Abstract
Background
Traditional methods of staging chronic rhinosinusitis (CRS) through imaging do not differentiate between degrees of partial mucosal sinus inflammation, thus limiting their utility as imaging biomarkers. We hypothesized that software-aided, quantitative measurement of sinus inflammation would generate a metric of disease burden that would correlate with clinical parameters in patients with suspected sinus disease.
Methods
Adults with rhinologic complaints undergoing CT imaging were recruited at an urban, academic, tertiary care center (n=45 with Lund-Mackay [LM] scores ≥ 4). 3D volumetric image analysis was performed using a semi-automated method to obtain a “Chicago-modified Lund-Mackay” (Chicago MLM) score, which provides a continuous scale to quantify extent of opacification. Linear regression was used to test the association of the Chicago MLM score with concurrent symptoms (total nasal symptom scores [TNSS]) and disease-specific quality of life (Sinonasal Outcome Test-22 [SNOT22]).
Results
Chicago MLM scores were significantly associated with both symptoms (p=0.037) and disease-specific quality of life (p=0.007). Inflammation in the ethmoid and sphenoid sinuses appeared to influence these associations. These findings were even more robust when analysis was limited to patients with more severe disease (LM>6).
Conclusions
The quantitative measurement of sinus inflammation by computer-aided 3D analysis correlates modestly with both symptoms and disease-specific quality of life. Posterior sinuses appear to have the greatest impact on these findings, potentially providing an anatomic target for clinicians to base therapy. The Chicago MLM score is a promising imaging biomarker for clinical and research use.
Keywords: Spiral Computed Tomography, Quality of Life, Sinusitis, Biomarkers, Paranasal Sinus Diseases
Introduction
Chronic rhinosinusitis (CRS) is a highly prevalent airway disease affecting 1 in 8 adults in the United States with a large associated economic burden.1 This disorder has major adverse effects on patient quality of life and significantly hinders patients’ daily activities and social function.2 However, despite its public health impact, there are no well-defined biomarkers of CRS, a barrier that inhibits research and makes patient management more challenging in terms of assessing severity of disease and response to treatment. One area that suffers most is the evaluation of treatment efficacy; thus, there are no Food and Drug Administration (FDA)-approved treatments for this disease to date.
Computed tomography (CT) imaging is a commonly used diagnostic tool that can delineate the extent of mucosal inflammation in the paranasal sinuses in CRS patients. Currently, the most widely used radiographic staging method for CRS is the Lund-Mackay (LM) score, where an examiner subjectively assigns a score of 0 (no abnormalities), 1 (partial opacification), or 2 (complete opacification) for each of the 10 sinuses and a 0 or 2 (for clear or blocked) for the osteomeatal complex on coronal images from CT imaging to attain a score that ranges from 0–24.3 While the LM system offers a simple method to determine burden of disease and has low inter-observer variability, it does not differentiate the extent of mucosal inflammation in each anatomic region and therefore falls short of being an effective disease assessment tool.
Subsequent studies have attempted to improve the LM score by for instance, expanding the opacification scales;4, 5, 6 however, the superiority of these methods over the traditional LM score is unclear, since increasing the number of subgrades inherently leads to lower inter-observer agreement and all these systems require human interpretation, which has potential for bias and error.
More recent studies have focused on creating an objective scoring system by utilizing software-based tools and measuring sinus inflammation using volumetric approaches. Pallanch et al. correlated changes in the percent of sinus opacification before and after triamcinolone treatment with changes in symptoms and endoscopic findings.7 Sedaghat and Bhattacharyya incorporated Hounsfield units into LM scores in an attempt to better utilize density information from the CT images.8 Both studies found that image scores better correlated with some (but not all) of the symptoms, suggesting the superiority of the objective software-based systems to subjective, visual scoring systems.
