Abstract
Centrifugal precipitation chromatography (CpC) is a powerful chromatographic technique invented in the year 2000 but so far very little applied. The method combines dialysis, counter-current and salting out processes. The separation rotor consists of two identical spiral channels separated by a dialysis membrane (6-8K MW cut-off) in which the upper channel is eluted with an ammonium sulfate gradient and the lower channel with water. In the present study, the method was successfully applied for separation and purification of R-Phycoerythrin (R-PE), a protein widely used as a fluorescent probe, from the red alga Gracilaria lemaneiformis. The separation was performed with the elution of ammonium sulfate from 50% to 0% in 21.5 h at a flow rate of 0.5 ml/min, while the lower channel was eluted with water at a flow rate of 0.05 ml/min after sample charge, and the column was rotated at 200 rpm. After a single run, the absorbance ratio A565/A280 (a criterion for the purity of R-PE) was increased from 0.5 of the crude to 6.5. The purified R-PE exhibited a typical “three peaks” spectrum with absorbance maximum at 497, 538 and 565 nm. The Native-PAGE showed one single protein band and 20 kDa (subunits α and β) and 30 kDa (subunit γ) can be observed in SDS-PAGE analysis which were consistent with the (αβ)6γ subunit composition of R-PE. The results indicated that CpC is an efficient method to obtain protein with the high purity from a complex source.
Keywords: Centrifugal precipitation chromatography, R-Phycoerythrin, Gracilaria lemaneiformis, purification
1. Introduction
R-Phycoerythrin (R-PE), a water-soluble protein with highly fluorescent properties, is commonly used as fluorescent tag in many biochemical techniques [1], such as immunology, cell biology [2], and flow cytometry [3]. Besides, it is also applied in food and cosmetics as a natural colorant. The molecular weight is 240 kDa, consisting of 6α (about 20 kDa), 6β (about 20 kDa) and 1γ (about 30 kDa) subunits. The maximum absorption wavelength of R-PE were located at 565, 539 and 498 nm [4].
The red alga, Gracilaria lemaneiformis, is mainly distributed in the coasts of Shandong, Liaoning, Guangdong and Fujian provinces, and is cultivated at an industrial scale in South China [5]. The red alga is rich in sugar, protein, lipids, and minerals [6], and has extensive pharmacological application [7]. This important alga is a source for production of R-PE.
Previously, many methods were used for separation and purification of R-PE, such as a combination of precipitation with ammonium sulfate and Q-Sepharose column chromatography [8,9], polyacrylamide gel electrophoresis [10], hydroxyapatite column chromatography [11], DEAE-Sepharose chromatography [12], and so forth. However, these purification procedures are often very tedious and time-consumming. Furthermore, the purity of product was not high, i.e., the absorbance ratio A565/A280 (a criterion for the purity of R-PE) was about 3.0 in the reported data, which dose not meet the requirements for the high purity R-PE (A565/A280>4.0).
Centrifugal precipitation chromatography (CpC) based upon counter-current process combined with protein precipitation was developed for separation of molecules according to their solubility in ammonium sulfate (AS) [13]. There is no sample loss and denaturation by solid support and is very suitable for protein separation as shown in practical applications [14,15]. In the present study, CpC was used to obtain high-purity R-PE from Gracilaria lemaneiformis.
2. Experimental
2.1. Apparatus
The Centrifugal precipitation chromatography instrument was manufactured by Pharma-tech Research Corporation (Baltimore MD, USA) with the rotor fabricated at the machine shop of National Institute of Health, Bethesda, MD, USA. A dialysis membrane with molecular weight cut-off 30 000 (Pall Corp., USA) is sandwiched between two disks (high-density polyethylene, 13.2 cm diameter and 1.5 cm in thickness) with mutually mirror-imaged spiral grooves (1.5 mm wide, 2.0 mm deep and ca. 2 m in length) to form two spiral channels. The capacity of each channel is 5 ml. An inlet and an outlet are made at the different terminals of each spiral channel. The upper and lower channels are connected to a Star-chrom HPLC gradient pumping system (D-Star Instrument Inc. USA) and a D-Star DSP-20 pump (D-Star Instrument Inc. USA), respectively. The revolution speed of centrifuge is regulated with an electronic controller (Pharma-tech research corp. USA). The fractions were collected with a Retriever 500 fraction collector (Teledyne ISCO, USA) and assayed by Cary 3E UV-Visible Spectrophotometer (Varian, USA). The centrifugal precipitate chromatography separation system was shown in Fig. 1. The Native-PAGE and SDS-PAGE was performed using a Xcell SureLock Mini-Cell electrophoresis system (Invirogen, USA).
