburn et al. 2007; Joyner et al. 2010; Mullins et al. 2011; Mullins et al. 2013). The potential distribution of B. anthracis can be interpreted as landscapes characterized by biotic and abiotic conditions that support the persistence of B. anthracis spores. These regions present a potential risk of anthrax transmission to susceptible species using these landscapes.
Anthrax transmission is complex and poorly understood. Under certain environmental conditions, the pathogen can persist for extended periods (Hugh-Jones and Blackburn 2009), and the onset of an anthrax epizootic is likely triggered by seasonal climate events (Hampson et al. 2011; Blackburn and Goodin 2013; Turner et al. 2013). In higher latitudes anthrax tends to be a summertime disease, and anthrax outbreaks have been associated with rain during a hot, dry summer in North America and Australia (Turner et al. 1999; Parkinson et al. 2003; Blackburn and Goodin 2013). More recently, serological evidence confirms exposure may be frequent in wildlife populations with individuals surviving sub-lethal infections (Bagamian et al. 2013). Exposure is most likely through ingestion during grazing or foraging (Turner et al. 2013), including grazing at carcass sites of previous anthrax deaths (Turner et al. 2014) with localized secondary cases from (short-term) mechanical transmission from necrophagous or hematophagous flies on certain landscapes (Blackburn et al. 2014b; Blackburn et al. 2014c).
Anthrax remains a problem for wildlife and livestock in many parts of North America (Blackburn et al. 2007; Blackburn and Goodin 2013; Blackburn et al. 2014c). Recent outbreaks have been reported in free ranging plains bison (Bison bison) in Canada (Shury, Frandsen and O’Brodovich 2009) and white-tailed deer (Odocoileus virginianus) in west Texas (Blackburn and Goodin 2013). In southwest Montana, a large outbreak occurred in summer 2008 affecting a managed bison herd and free ranging elk on private land (Blackburn et al. 2014a). That outbreak was the first reported anthrax in western Montana since the mid-1950s (Hugh-Jones and Blackburn 2009). Subsequent cases were reported in the same bison herd in 2010 (Blackburn et al. 2014a). An ENM-based prediction of potential anthrax risk surrounding the 2008 outbreak area identified habitat across the region as suitable for pathogen persistence on public and private land (Morris et al. 2015). However, statewide anthrax risk across Montana remains unknown.
Anthrax management is challenging, expensive, and requires a multi-disciplinary and collaborative approach (Hugh-Jones and De Vos 2002; Blackburn et al. 2007). Effective vaccination is available for livestock, but the injectable vaccine is untenable for large wildlife populations across vast landscapes (Blackburn et al. 2007). Therefore, anthrax management in wildlife relies on surveillance and decontamination of infectious carcasses to prevent the formation of new infectious zones (Hugh-Jones and De Vos 2002). However, anthrax carcasses are difficult to find (Blackburn et al. 2014b) and the spatial distribution of risk is frequently under-estimated (Bellan et al. 2013). A priori knowledge of high risk regions could greatly enhance management efforts. Predicting risk is also important for identifying stakeholders, as Montana is ~65% private land, ~29% federal land, and ~6% state land and anthrax susceptible wildlife hosts are found on each. Establishing effective vaccination strategies for livestock and surveillance systems for wildlife necessitates identifying stakeholders and understanding disease risk across Montana.
The purpose of this study was to define potential anthrax risk across the state in an effort to understand risk across species, inform management, and identify stakeholders. The objectives of this analysis were to: 1) identify potential anthrax risk across Montana; 2) explore land ownership and management associated with landscapes predicted as potential anthrax risk; 3) quantify the spatial distribution of susceptible wildlife and livestock and their overlap with risk zones; and 4) discuss the implications of land ownership for anthrax management in Montana.
METHODS
Ecological Niche Model
To estimate the distribution of B. anthracis across Montana, we used an ENM for B. anthracis in the US developed by our research group. The genetic algorithm for rule set prediction (GARP) modeling system has been described in detail elsewhere (Stockwell and Peters 1999). Briefly, GARP is an iterative algorithm that searches for non-random relationships between point occurrence data and environmental coverages (raster files of climatic or environmental data). GARP models relationships using a series of If/Then logic statements (e.g. range rules or logistic regression) that are used to predict species’ presence or absence. GARP performs well across the spectrum of species’ prevalence on the landscape from rare to common making it useful for management oriented studies (McNyset and Blackburn 2006). GARP has been used to predict B. anthracis across multiple landscapes (Blackburn 2010; Joyner et al. 2010; Mullins et al. 2011; Blackburn et al. 2015).