In prior work, our group developed a quantitative image analysis method and applied it to the measurement of sinus inflammation.9 The Chicago modified Lund-Mackay (Chicago MLM) scoring system (previously referred to as “modified Lund-Mackay system”9) uses specialized software to calculate the mucosa-to-sinus volume ratio through three-dimensional analysis of axial CT images. Unlike the LM system, the Chicago MLM system provides a quantitative and objective method to measure differing degrees of mucosal inflammation on a continuous scale from 0 to 1 (Figure 1). Chicago MLM scores from unselected patients undergoing CT scanning for various reasons positively correlated with the severity of sinonasal symptoms, whereas LM scores showed no such association in the same patients; however, neither LM nor Chicago MLM scores showed any association with disease-specific quality of life measurements, perhaps due to the relatively small sample size and the overall low severity of sinonasal disease among the unselected study subjects.
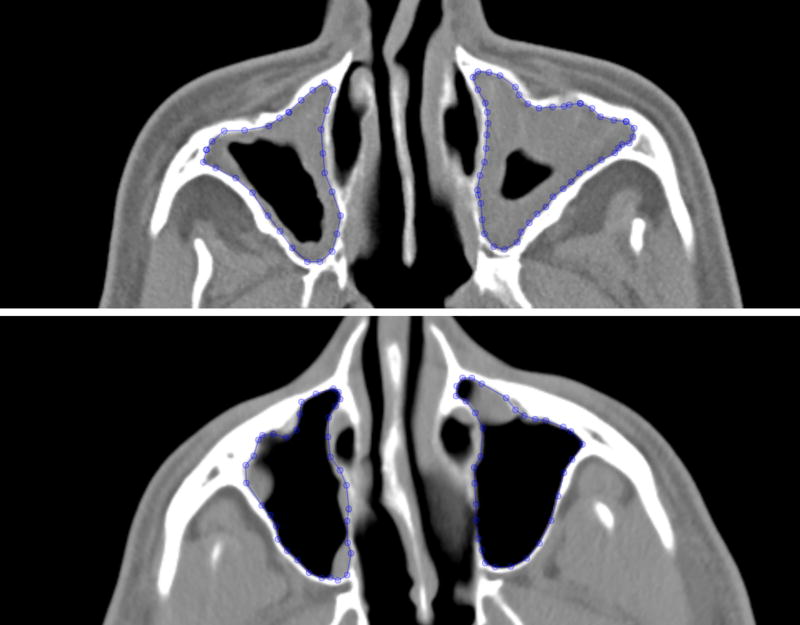
Figure 1.
Axial sinus CT images in two different patients with the manual outlines of the maxillary sinuses shown. Both patients received LM scores of 2 for their maxillary sinuses (1 for each sinus), but the Chicago MLM scores were 3.01 (top) and 1.59 (bottom).
To further characterize and compare the efficacy of the Chicago MLM relative to the LM method for patients with sinusitis, the present study was undertaken by focusing on CT images of patients with rhinologic complaints who have higher-than-baseline inflammation in the sinuses. We hypothesized that compared with LM scores, Chicago MLM scores would positively and more strongly correlate with both sinonasal symptoms and with quality of life scores in patients with more than minimal sinus disease.
Methods
Subjects Adults (≥ 18 years old) were recruited for this study with the following inclusion criteria: those who were a) undergoing imaging of the sinuses by CT for sinus complaints and b) who were able to provide written informed consent. Image data were provided to the study staff by the Human Imaging Research Office10 and were analyzed by 3D volumetric analysis. We excluded patients with: a total LM score less than 4 to focus on patients with a higher level of mucosal inflammation; failure to fully complete the questionnaires; and grossly distorted sinus anatomy (developmental abnormalities, radical surgery for tumors) preventing clear identification of the sinuses. The study was approved by the University of Chicago Institutional Review Board. Subjects were recruited over two time periods: July 2012 to August 2013 and May 2015 to August 2015. During this time, total of 96 patients were recruited, of whom 45 met the inclusion criteria. All patients getting sinus scans had rhinologic complaints (such as congestions or post nasal drip), including 2 patients undergoing evaluation prior to stem cell transplantation. The first period represented subjects who were analyzed in our initial study9 and who further met the LM ≥ 4 criterion (n=23). The second period added new subjects for the present study (n=22).
