Summary
Remarkably few hormones have been identified that stimulate appetite. The recent discovery of asprosin, a hormone that activates AgRP neurons to increase food intake and body weight, begins to fill this gap (Duerrschmid et al., 2017; Romere et al., 2016).
The fundamental question of how we become hungry remains a mystery. Textbook models of energy homeostasis suggest that fasting stimulates the production of hormones that promote hunger (orexigens) and suppresses those that block it (anorexigens). This model has been supported by the discovery of numerous satiety signals that decrease food intake when administered in experimental models and are thought to promote meal termination (Figure 1A). In contrast, comparatively few orexigenic hormones have been identified, and the mechanisms responsible for initiating feeding remain poorly understood.
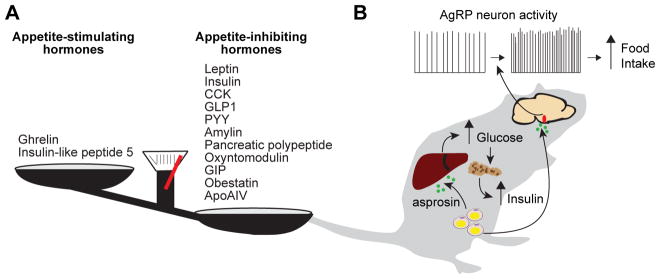
Figure 1. Discovery and characterization of the novel orexigenic hormone, asprosin.
(A) List of hormones previously shown to affect food intake.
(B) Proposed mechanism of action of asprosin.
The only well-studied orexigenic hormone is the peptide ghrelin, which is secreted from the stomach; however, whether ghrelin plays a physiologic role in the regulation of feeding remains controversial (reviewed in Mani and Zigman, 2017). As would be expected of an appetite-stimulating hormone, the plasma concentration of ghrelin has been reported to rise before meals and fall quickly after food intake. Additionally, peripheral administration of ghrelin increases food intake and activates a key population of neurons in the hypothalamus that promote hunger – AgRP neurons. On the other hand, knockout strains lacking ghrelin, its receptor, or its processing enzyme have little or no bodyweight phenotype. Furthermore, acute ablation of ghrelin-secreting cells in adult animals causes no weight loss, and plasma levels of ghrelin must be increased several-fold above normal to stimulate food intake (McFarlane et al., 2014). This suggests that ghrelin is unlikely to be a key signal that triggers meal initiation, and few other plausible candidates have been proposed (Figure 1A).
How could such candidates be discovered? Traditionally, forward genetics has been one of the most powerful ways to identify new components of the energy homeostasis system. This approach has led to the discovery of multiple genes required to suppress feeding, including most prominently leptin, an anorexigenic hormone secreted by adipose tissue, and the leptin receptor (Farooqi and O’Rahilly, 2014). One limitation of this approach, however, is that it is easier to find genes that suppress feeding than those that promote it. This is because loss of a critical hunger promoting gene might be lethal, or alternatively might cause stunted growth and failure to thrive, which are common phenotypes that are not necessarily caused by a primary deficit in appetite. Indeed, 31% of non-lethal gene knockouts in mice result in decreased body weight, and only a small fraction of these are likely to be directly related to impaired hunger (Reed et al., 2008).
Given this context, it is particulary exciting that a new orexigenic hormone named asprosin was recently identified (Duerrschmid et al., 2017; Romere et al., 2016). The discovery of asprosin arose from studying humans with an inherited form of lipodystrophy known as neonatal progeroid syndrome (NPS). Inherited lipodistrophies are a genetically heterogeneous group of diseases characterized by loss of fat that can begin at birth or develop later in life. Many patients with these disorders develop metabolic and behavioral abnormalities including dyslipidemia, profound insulin resistance, and hyperphagia, which are thought to be secondary to lack of adipose tissue and decreased circulating leptin (Patni and Garg, 2015). However, while the two NPS patients studied by Duerrschmid et al. (2017) and Romere et al. (2016) exhibited generalized lipoatrophy from birth, they differed from typical patients with inherited lipodystrophy in two important ways. First, they did not develop hyperinsulinemia or insulin resistance. Second, they consumed less food than age matched controls. These surprising findings raised the question of why these NPS patients do not display typical lipodystophic phenotypes.
To address this question, Romere et al. (2016) performed genetic analysis of the two NPS patients. This revealed heterozygous 3′ truncating mutations in the FBN1 gene, which encodes the extracellular matrix protein fibrillin-1. Although similar mutations had been described previously, their functional significance was unclear (Jacquinet et al., 2014). Here, the authors showed that these mutations disrupt a furin cleavage site in the C-terminal region of profibrillin, thereby preventing the formation of both mature fibrillin-1 and also the production of a previously uncharacterized 140 amino acid C-terminal cleavage product. The authors named this peptide asprosin and showed that it can be secreted from adipocytes, although other sources were not excluded. Unexpectedly, this hormone was shown to promote hepatic glucose production and insulin resistance, explaining why NPS patients that lack this hormone appear to be protected from insulin resistance.
While these findings do not explain why NPS patients appear to eat less food than controls, the initial study did offer clues as to asprosin’s possible role as an orexigen. Specifically, circulating asprosin levels increased with overnight fasting in mice, rats, and humans, and, conversely, plasma asprosin levels in mice rapidly dropped following food consumption. This suggested that asprosin may act as a circulating hunger signal.
To investigate this possibility, Duerrschmid et al. (2017) introduced the NPS-associated mutation to mice (Fbn1NPS/+ mice), and showed that these animals phenotypically recapitulate the human mutants with low circulating asprosin levels, markedly decreased fat mass and body weight, and hypophagia. These animals were also completely protected from the development of diet-induced obesity compared to littermate controls. Of note, these effects were observed in heterozygous mice and humans, because, for reasons that remain unclear, the NPS mutation appears to act as a dominant negative.