We extracted the predicted distribution of B. anthracis across Montana from a 1 Km experiment of the continental US (Blackburn et al. In Review). We performed 10 GARP experiments using 200 models and the best subsets procedure (Anderson et al. 2003) to arrive at a subset of ten models in each experiment. To develop models, we used 50 spatially unique anthrax outbreaks from the A1.a sublineage, as the A1.a sublineage in dominant in regions spanning from south Texas through the Dakotas (Mullins et al. 2015). We expanded the outbreak dataset of Mullins et al. (2013) to include new confirmed A1.a outbreaks from Texas and Colorado. Outbreaks occurred between 2000 and 2012 and included culture confirmed mortality events in livestock and wildlife. We used the same environmental variables as Mullins et al. (2013): elevation, annual temperature range, annual mean temperature, precipitation of the driest month, precipitation of the wettest month, annual precipitation (WorldClim database (Hijmans et al. 2005)), normalized difference vegetation index (NDVI), NDVI annual amplitude and mean annual NDVI (Hay et al. 2006). We also added two soils variables to improve model accuracy: average soil pH and average organic content from the harmonized world soils database (HWSD; FAO/IIASA/CISS-CAS/JRC 2008). From the 10 experiments, we ranked and selected the best performing experiment from the accuracy metrics. The 10 experiments were based on 10 different random draws of 50 locations to build models and 16 outbreak locations to post hoc evaluate models. We calculated total and average omission (false negatives), total and average commission (the proportion of pixels predicted present) and the area under the curve (AUC) following McNyset (2005). The AUC compares model predictions to a random distribution with an AUC approaching 0.5 indicating a random distribution and an AUC approaching 1 indicating a perfect model (Zweig and Campbell 1993). While not a perfect metric (Lobo et al. 2008), AUC when evaluated together with omission and commission metrics, is useful for evaluating GARP models (McNyset 2005; Joyner et al. 2010). To define potential anthrax risk across Montana, we used the conservative cutoff described by Morris et al. (2015), which is agreement between 9 or 10 models in the final best subset.
Land Ownership
We downloaded owner parcel data for Montana from the Montana state library geographic information services (http://nris.mt.gov/gis/) to map land ownership. We collapsed land ownership into three primary categories: private, state, and federal. Federal land included the US Fish and Wildlife Service, Department of Defense, US Forest Service, US Bureau of Reclamation, and National Park Service land. State land included Montana State Trust lands, Montana University System, Montana Fish Wildlife and Parks, Montana Department of Transportation, Montana Department of Corrections, Montana Department of Natural Resources, and local government. We mapped each land ownership delineation and overlaid the ENM derived potential anthrax risk surface. We calculated the percentage of each land ownership category that overlapped pixels predicted as potential anthrax risk.
Species Distribution
We downloaded species’ ranges within Montana from the Montana Fish, Wildlife, and Parks (FWP) GIS database (http://fwp.mt.gov/doingBusiness/reference/gisData/dataDownload.html) for six ungulate species susceptible to anthrax: bighorn sheep (Buechner 1960; Van Ness 1981), elk (Blackburn et al. 2014a), moose (Dragon et al. 1999), mule deer (Blackburn 2006), pronghorn (Langford and Dorward 1977), and white-tailed deer (Blackburn et al. 2014b). Range data are generalized across seasons at ~1.6 Km2 resolution and are based on 1:100,000 scale Public Land Survey boundaries. Ranges do not include distribution estimates on National Park land. We mapped the predicted distribution for each species, overlaid distribution maps with the ENM-based potential anthrax risk map and land ownership delineations. We calculated the total area of each species’ range, the percent overlap with pixels predicted as potential anthrax risk, and the percent overlap with public and private land classifications.
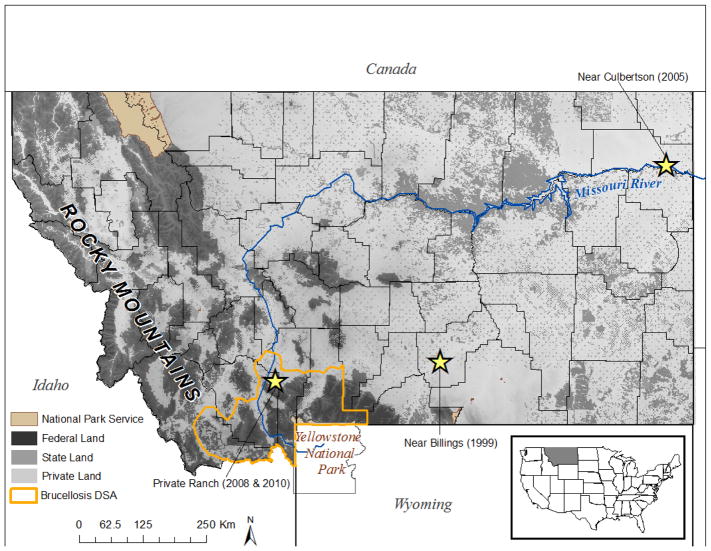
Approximately ~93,714 Km2 of Montana’s land area (~25% of the state) is designated as Bureau of Land Management (BLM) grazing allotments. A grazing allotment is land managed for grazing livestock, and can include private, state, and public lands. We downloaded BLM grazing allotment GIS data (https://data.doi.gov) from the Department of the Interior to represent the potential spatial distribution of grazing livestock across Montana. We overlaid grazing allotment polygons, which reflect pasture outlines, with the potential anthrax risk surface and land ownership categories. We calculated the percentage of grazing allotment area that overlapped pixels predicted as potential anthrax risk. We also calculated the percentage of overlap in each land ownership category. We also mapped the designated surveillance area (DSA) for brucellosis in Montana to illustrate the spatial extent of predicted brucellosis risk across the state.