Clinical Metrics
Immediately prior to CT imaging, patients were asked to complete two validated surveys: the Total Nasal Symptom Score (TNSS), which measures nasal symptom severity, and the Sinonasal Outcomes Test-22 (SNOT22), which measures disease-specific quality of life. TNSS is a 4-item questionnaire used to rate the severity of sinonasal symptoms (sneezing, runny nose, stuffy nose, and other symptoms) on a 4-point scale from 0 (none) to 3 (severe), with a range of 0 to 12.11 The SNOT22 is a 22-item questionnaire used to rate quality of life measures of sinonasal function on a 6-point scale from 0 (no problem) to 5 (problem as bad as it can be), with a total range of 0 to 110.12 For both questionnaires, higher scores indicate more severe symptoms or degraded quality of life. Demographic information such as age, gender, presence of hay fever/allergic rhinitis (as defined by self-report from the question “have you ever been diagnosed with hay fever/allergic rhinitis”), and smoking status was also collected at this time.
3D Volumetric Image Analysis
CT scanning of the paranasal sinuses was performed using a Brilliance CT 64-channel scanner and a Brilliance CT 16P scanner (Philips Healthcare, Cleveland, OH). CT scans with section thickness of 3mm were analyzed. Axial CT images were manually segmented using in-house software (ABRAS), which allows users to visualize and manipulate all sections of a CT scan.13 For each axial CT section, each sinus was manually outlined and labeled by three trained observers (M.R., M.K.F., S.L.) and every axial slice that contained a sinus cavity was delineated. ABRAS allows the user to label the anatomic location (maxillary, anterior or posterior ethmoid, sphenoid, or frontal) of each individual sinus outline. All outlines were independently reviewed for their accuracy by a board-certified rhinologist (J.M.P.) or a board-certified radiologist with certificate of added qualification in neuroradiology (D.T.G.).
Chicago Modified Lund-Mackay Scoring System
Outlines of the sinuses were exported to an in-house volumetric analysis software tool.14 This program uses a Hounsfield unit (HU) threshold to identify pixels belonging to the mucosa within the sinus outline in a single CT section image. The HU range −500 to +150 (non-inclusive) was used to define mucosal pixels. The software then examined all CT sections to calculate: 1) the total volume of mucosal inflammation, 2) the total sinus volume, as measured by the sinus outline, and 3) the ratio of mucosal inflammation to sinus volume for each sinus. Chicago MLM scores were calculated for each sinus by multiplying the mucosa-to-sinus volume ratio (a continuous value between 0 and 1) by 2 to match the range of values of the traditional LM system and summed to obtain the total Chicago MLM score for the sinuses (maxillary, anterior and posterior ethmoid, frontal and sphenoid). Traditional LM scores were assigned to each sinus by a board-certified rhinologist (J.M.P.) or a board-certified radiologist (D.T.G.). Of note, the osteomeatal complex (OMC) was not included in the Chicago MLM scoring system since this structure cannot be well visualized on axial scans for the purpose of outlining structure boundaries. Therefore, the main comparison was between LM without OMC and Chicago MLM. Investigators outlining, reviewing, and scoring the scans were blinded to all clinical data of the subjects.
Statistical Analysis
The Mann-Whitney U test was performed to compare the LM without OMC and Chicago MLM scores. Associations of Chicago MLM scores with TNSS and SNOT22 scores were evaluated using univariate linear regression models with Chicago MLM as the dependent variable and TNSS or SNOT22 scores as the independent variable. Bivariate linear regression was performed to assess the role of individual confounding factors such as age, gender, presence of hay fever or allergic rhinitis, symptoms of allergy, tobacco use, presence of a cold on the day of scan, presence of a cold within 3 weeks prior to the scan, season, mean time spent outside per day, outdoor occupation, and exposure to chemicals or pollutants. Multivariate linear regression models were constructed to include age and tobacco use; these covariates were chosen as they may affect mucosal inflammation.15, 16, 17, 18, 19 Associations between LM without OMC scores and TNSS and SNOT22 scores were evaluated in a similar fashion. Lastly, associations between individual sinus Chicago MLM scores and TNSS or SNOT22 scores were evaluated. All analyses were performed using R-Console (www.r-project.org).