To prove that loss of circulating asprosin is the mechanism by which the FBN1 mutation causes hypophagia, the authors peripherally injected recombinant asprosin and showed that this rescued 24-hour food intake in the mutant mice. They further showed that the hormone can cross the blood-brain barrier and that intracerebroventricular (ICV) injection of recombinant asprosin stimulates appetite in wild-type mice, indicating a central mechanism of action.
This raises the question of where in the brain asprosin is acting to promote feeding. Given the critical role that AgRP neurons play in generating hunger, the authors examined the activity of AgRP neurons in slices taken from Fbn1NPS/+ mice and control animals. They found that AgRP neurons from the small, hypophagic mutant mice exhibited decreased firing frequency and membrane potential compared to AgRP neurons from littermate controls. Importantly, ICV injection of asprosin rescued these electrophysiologic phenotypes. Additional characterization showed that asprosin’s effects on AgRP neuron activity are not dependent upon synaptic inputs, suggesting that asprosin acts directly on AgRP neurons to exert its effects on appetite.
Finally, to test whether AgRP neurons are necessary for mediating asprosin’s effect on food intake, the authors ablated AgRP neurons in neonatal mice using diphtheria toxin (DT). Of note, previous studies have shown that while ablation of AgRP neurons in adult mice causes rapid death by starvation, neonatal animals are able to compensate for the loss of this neuronal population and go on to have relatively normal feeding behavior as adults (Luquet et al., 2005). However, neonatal ablation of AgRP neurons completely blocked the orexigenic effects of asprosin treatment in these mice as adults (Duerrschmid et al., 2017). This experiment, combined with the electrophysiology and pharmacologic data described above, are consistent with a model wherein asprosin directly activates AgRP neurons to stimulate feeding.
Much remains to be learned about asprosin. While slice electrophysiology can reveal potential functional interactions, in vivo recording is necessary to determine whether asprosin modulates AgRP neurons in a living animal, as well as the timescale over which any such modulation occurs. In this regard, asprosin induces a gradual and sustained increase in food intake over hours rather than causing the rapid, voracious appetite associated with ghrelin administration or optogenetic stimulation. This suggests asprosin might induce a slow modulation of AgRP neuron activity, as has recently been shown for leptin (Beutler et al., 2017), rather than the rapid activation of AgRP neurons caused by pharmacologic doses of ghrelin.
A second question regards the identity of the asprosin receptor. Initial characterization shows that asprosin induces cAMP production and activates PKA, suggesting it binds to a Gαs-coupled GPCR. Interestingly, it has previously been shown that CNO-mediated activation of a Gαs-coupled DREADD in AgRP neurons causes a similar gradual and sustained increase in food intake (Nakajima et al., 2016). This provides hints of a possible mechanism by which asprosin might stimulate appetite. In addition to clarifying mechanism, the identification of the asprosin receptor would also be valuable as a potential new drug target for the treatment of obesity and metabolic disease. As proof-of-concept, these studies showed that monoclonal antibody sequestration of asprosin in vivo has a number of favorable metabolic effects, including decreased food intake in wild-type mice, weight loss in mice with diet induced obesity, and attenuation of hyperinsulinemia in obese mice.
More broadly, it remains unclear how asprosin fits into the larger energy homeostasis system, and whether its effect on AgRP neurons is related to the striking, generalized lipodystrophy observed in NPS patients (Figure 1B). While it is attractive to conceive of this hormone as a homeostatic regulator of hunger, the finding that circulating asprosin is increased in multiple models of obesity does not fit neatly with this hypothesis, since one would expect such a hormone to be down-regulated when body weight increases. Additionally, although it has been shown that asprosin is secreted from adipocytes, it is not clear that adipose tissue is the sole or even the most important source of asprosin, given that the FBN1 gene is a broadly expressed. Finally, it is also critical to determine how asprosin expression and secretion is regulated by nutritional state, including the role of specific hormones, nutrients, metabolites, or other factors.
Perhaps the most important lesson from these studies is that there are still fundamental discoveries about feeding regulation to be made using human genetics. Mutations that specifically reduce appetite have been challenging to identify, and the discovery of asprosin was enabled by careful clinical characterization of rare individuals with dramatic metabolic phenotypes. Given that inherited leanness is likely undiagnosed in most cases, there may be many other hormones like asprosin that await discovery by forward genetics.
References
- Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23:1444–1453. doi: 10.1038/nm.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, O’Rahilly S. 20 Years of Leptin: Human Disorders of Leptin Action. J Endocrinol. 2014;223:T63–70. doi: 10.1530/JOE-14-0480. [DOI] [PubMed] [Google Scholar]
- Jacquinet A, Verloes A, Callewaert B, Coremans C, Coucke P, de Paepe A, Kornak U, Lebrun F, Lombet J, Pierard GE, et al. Neonatal progeroid variant of Marfan syndrome with congenital lipodystrophy results from mutations at the 3′ end of FBN1 gene. Eur J Med Genet. 2014;57:230–234. doi: 10.1016/j.ejmg.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mani BK, Zigman JM. Ghrelin as a Survival Hormone. Trends Endocrinol Metab. 2017;28:843–854. doi: 10.1016/j.tem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20:54–60. doi: 10.1016/j.cmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, Wess J. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun. 2016;7:10268. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N, Garg A. Congenital generalized lipodystrophies--new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11:522–534. doi: 10.1038/nrendo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genet. 2008;9:4-2156-9-4. doi: 10.1186/1471-2156-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, Saha PK, Del Solar M, Zhu B, York B, et al. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell. 2016;165:566–579. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]