RESULTS
Ecological Niche Model
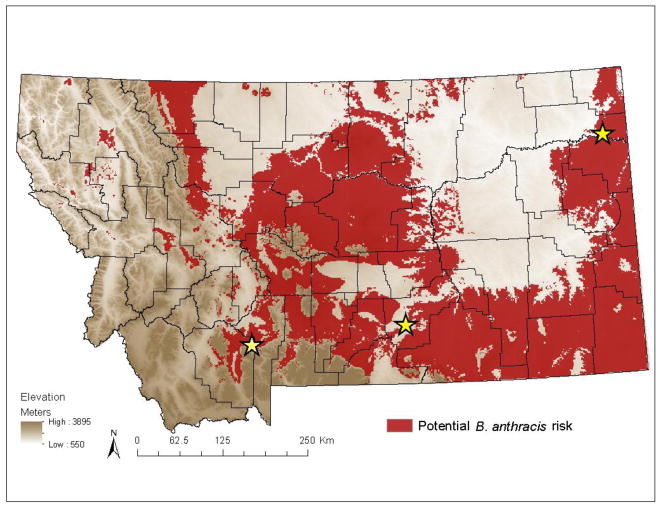
The model for the US had low omission (total = 0%, average = 0.6%) and a high AUC score (0.9446), suggesting an excellent model set (McNyset 2005). Using the conservative cutoff, ~140,539 Km2 were defined as potential anthrax risk in Montana (~37% of the total state area; Figure 2). Potential anthrax risk was predicted in the following areas: central Montana with a band expanding to the northwest, small regions along the north central border and northwest, the southern and eastern borders, and particularly the southeast corner where Montana meets North and South Dakota. North and South Dakota both have a long history of anthrax with recent epizootics (Kenefic et al. 2008; Mongoh et al. 2008).
Figure 2.
Regions predicted as potential anthrax risk across Montana derived from ecological niche models. High risk regions are shown in red. Historic anthrax events are represented with stars.
Land Ownership
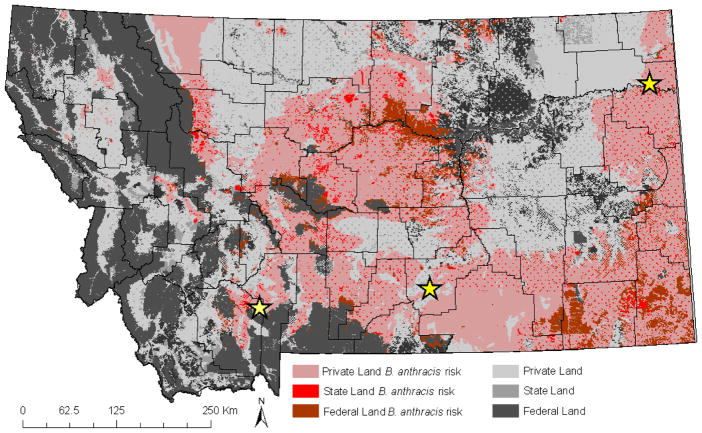
Areas of potential anthrax risk were predicted across federal, state, and private lands (Figure 3, Table 1). Approximately 14% of federal land in Montana was predicted as potential anthrax risk. The majority of overlap between federal land and potential anthrax risk was identified in small pockets in central and southeastern Montana. State owned land accounts for ~6% of Montana, and ~48% of that was predicted as potential anthrax risk. The largest regions of overlap between state-owned land and potential anthrax risk were in central and south central Montana. The majority of Montana is privately owned (~65%) and we predicted ~47% of private land as potential anthrax risk across southwestern, central and southeastern Montana.
Figure 3.
Land ownership associated with potential anthrax risk across Montana, as defined by ecological niche models. Land ownership is in shades of gray, and regions that overlap potential anthrax risk are in shades of red. Historic anthrax events are represented with stars.
Table 1.
The total area of each land ownership category in Montana and the percentage of each land ownership area that overlaps regions predicted as potential anthrax risk.
| Ownership | Area (Km2) | % area predicted as potential anthrax risk |
|---|---|---|
| Federal | 111,001 | 14% |
| State | 23,694 | 38% |
| Private | 247,434 | 47% |
| Total | 382,128 | 37% |
Species Range Overlap with Potential Anthrax Risk
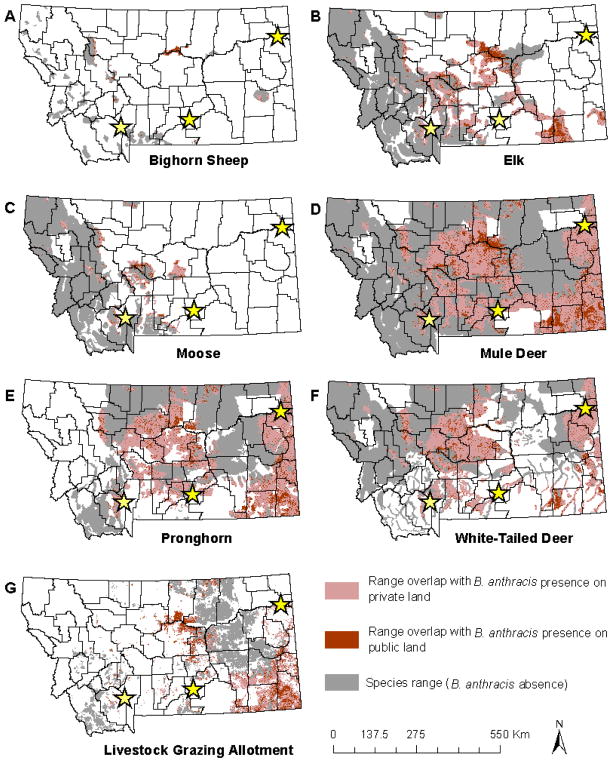
Geographic ranges for all six wildlife species overlapped with regions of potential anthrax risk. Overlap occurred on both public (state and federal) and private land (Figure 4, Table 2). Bighorn sheep had the smallest range of the six species and are primarily concentrated in western Montana. Approximately 11% of the range was in anthrax risk zones with ~56% on public and ~44% on private land. Elk are concentrated in the west and southwest reaching east into central Montana. Approximately 42,650 Km2 of the elk range overlapped anthrax risk zones, primarily in central Montana. Approximately 27% of overlap was on public and ~73% was on private land. Moose range across western Montana. An area of 10,337 Km2 overlapped regions of potential anthrax risk. Approximately 25% of the overlapping area was on public and ~75% was on private land. Mule deer range throughout much of the state and had the amount of overlap with potential anthrax risk areas. Approximately 20% of the overlap was on public and 80% was on private land. The pronghorn range stretches across the eastern half of the state and areas of overlap potential anthrax risk were identified in central and eastern Montana. Approximately 18% of overlap was on public and 82% of overlap was on private land. White-tailed deer range in the northern half of the state, and areas of overlap with potential anthrax risk were in central Montana and along the eastern border of Montana.