Results
Subject Demographics and CT Staging
There were 45 subjects (male: 23, female: 22) who met entry criteria. The mean age was 49.3 years. The patient demographic data is summarized in Supplementary Table 1. The median LM score with OMC was 8 (range 4 to 21), reflecting mild-to-moderate disease. The median LM without OMC score was 7 (range 1 to 17), while the median Chicago MLM score was 4.28 (range 1.30 to 18.03) (Supplementary Table 1). This difference was statistically significant as the LM method generally over-estimated the extent of inflammation compared with the Chicago MLM in our study group (p=0.015).
Relationship between CT Staging, Symptoms, and Quality of Life
In univariate models, increased mucosal inflammation as captured by the Chicago MLM score was significantly associated with increased symptoms (i.e., greater TNSS scores) as well as worse quality of life (i.e., increased SNOT22 scores) (β=0.455, p=0.037; β=0.072, p=0.007, respectively) (Table 1). These associations remained significant after adjustment for age and smoking status in multivariate models (Tables 2 and 3). In contrast, the associations between LM without OMC scores and TNSS or SNOT22 scores failed to achieve statistical significance (β=0.139, p=0.465; β=0.034, p=0.145, respectively) (Table 1). Inclusion of the OMC in the LM score (as originally described in its scoring) did not alter these results (Table 1). The association between Chicago MLM scores and TNSS or between Chicago MLM scores and SNOT22 was not affected by the following comorbidities or demographics, such as: age, presence of hay fever/allergic rhinitis, tobacco use, presence of cold on the day of or within 3 weeks prior to the scan, outdoor occupation, hours spent outside, and exposure to chemicals or pollutants (data not shown). The correlations remained significant (p < 0.05) with improved β values when LM cut off was increased to 5 and to 6. At LM cutoff of 6, LM and LM w/o OMC scores vs. SNOT22 also approached significant p-values (Supplementary tables 7-1 and 7-2). Next, the association of Chicago MLM scores with specific subdomains of the SNOT22 was examined. Chicago MLM scores tended to correlate with sinonasal-specific components such as blockage and congestion of the nose and post-nasal discharge, while these scores did not correlate significantly with more systemic symptoms (e.g., being sad or embarrassed) (Supplementary Table 6-1). Note that results in Supplementary Table 6-1 is an exploratory data, as data in this table were not corrected for multiple comparisons given the limits of sample size.
Table 1.
Linear regression models for LM (with/without OMC) and Chicago MLM score vs. TNSS and SNOT22
| Model | LM (without OMC) | LM (with OMC) | Chicago MLM |
|---|---|---|---|
|
| |||
| TNSS | β = 0.139 | β = 0.165 | β = 0.455 |
| (p= 0.465) | (p=0.500) | (p= 0.037)* | |
| (R2=0.013) | (R2=0.011) | (R2=0.098) | |
|
| |||
| SNOT22 | β = 0.034 | β = 0.051 | β = 0.072 |
| (p=0.145) | (p=0.094) | (p=0.007)** | |
| (R2=0.049) | (R2=0.064) | (R2=0.156) | |
Statistically significant (p<0.05)
R2= Multiple R-squared
LM = Lund-Mackay; MLM = Modified Lund-Mackay; OMC = osteomeatal complex; SNOT22 = Sino-Nasal Outcome Test 22; TNSS = Total Nasal Symptom Score
Table 2.
Multivariate regression models for Chicago MLM score vs. TNSS
| TNSS vs. Age | TNSS vs. Tobacco | TNSS vs. Age vs. Tobacco | |
|---|---|---|---|
|
| |||
| TNSS | β = 0.467 | β = 0.465 | β = 0.476 |
| (p=0.034)* | (p=0.032)* | (p=0.030)* | |
| (R2=0.063) | (R2=0.089) | (R2=0.073) | |
|
| |||
| Age | β = 0.025 | β = 0.023 | |
| (p= 0.552) | (p=0.582) | ||
|
| |||
| Tobacco use (Currently) | β = −3.531 | β = −3.468 | |
| (p=0.218) | (p=0.230) | ||
Statistically significant (p<0.05)
R2= Adjusted R-squared
MLM = Modified Lund-Mackay; TNSS = Total Nasal Symptom Score
Table 3.
Multivariable regression models for Chicago MLM score vs. SNOT22
| SNOT22 vs. Age |
SNOT22 vs. Tobacco |
SNOT22 vs. Age vs. Tobacco |
|
|---|---|---|---|
|
| |||
| SNOT22 | β = 0.075 | β = 0.070 | β = 0.073 |
| (p= 0.006)* | (p= 0.009) * | (p= 0.008) * | |
| (R2=0.1296) | (R2=0.136) | (R2=0.128) | |
|
| |||
| Age | β = 0.033 | β = 0.031 | |
| (p= 0.418) | (p= 0.449) | ||
|
| |||
| Tobacco use (Currently) | β = −2.744 | β = −2.630 | |
| (p= 0.325) | (p= 0.348) | ||
Statistically significant (p<0.05)
R2= Adjusted R-squared
MLM = Modified Lund-Mackay; SNOT22 = Sino-Nasal Outcome Test 22
The next analyses examined whether the association between Chicago MLM scores and symptoms or quality of life was influenced by specific sinuses. Increases in sphenoid Chicago MLM scores (obtained by adding MLM of each side of the sinuses) were significantly associated with increased symptoms (β=1.563, p=0.004), and posterior ethmoid approached near significance (β=0.901, p=0.051) (Table 4). Increased Chicago MLM scores of each individual sinus were significantly associated with worse quality of life with the exception of the maxillary sinus, which was nearly significant (anterior ethmoid β=8.795, p=0.006; posterior ethmoid β=7.749, p=0.035; sphenoid β =12.149, p=0.005; frontal β=8.042, p=0.020; maxillary β=5.901, p=0.072).
Table 4.
Linear regression models for individual sinus Chicago MLM score vs. TNSS or SNOT22
| Sinuses | TNSS | SNOT22 |
|---|---|---|
|
| ||
| Anterior ethmoid | β =0.754 | β =8.795 |
| (p=0.068) | (p=0.006)** | |
| (R2=0.075) | (R2=0.161) | |
|
| ||
| Posterior ethmoid | β = 0.901 | β = 7.749 |
| (p= 0.051) | (p=0.035)* | |
| (R2=0.086) | (R2=0.100) | |
|
| ||
| Sphenoid | β = 1.563 | β =12.149 |
| (p=0.004)* | (p=0.005)** | |
| (R2=0.173) | (R2=0.165) | |
|
| ||
| Frontal | β = 0.617 | β =8.042 |
| (p=0.164) | (p=0.020)* | |
| (R2=0.045) | (R2=0.119) | |
|
| ||
| Maxillary | β =0.571 | β =5.901 |
| (p=0.168) | (p=0.072) | |
| (R2=0.044) | (R2=0.074) | |
Statistically significant (p<0.05)
R2= Multiple R-squared
MLM = Modified Lund-Mackay; SNOT22 = Sino-Nasal Outcome Test 22
Discussion
LM is the most commonly used imaging scoring system for CRS; however, nearly all studies thus far have found that LM scores do not correlate well with patient symptoms or quality of life measures.20, 21, 22, 23 Moreover, one study showed that differences in LM score before and after intervention did not correlate with the extent of symptom improvement, which further suggests that the LM score is not an ideal tool to evaluate the efficacy of new treatments.7 Image-based measurement of sinus inflammation by computer-aided 3D analysis and the Chicago MLM score correlated with both symptoms and disease-specific quality of life, which supports our prior work and compares favorably with other ongoing efforts in the field.7, 24 The finding that mucosal inflammation (as measured by the Chicago MLM score) significantly correlates with disease-specific quality of life score (as measured by SNOT22) is especially notable, given the numerous factors that can affect this score. We also observed a trend of strengthened associations when the LM cutoff was increased to 5 and 6. While doing so decreases the number of subjects available for analysis, the observed trend suggests that focusing on even more severe disease may be fruitful in the future.