Figure 4.
Wildlife and livestock distribution, and overlap with potential anthrax risk in each land ownership category. The distribution of 6 anthrax susceptible wildlife species and livestock grazing allotments were mapped: bighorn sheep (panel A), elk (panel B), moose (panel C), mule deer (panel D), pronghorn (panel E), white-tailed deer (panel F), and livestock grazing allotments (panel G). Historic anthrax events are represented with stars.
Table 2.
The total area of species distribution estimates across Montana, the percentage of overlap between the species range and anthrax risk predictions, and the percentage of overlap between potential anthrax risk and the species range that is on public and private land.
| Species | Total Range Area (KM2) | % overlap with potential anthrax risk | % overlap on public land | % overlap on private land |
|---|---|---|---|---|
| Bighorn Sheep | 19,783 | 11% | 56% | 44% |
| Elk | 154,279 | 28% | 27% | 73% |
| Moose | 103,946 | 10% | 25% | 75% |
| Mule Deer | 339,893 | 37% | 20% | 80% |
| Pronghorn | 208,339 | 45% | 18% | 82% |
| White Tailed Deer | 217,899 | 35% | 16% | 84% |
| Livestock Grazing Allotment | 93,714 | 41% | 35% | 65% |
Livestock grazing allotments covered an area of 93,714 Km2 statewide and were primarily in the east and south Montana. Approximately 38,174 Km2 of the grazing allotment area overlapped regions predicted as potential anthrax risk. Approximately 35% of the overlap was on public and ~65% was on private land.
DISCUSSION
Our results suggest anthrax could be circulating in species and locations in Montana without a documented disease history. The 2008 outbreak was the first reported in southwestern Montana for decades, there were additional bison cases at this site in 2010 (Blackburn et al. 2014a), and livestock outbreaks were reported in Billings and Culbertson prior to 2008 (Blackburn et al. 2007). Our objective was to use an updated ENM-based prediction of potential anthrax risk across Montana and explore risk to wildlife and livestock across land ownership. We found that a significant portion of the Montana landscape is predicted as potential anthrax risk, and particularly the central and southeastern regions of the state. These high risk regions were on public and private land and overlapped the predicted distribution of multiple wildlife species and livestock grazing lands.
Our predictions provide a preliminary, yet valuable, insight into locations at risk of future epizootics. It is also important to note that our modeling efforts were based on mortality data, which are known to underestimate the intensity of outbreaks (Bellan et al. 2013) and potentially underestimate the geographic extent of outbreaks (Bagamian et al. 2013). It is possible that our predictions are under estimating the true extent of potential anthrax risk across the state, as anthrax carcasses can be difficult to detect on remote landscapes (Blackburn et al. 2014a) and there is limited anthrax surveillance in much of the state. There is also a history of under-reporting of anthrax in North America (Hugh-Jones and De Vos 2002), and enhanced surveillance and reporting would greatly increase our ability to predict the true distribution of the disease. Specifically, ante-mortem serological surveillance for antibodies to protective antigen could be used to estimate background prevalence. Bacteriological testing of materials (bone, tissue) from carcass surveys would inform anthrax-specific mortality estimates. Likewise, such data would serve to validate and improve ENM predictions.
We found regions of potential anthrax risk on federal, state, and private lands. Approximately 46% of privately owned land in Montana was classified as potential anthrax risk; private land comprises the majority of land ownership statewide. Approximately 38% of state land, and ~14% of federal land were also classified as potential anthrax risk. These findings highlight a need for collaborative anthrax management between federal, state, and private stakeholders across Montana. There are significant economic costs associated with large scale outbreaks related to the loss of livestock and associated market impacts, the loss of hunted wildlife populations and associated revenue, and cleanup costs. Our findings suggest that both public and private stakeholders are at risk of incurring these costs; however, implementing preventative measures could greatly reduce the risk of a large scale outbreak. Livestock operations in potential anthrax risk zones could implement preventative vaccination programs. Wildlife surveillance efforts could also be initiated in potential anthrax risk zones during summer months to identify and decontaminate carcasses to prevent/reduce the formation of new infectious zones. Surveillance efforts should be implemented on public and private lands, as there is evidence that multiple species could encounter the pathogen across land ownership categories.