These results contradict other prior studies, likely related to the fact that the Chicago MLM score better captures disease burden.20, 21, 22, 23, 25 Interestingly, comorbidities and demographic characteristics did not substantially affect these findings in our analyses. When individual sinuses were assessed, positive and statistically significant associations were found between posterior ethmoid sinus and sphenoid sinus Chicago MLM scores and quality of life scores, and near-significant association between posterior ethmoid sinus and symptom scores. These data indicate that inflammation in more posterior sinuses may have a greater role in patient symptom burden compared with that in other sinuses and should influence thinking about equal scoring weights for each sinus in other staging systems. The varying contributions to disease burden from the different sinuses indicate that anatomic location of the inflammation can be useful knowledge for clinicians in the assessment of symptoms and, potentially, in the targeting of therapy.
The present study included any patient with rhinologic complaints and examined a snapshot of their sinus inflammation and symptoms, both of which can vary over time. Due to logistical limits and issues related to radiation exposure, scores from multiple time points were not obtained. These issues plague many rhinosinusitis studies and are a barrier to progress given the lack of other objective and measurable metrics that correlate with disease. Many studies have examined the relationship between CT-based scores and symptoms or quality of life burden in a group of patients who meet the criteria of CRS.7, 8, 21, 23, 25 The present study attempted to capture patients with a spectrum of disease but with a minimum threshold by selecting patients with a LM score greater than or equal to 4. Although the use of image-based criterion (LM>4) adds a potential for bias since patients may have rhinologic complaints and meet clinical criteria for CRS without significant evidence on imaging studies, we opted to utilize a validated, objective measure of at least minimal sinus inflammation so as to be applicable to the typical patient in an rhinology practice.26, 27 Moreover, the median LM score in the present study was only 8 out of a total possible score of 24. Although these patients did not present at the extreme end of inflammation, statistically significant yet moderate correlations were observed. One of the limitations of the present study is the small sample size (n=45), which limits the power of the study to account for numerous variables that can affect mucosal inflammation beyond those examined in this study and may explain moderate correlations (as captured by R2 values). Thus, it is not known whether these results are generalizable to other clinical settings, which is a challenge for many studies in this field. A follow-up study with a larger sample size that can account for numerous variables which affect mucosal inflammation and patients with greater disease burden will be the necessary next step to further assess the implications and utility of the Chicago MLM score in CRS patients. Indeed, increasing the minimum LM score for study entry from 4 to 6 and re-analyzing the data tended to strengthen the reported results, suggesting that this tool would be even more useful in patients with medium-to-severe disease burden. We used TNSS score to assess symptom severity for ease in logistics of administering quick survey to patients waiting to get their CT scans, however, TNSS score contains sneezing component, which is more specific to allergic rhinitis. To ensure that this issue did not affect our results, we reanalyzed our data using the TNSS without the sneezing score and found that the results were unchanged and indeed were more robust (Supplementary tables 2-2, 3-2, 5-3). Using other measures that are more specific to CRS may be useful for future studies. Finally, the strength of these relationships is limited, likely due to the complex and multiple factors that affect both sinus inflammation and symptoms and quality of life. Future work will be needed to demonstrate that these statistically significant correlations are clinically meaningful.
Exclusion of the osteomeatal complex (OMC) was necessary due to difficulties in visualizing this region on axial scans, which were required by the semi-automated methodology. In order to compare Chicago MLM to LM, we used LM scores without OMC component for our main analysis. Obstruction of OMC is a highly relevant cause of CRS symptoms since it serves as a common drainage pathway for three out of five sinuses. Adapting this method for use with coronal CT scan images will prove useful in future work. Lastly, although the method is semi-automated and utilizes a software tool, it requires manual sinus outlines, which is time-consuming and subject to observer variability. Full automation of the entire process may help create an easy-to-use metric useful for surgeons, allergists, radiologists, and other clinicians who provide care for CRS patients.
Conclusion
In summary, the data support the utility of CT-based volumetric analysis of the sinuses to generate an objective imaging biomarker for CRS. Such an imaging biomarker may prove useful in therapeutic trials for this major disease.
Supplementary Material
Acknowledgments
We thank Gregory A. Christoforidis, M.D., for useful discussions and intellectual contributions. MR, JG, and SL were supported by the Pritzker School of Medicine’s Summer Research and Scholarship and Discovery Programs. This work was supported by the National Institute on Aging (AG036762), the National Institute of Allergy and Infectious Disease (Chronic Rhinosinusitis Integrated Studies Program- AI106683), and The University of Chicago Institute for Translational Medicine via the National Center for Advancing Translational Sciences (5UL1 TR 000430-09). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SGA receives loyalties and licensing fees through the University of Chicago for computer-aided diagnosis technology.
Footnotes
Disclosure: SGA receives loyalties and licensing fees through the University of Chicago for computer-aided diagnosis technology. All other authors declare no conflicts
This work was presented at the 2016 The American Academy of Allergy, Asthma & Immunology Annual meetings in Los Angeles, CA (March 6, 2016)
References
- 1.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;(260):1–161. [PubMed] [Google Scholar]
- 2.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113(1):104–9. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 3.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;31(4):183–4. [PubMed] [Google Scholar]
- 4.Zinreich SJ. Rhinosinusitis: radiologic diagnosis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S27–34. doi: 10.1016/S0194-59989770004-4. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair S. Correlation between Symptoms and Radiological Findings in Patients of Chronic Rhinosinusitis: A Modified Radiological Typing System. Rhinology. 2009;47:181–86. [PubMed] [Google Scholar]
- 7.Pallanch JF, Yu L, Delone D, et al. Three-dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: clinical correlations and comparison to Lund-Mackay scoring. Int Forum Allergy Rhinol. 2013;3(12):963–72. doi: 10.1002/alr.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedaghat AR, Bhattacharyya N. Chronic rhinosinusitis symptoms and computed tomography staging: improved correlation by incorporating radiographic density. Int Forum Allergy Rhinol. 2012;2(5):386–91. doi: 10.1002/alr.21042. [DOI] [PubMed] [Google Scholar]
- 9.Garneau J, Ramirez M, Armato SG, 3rd, et al. Computer-assisted staging of chronic rhinosinusitis correlates with symptoms. Int Forum Allergy Rhinol. 2015 doi: 10.1002/alr.21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armato SG, 3rd, Gruszauskas NP, Macmahon H, et al. Research imaging in an academic medical center. Acad Radiol. 2012;19:762–771. doi: 10.1016/j.acra.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Fairley JW, Yardley MPJ, Durham LH, Parker AJ. Reliability and validity of a nasal symptom questionnaire for use as an outcome measure in clinical research and audit. Clin Otolaryngol. 1993;18:436–437. [Google Scholar]