The ranges of six important wildlife species across Montana overlapped potential anthrax risk areas on public and private land. The percentage of potential anthrax risk zones comprising a species range on public land ranged from ~16% to ~56%, and the potential anthrax risk zones on private land ranged from ~44% to ~84%. All species had range overlap with anthrax risk zones on both public and private land. The majority of bighorn sheep overlap with potential anthrax risk was on public land. Elk, moose, mule deer, and white-tailed had the majority of overlap with potential risk on private land. The FWP species’ range estimates used were based on general land surveys and do not provide detailed occupancy estimates or seasonal shifts in distribution; however, these general distributions are meaningful for state level analyses and are used for other management purposes. The range estimates did not include NPS land, as FWP does not have management authority in these regions, and future studies should explore potential anthrax risk in Montana NPS land to inform management. There is a significant bison population in Yellowstone National Park, and bison were greatly affected in the 2008 outbreak. Vaccination in the Yellowstone herd is untenable, in contrast to the bison herd affected in the 2008 outbreak (Blackburn et al. 2014a).
The risk of susceptible wildlife encountering B. anthracis on public lands is concerning, as all six species included in our analyses are hunted populations. Hunting is the primary source of funding for wildlife conservation in the United States, and big game hunting in particular (Peterson 2004; Geist 2006; Williams 2010). In 2014 there were ~144,638 mule and white-tailed deer hunting licenses and ~107,633 elk hunting licenses issued in Montana (http://fwp.mt.gov/hunting/planahunt/harvestReports.html). Deer and elk hunting provides over $50 million to Montana conservation efforts annually, which accounts for over 60% of Montana conservation agency revenue (Gude et al. 2012; Schorr et al. 2014). Approximately 16% of the white-tailed deer range, 20% of the mule deer range, and 27% of the elk range overlapped regions of potential anthrax risk. Additionally, ~14,858 pronghorn licenses, ~357 moose licenses, and ~388 bighorn sheep licenses were issued in 2014. These findings suggest that a large scale anthrax outbreak with significant wildlife mortalities on public land would likely have ramifications for hunting opportunities. Outbreaks have led to significant die offs in other wildlife species (Clegg et al. 2007; Muoria et al. 2007) including mortalities comprising up to 10% of the wild wood bison (Bison bison athabascae) population during outbreaks in Northern Canada (Salb et al. 2014). In addition to population effects, an anthrax outbreak could potentially create a public health concern for hunters. The archery season for bighorn sheep, elk, deer, and pronghorn starts in early September, and the 2008 anthrax epizootic in southwest Montana was primarily in late August (Blackburn et al. 2014a). A late summer anthrax outbreak could potentially overlap with the start of archery season.
Overlap between wildlife species’ ranges and potential anthrax risk zones were identified on private land for all six species. We found that over 70% of overlap between wildlife species ranges and potential anthrax risk for cervids and pronghorn was on private land. These findings are in line with the recent trend of wildlife increasingly using private lands in some regions of Montana, namely the greater Yellowstone ecosystem (GYE; (Gosnell et al. 2006; Haggerty and Travis 2006)). The recent shift from resident ranchers to absentee land owners has been linked to wildlife increasingly using private land. Resident ranchers whose livelihoods were dependent on livestock production considered wildlife a nuisance. Part-time residents with financial assets independent of livestock are generally tolerant or promote wildlife on their land (Haggerty and Travis 2006). Many of these non-traditional land owners have closed their properties to hunting opportunities, and the amount of land closed to all hunting in the GYE increased from 8% in 1979 to over 22% in 2003 (Haggerty and Travis 2006). Security from hunting and forage availability are likely related to big game species, such as elk, spending more time on private land (Haggerty and Travis 2006, Burcham, Edge and Marcum 1999, Proffitt et al. 2013). Wildlife on private land has resulted in a call for collaborative management between public and private stakeholders (Cross et al. 2010; Proffitt et al. 2013). The potential anthrax risk to wildlife on private land that we reported reiterates the need for collaborative management.
We also found that there was extensive overlap between livestock grazing allotments and regions predicted as potential anthrax risk statewide. Approximately 35% of this overlap was on public land and ~65% was on private land. These findings suggest that grazing livestock across the state could be affected by anthrax. Areas of overlap between potential anthrax risk and livestock provided here can be used to inform priority regions for livestock surveillance and preventative vaccine. There was also overlap between livestock grazing allotments and wildlife ranges across potential anthrax risk zones (Figure 4). For example, there is apparent overlap between mule deer, pronghorn, and grazing allotments in high risk regions in southeastern Montana. Bacillus anthracis is maintained in the environment for extended time periods and susceptible species that interact with infectious carcass sites could potentially become infected, which could result in spillover between species. For example, moose cases have been associated with large bison outbreaks in Canada due to either moose contacting infectious bison carcass sites or widespread environmental contamination (Dragon et al. 1999). There was also hypothesized spillover from a white-tailed deer outbreak in southwest Texas in 1997 to neighboring livestock populations (Hugh-Jones and De Vos 2002), which was confirmed in the region in 2009 and 2010 (Blackburn et al. 2014c). The overlap between livestock and wildlife in potential anthrax risk zones on public and private lands suggests a multi-species outbreak affecting public and private land owners is plausible statewide.