- 12.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 13.Starkey A, Sensakovic W, Armato S., 3rd Abras: a portable application for observer studies and visualization. Int J Comput Assist Radiol Surg; Computer Assisted Radiology and Surgery: Proceedings of the 25th International Congress and Exhibition; Berlin, Germany. June 22–25, 2011; 2011. pp. 193–195. CARS 2011. [Google Scholar]
- 14.Sensakovic WF, Pinto JM, Baroody FM, et al. Automated segmentation of mucosal change in rhinosinusitis patients. [10.1117/12.844282];Proceedings of SPIE 7624, Medical Imaging 2010: Computer-Aided Diagnosis. 2010 Mar 09; 76243N. [Google Scholar]
- 15.Cho SH, Hong SJ, Han B, et al. Age-Related Differences in the Pathogenesis of Chronic Rhinosinusitis. J Allergy Clin Immunol. 2012 Mar;129(3):858–860. doi: 10.1016/j.jaci.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins JG. Prevalence of Selected Chronic Conditions: United States, 1990–1992. Vital Health Stat 10. 1997 Jan;(194):1–89. [PubMed] [Google Scholar]
- 17.Loftus PA, Wise SK, Nieto D, et al. Intranasal Volume Increases with Age: Computed Tomography Volumetric Analysis in Adults. Laryngoscope. 2016 Oct;126(10):2212–15. doi: 10.1002/lary.26064. [DOI] [PubMed] [Google Scholar]
- 18.Lieu JC, Feinstein AR. Confirmations and Surprises in the Association of Tobacco Use With Sinusitis. Arch Otolaryngol Head Neck Surg. 2000 Aug;126(8):940–6. doi: 10.1001/archotol.126.8.940. [DOI] [PubMed] [Google Scholar]
- 19.Shi JB, Fu QL, Zhang H, et al. Epidemiology of Chronic Rhinosinusitis: Results from a Cross-Sectional Survey in Seven Chinese Cities. Allergy. 2015 May;70(5):533–9. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya T, Piccirillo J, Wippold FJ., 2nd Relationship between patient-based descriptions of sinusitis and paranasal sinus computed tomographic findings. Arch Otolaryngol Head Neck Surg. 1997;123(11):1189–92. doi: 10.1001/archotol.1997.01900110039006. [DOI] [PubMed] [Google Scholar]
- 21.Stewart MG, Sicard MW, Piccirillo JF, Diaz-Marchan PJ. Severity staging in chronic sinusitis: are CT scan findings related to patient symptoms? Am J Rhinol. 1999;13(3):161–7. doi: 10.2500/105065899781389704. [DOI] [PubMed] [Google Scholar]
- 22.Wabnitz DA, Nair S, Wormald PJ. Correlation between preoperative symptom scores, quality-of-life questionnaires, and staging with computed tomography in patients with chronic rhinosinusitis. Am J Rhinol. 2005;19(1):91–6. [PubMed] [Google Scholar]
- 23.Hopkins C, Browne PB, Slack R, et al. The Lund-Mackay Staging System for Chronic Rhinosinusitis: How Is It Used and What Does It Predict? Otolaryngol Head Neck Surg. 2007;137:555–61. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Deeb R, Malani PN, Gill B, et al. Three-dimensional volumetric measurements and analysis of the maxillary sinus. Am J Rhinol Allergy. 2011;25(3):152–6. doi: 10.2500/ajra.2011.25.3605. [DOI] [PubMed] [Google Scholar]
- 25.Ryan WR, Ramachandra T, Hwang PH. Correlations between Symptoms, Nasal Endoscopy, and in-Office Computed Tomography in Post-Surgical Chronic Rhinosinusitis Patients. Laryngoscope. 2011;121:674–678. doi: 10.1002/lary.21394. [DOI] [PubMed] [Google Scholar]
- 26.Ashraf N, Bhattacharyya N. Determination of the ‘Incidental’ Lund Score for the Staging of Chronic Rhinosinusitis. Otolaryngol Head Neck Surg. 2001:483–86. doi: 10.1067/mhn.2001.119324. [DOI] [PubMed] [Google Scholar]
- 27.Nazri M, Bux SI, Tengku-Kamalden TF, et al. Incidental Detection of Sinus Mucosal Abnormalities on CT and MRI Imaging of the Head. Quant Imaging Med Surg. 2013:82–88. doi: 10.3978/j.issn.2223-4292.2013.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.