The complexities associated with anthrax management parallel many aspects of brucellosis management efforts in the GYE region of Montana. Both diseases affect wildlife and livestock on public and private land and can result in severe economic losses for stakeholders. A recent study suggested that brucellosis sero-prevalence could potentially be expanding outside of the boundaries of the DSA, and that targeted surveillance efforts at the borders of the DSA were warranted (Proffitt et al. 2015). The findings of our study suggest that anthrax could pose a risk to susceptible species using landscapes without a documented history of the disease and enhanced surveillance and serological testing are important management strategies (Bagamian et al. 2013). Successful brucellosis management is dependent on collaborations between state and federal agencies and private land owners (Cross et al. 2010), and has to account for societal tolerance of potential management strategies (Proffitt et al. 2015). Anthrax management efforts should contribute to the existing dialogue and relationships among stakeholders resulting from brucellosis management strategies in the region.
CONCLUSION
Wildlife and livestock could be at risk of contracting anthrax on public and private land across an extensive area of Montana. Effective management must be a collaboration between public and private stakeholders, as this is a multi-species concern that is potentially associated with economic losses and public health threats. Areas defined here could inform vaccination efforts for livestock (domestic cattle, bison, or small ruminants) and targeted surveillance could be initiated during the risk period across potential anthrax risk zones. In times of confirmed cases, methods used to search for carcasses in wooded areas of Canada (Dragon et al. 1999) could be used to detect carcasses in heavily forested landscapes. Serological screening of carnivores or scavengers (Bellan et al. 2013) could also be employed to determine if they have fed on infected carcasses in the region (Blackburn et al. 2014a). Improved anthrax surveillance and reporting across Montana could greatly enhance our knowledge of anthrax ecology and prevalence and improve our ability to effectively manage anthrax across the state.
Figure 1.
A map of Montana showing private, state, and federal land ownership. National park service land and the Designated Surveillance Area (DSA) for Brucellosis are also delineated. Historic anthrax events are represented with stars.
Acknowledgments
Funding for this study was provided by the National Institutes of Health Grant 1R01GM117617-01 to JKB, the College of Liberals Arts and Sciences and the Emerging Pathogens Institute at the University of Florida. We thank M.E. Hugh-Jones for information on anthrax in mule deer.
References
- Anderson R, Lew D, Peterson A. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol Model. 2003;162:211–232. [Google Scholar]
- Bagamian KH, Alexander KA, Hadfield TL, Blackburn JK. Ante-and postmortem diagnostic techniques for anthrax: Rethinking pathogen exposure and the geographic extent of the disease in wildlife. J Wildl Dis. 2013;49:786–801. doi: 10.7589/2013-05-126. [DOI] [PubMed] [Google Scholar]
- Bellan SE, Gimenez O, Choquet R, Getz WM. A hierarchical distance sampling approach to estimating mortality rates from opportunistic carcass surveillance data. Methods Ecol Evol. 2013;4:361–369. doi: 10.1111/2041-210x.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JK. Emerging and Endemic Pathogens. Springer; 2010. Integrating geographic information systems and ecological niche modeling into disease ecology: a case study of Bacillus anthracis in the United States and Mexico; pp. 59–88. [Google Scholar]
- Blackburn JK. Evaluating the spatial ecology of anthrax in North America: Examining epidemiological components across multiple geographic scales using a GIS-based approach 2006 [Google Scholar]
- Blackburn JK, Asher V, Stokke S, Hunter DL, Alexander KA. Dances with anthrax: wolves (Canis lupus) kill anthrax bacteremic plains bison (Bison bison bison) in southwestern Montana. J Wildl Dis. 2014a;50:393–396. doi: 10.7589/2013-08-204. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Goodin DG. Differentiation of springtime vegetation indices associated with summer anthrax epizootics in west Texas, USA, deer. J Wildl Dis. 2013;49:699–703. doi: 10.7589/2012-10-253. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Hadfield TL, Curtis AJ, Hugh-Jones ME. Spatial and Temporal Patterns of Anthrax in White-Tailed Deer, Odocoileus virginianus, and Hematophagous Flies in West Texas during the Summertime Anthrax Risk Period. Ann Assoc Am Geogr. 2014b;104:939–958. [Google Scholar]
- Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am J Trop Med Hyg. 2007;77:1103–1110. [PubMed] [Google Scholar]
- Blackburn JK, Mullins JC, Rashid M, Van Ert M, Bowen RA, Hugh-Jones ME, Hadfield TL. A revised prediction of the Western North America lineage of Bacillus anthracis for the continental United States: pathogen re-emergence or under-reporting? [Google Scholar]
- Blackburn JK, Odugbo MO, Van Ert M, O’Shea B, Mullins J, Perrenten V, Maho A, Hugh-Jones M, Hadfield T. Bacillus anthracis Diversity and Geographic Potential across Nigeria, Cameroon and Chad: Further Support of a Novel West African Lineage. PLoS Negl Trop Dis. 2015;9:e0003931. doi: 10.1371/journal.pntd.0003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JK, Van Ert M, Mullins JC, Hadfield TL, Hugh-Jones ME. The Necrophagous Fly Anthrax Transmission Pathway: Empirical and Genetic Evidence from Wildlife Epizootics. Vector-Borne Zoonotic Dis. 2014c;14:576–583. doi: 10.1089/vbz.2013.1538. [DOI] [PubMed] [Google Scholar]
- Brook RK, McLachlan SM. Transdisciplinary habitat models for elk and cattle as a proxy for bovine tuberculosis transmission risk. Prev Vet Med. 2009;91:197–208. doi: 10.1016/j.prevetmed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Buechner HK. The Bighorn Sheep in the United States, Its Past, Present, and Future. Wildl Monogr. 1960:3–174. doi: 10.2307/3830515. [DOI] [Google Scholar]
- Carstensen M, O’Brien DJ, Schmitt SM. Public acceptance as a determinant of management strategies for bovine tuberculosis in free-ranging US wildlife. Vet Microbiol. 2011;151:200–204. doi: 10.1016/j.vetmic.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Cheville NF, McCullough DR, Paulson LR. Brucellosis in the greater Yellowstone area. National Academies Press; 1998. [PubMed] [Google Scholar]
- Clegg S, Turnbull P, Foggin C, Lindeque P. Massive outbreak of anthrax in wildlife in the Malilangwe Wildlife Reserve, Zimbabwe. Vet Rec. 2007;160:113–118. doi: 10.1136/vr.160.4.113. [DOI] [PubMed] [Google Scholar]
- Costa J, Peterson AT, Beard CB. Ecologic niche modeling and differentiation of populations of Triatoma brasiliensis neiva, 1911, the most important Chagas’ disease vector in northeastern Brazil (hemiptera, reduviidae, triatominae) Am J Trop Med Hyg. 2002;67:516–520. doi: 10.4269/ajtmh.2002.67.516. [DOI] [PubMed] [Google Scholar]
- Cross P, Cole E, Dobson A, Edwards W, Hamlin K, Luikart G, Middleton A, Scurlock B, White P. Probable causes of increasing brucellosis in free-ranging elk of the Greater Yellowstone Ecosystem. Ecol Appl. 2010;20:278–288. doi: 10.1890/08-2062.1. [DOI] [PubMed] [Google Scholar]
- Dragon D, Elkin B, Nishi J, Ellsworth T. A review of anthrax in Canada and implications for research on the disease in northern bison. J Appl Microbiol. 1999;87:208–213. doi: 10.1046/j.1365-2672.1999.00872.x. [DOI] [PubMed] [Google Scholar]
- FAO/IIASA/CISS-CAS/JRC. Harmonized world soil database (version 1.0) FAO; Rome, Italy and Laxenburg, Austria: 2008. [Google Scholar]
- Geist V. The North American model of wildlife conservation: a means of creating wealth and protecting public health while generating biodiversity. Gaining Ground Purs Ecol Sustain. 2006:285–293. [Google Scholar]
- Gortazar C, Diez-Delgado I, Barasona JA, Vicente J, De La Fuente J, Boadella M. The wild side of disease control at the wildlife-livestock-human interface: a review. Front Vet Sci. 2015;1:27. doi: 10.3389/fvets.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell H, Haggerty JH, Travis WR. Ranchland ownership change in the Greater Yellowstone Ecosystem, 1990–2001: implications for conservation. Soc Nat Resour. 2006;19:743–758. [Google Scholar]
- Gude JA, Cunningham JA, Herbert JT, Baumeister T. Deer and elk hunter recruitment, retention, and participation trends in Montana. J Wildl Manag. 2012;76:471–479. [Google Scholar]
- Haggerty JH, Travis WR. Out of administrative control: absentee owners, resident elk and the shifting nature of wildlife management in southwestern Montana. Geoforum. 2006;37:816–830. [Google Scholar]
- Hampson K, Lembo T, Bessell P, Auty H, Packer C, Halliday J, Beesley CA, Fyumagwa R, Hoare R, Ernest E. Predictability of anthrax infection in the Serengeti, Tanzania. J Appl Ecol. 2011;48:1333–1344. doi: 10.1111/j.1365-2664.2011.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Tatem A, Graham A, Goetz S, Rogers D. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006;62:37–77. doi: 10.1016/S0065-308X(05)62002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Hugh-Jones M, Blackburn J. The ecology of Bacillus anthracis. Mol Aspects Med. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones M, De Vos V. Anthrax and wildlife. Rev Sci Tech-Off Int Epizoot. 2002;21:359–384. doi: 10.20506/rst.21.2.1336. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner TA, Lukhnova L, Pazilov Y, Temiralyeva G, Hugh-Jones ME, Aikimbayev A, Blackburn JK. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PloS One. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenefic L, Beaudry J, Trim C, Daly R, Parmar R, Zanecki S, Huynh L, Van Ert M, Wagner D, Graham T. High resolution genotyping of Bacillus anthracis outbreak strains using four highly mutable single nucleotide repeat markers. Lett Appl Microbiol. 2008;46:600–603. doi: 10.1111/j.1472-765X.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- Langford E, Dorward W. Erysipelothrix insidiosa recovered from sylvatic mammals in northwestern Canada during examinations for rabies and anthrax. Can Vet J. 1977;18:101. [PMC free article] [PubMed] [Google Scholar]
- Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17:145–151. [Google Scholar]
- McNyset K. Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecol Freshw Fish. 2005;14:243–255. [Google Scholar]
- McNyset K, Blackburn J. Does GARP really fail miserably? A response to Stockman et al.(2006) Divers Distrib. 2006;12:782–786. [Google Scholar]
- Miller RS, Farnsworth ML, Malmberg JL. Diseases at the livestock–wildlife interface: status, challenges, and opportunities in the United States. Prev Vet Med. 2013;110:119–132. doi: 10.1016/j.prevetmed.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongoh MN, Dyer NW, Stoltenow CL, Khaitsa ML. Risk factors associated with anthrax outbreak in animals in North Dakota, 2005: A retrospective case-control study. Public Health Rep. 2008;123:352–359. doi: 10.1177/003335490812300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LR, Proffitt KM, Asher V, Blackburn JK. Elk resource selection and implications for anthrax management in Montana. 2015 doi: 10.1002/jwmg.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins JC, Garofolo G, Van Ert M, Fasanella A, Lukhnova L, Hugh-Jones ME, Blackburn JK. Ecological niche modeling of Bacillus anthracis on three continents: evidence for genetic-ecological divergence? PloS One. 2013;8:e72451. doi: 10.1371/journal.pone.0072451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins JC, Van Ert M, Hadfield TL, Nikolich MP, Hugh-Jones M, Blackburn JK. Spatio-temporal patterns of an anthrax outbreak in white-tailed deer, Odocoileus virginanus, and associated genetic diversity of Bacillus anthracis. 2015 doi: 10.1186/s12898-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J, Lukhnova L, Aikimbayev A, Pazilov Y, Van Ert M, Blackburn JK. Ecological Niche Modelling of the Bacillus anthracis A1. a sub-lineage in Kazakhstan. BMC Ecol. 2011;11:32. doi: 10.1186/1472-6785-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoria PK, Muruthi P, Kariuki WK, Hassan BA, Mijele D, Oguge NO. Anthrax outbreak among Grevy’s zebra (Equus grevyi) in Samburu, Kenya. Afr J Ecol. 2007;45:483–489. [Google Scholar]
- Nakazawa Y, Williams RA, Peterson AT, Mead PS, Kugeler KJ, Petersen JM. Ecological niche modeling of Francisella tularensis subspecies and clades in the United States. Am J Trop Med Hyg. 2010;82:912–918. doi: 10.4269/ajtmh.2010.09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DJ, Schmitt SM, Fitzgerald SD, Berry DE, Hickling GJ. Managing the wildlife reservoir of Mycobacterium bovis: the Michigan, USA, experience. Vet Microbiol. 2006;112:313–323. doi: 10.1016/j.vetmic.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Parkinson R, Rajic A, Jenson C. Investigation of an anthrax outbreak in Alberta in 1999 using a geographic information system. Can Vet J. 2003;44:315. [PMC free article] [PubMed] [Google Scholar]
- Peterson MN. An approach for demonstrating the social legitimacy of hunting. Wildl Soc Bull. 2004;32:310–321. [Google Scholar]
- Proffitt KM, Anderson N, Lukacs P, Riordan MM, Gude JA, Shamhart J. Effects of elk density on elk aggregation patterns and exposure to brucellosis. J Wildl Manag. 2015;79:373–383. [Google Scholar]
- Proffitt KM, Gude JA, Hamlin KL, Garrott RA, Cunningham JA, Grigg JL. Elk distribution and spatial overlap with livestock during the brucellosis transmission risk period. J Appl Ecol. 2011;48:471–478. [Google Scholar]
- Proffitt KM, Gude JA, Hamlin KL, Messer MA. Effects of hunter access and habitat security on elk habitat selection in landscapes with a public and private land matrix. J Wildl Manag. 2013;77:514–524. [Google Scholar]
- Salb A, Stephen C, Ribble C, Elkin B. DESCRIPTIVE EPIDEMIOLOGY OF DETECTED ANTHRAX OUTBREAKS IN WILD WOOD BISON (BISON BISON ATHABASCAE) IN NORTHERN CANADA, 1962–2008. J Wildl Dis. 2014;50:459–468. doi: 10.7589/2013-04-095. [DOI] [PubMed] [Google Scholar]
- Schorr RA, Lukacs PM, Gude JA. The Montana deer and Elk hunting population: The importance of cohort group, license price, and population demographics on hunter retention, recruitment, and population change. J Wildl Manag. 2014;78:944–952. doi: 10.1002/jwmg.732. [DOI] [Google Scholar]
- Sells SN, Mitchell MS, Nowak JJ, Lukacs PM, Anderson NJ, Ramsey JM, Gude JA, Krausman PR. Modeling risk of pneumonia epizootics in bighorn sheep. J Wildl Manag. 2015;79:195–210. doi: 10.1002/jwmg.824. [DOI] [Google Scholar]
- Stockwell D, Peters D. The GARP modelling system: problems and solutions to automated spatial prediction. Int J Geogr Inf Sci. 1999;13:143–158. [Google Scholar]
- Turner A, Galvin J, Rubira R, Miller G. Anthrax explodes in an Australian summer. J Appl Microbiol. 1999;87:196–199. doi: 10.1046/j.1365-2672.1999.00869.x. [DOI] [PubMed] [Google Scholar]
- Turner WC, Imologhome P, Havarua Z, Kaaya GP, Mfune JK, Mpofu ID, Getz WM. Soil ingestion, nutrition and the seasonality of anthrax in herbivores of Etosha National Park. Ecosphere. 2013;4:art13. [Google Scholar]
- Turner WC, Kausrud KL, Krishnappa YS, Cromsigt JP, Ganz HH, Mapaure I, Cloete CC, Havarua Z, Küsters M, Getz WM. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proc R Soc B Biol Sci. 2014;281:20141785. doi: 10.1098/rspb.2014.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness GB. Anthrax. Diseases and parasites of white-tailed deer 1981 [Google Scholar]
- Watson R. Public wildlife on private land: Unifying the split estate to enhance trust resources. Duke Envtl Pol F. 2012;23:291. [Google Scholar]
- Williams S. Wellspring of wildlife funding 2010 [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine [published erratum appears in Clin Chem 1993 Aug; 39 (8): 1589] Clin Chem. 1993;39:561. [PubMed] [Google Scholar]