Fig. 1.

Separation of R-PE from Gracilaria lemaneiformis by centrifugal precipitatiton chromatography. The absorption wavelengths are 565, 615, 280 nm. The linear gradient was 50% saturated AS solution from 100% to 0 % in 21.5 h.
2.2. Reagents and materials
Ammonium sulfate, potassium monohydrogen phosphate and monopotassium phosphate were obtained from Fisher Scientific (USA). Centrifugal filter concentrator was purchased from Sigma (USA). NativePAGE 3-12% Bis-Tris Protein Gels, NativePAGE Running Buffer (20X), NativePAGE Sample Buffer (4X), NativePAGE™ Cathode Additive, NuPAGE 4-12% Bis-Tris Protein Gels and NuPAGE MES SDS Running Buffer (20X) were obtained from Invirogen (USA). SDS Protein Gel Loading Solution 2X was purchased from Quality Biological (USA). Coomassie Brilliant Blue R-250 was purchased from Bio-Rad (USA). Gracilaria lemaneiformis was obtained from Hai Zao Zhi Xing Co., Ltd (Qingdao, China) and authenticated by Xiaoli Li, Dalian Ocean University.
2.3. Preparation of crude sample
An amount of 200 g fresh Gracilaria lemaneiformis was cut into small pieces, and 600 mL of distilled water were added. The alga-water mixture was kept in dark at 4°C for 24 h to lyse the alga cells by hypotonicity. Then, the alga was further broken by a refiner for a short time to release the protein. The alga-water mixture obtained was filtered through gauze. The filterate was centrifuged at 7000 g at 4°C for 10 min and the resulting supernatant was precipitated with 65% saturated ammonium sulfate (AS). After centrifugation at 7000 g at 4°C for 10 min, 8.3 g of the precipitates was obtained which was used as the a crude sample for the CpC separation.
2.4. Separation procedure of centrifugal precipitation chromatography
The upper and lower channels of CpC were named as the AS channel and water channel, respectively, and completely filled with 50% saturated AS solution at the start. After the sample (0.5 ml) was loaded into the inlet terminal of the water channel, 50% saturated AS solution was eluted through the AS channel with a linear gradient from 100% to 0% at a flow rate of 0.5 ml/min, while the water channel was eluted with water at a flow rate of 0.05 ml/min. Then, the column was rotated at a given speed. The eluents were collected into test tubes at 20 min intervals and analyzed at designated wavelengths (615, 565, 280 nm) by a UV-Visible Spectrophotometer and the absorbance values plotted.
2.5. Analysis of purified R-PE
The purity of R-PE was calculated by the following formula:
where A565 is the absorbance of the sample at 565 nm and A280 is the absorbance of the sample at 280 nm.
The absorption spectrum of the purified R-PE was recorded using a UV-Visible Spectrophotometer with the scan wavelength from 250 to 700 nm.
For Native-PAGE analysis, 25 μl sample was mixed with 25 μl of Native-PAGE sample buffer and 50 μl of deionized water. The upper cathode buffer chamber was filled with Native-PAGE dark blue cathode buffer (1X), and the lower anode buffer chamber was filled with Native-PAGE anode buffer. The sample was analyzed on 3-12% Bis-Tris protein gel at a voltage of 150 V for 115 min and stained with CBB R250.
For SDS-PAGE analysis, the concentrated samples were mixed with 50 μl of SDS protein gel loading solution and then heated at 100°C for 5 min. The samples were analyzed on a NuPAGE 4-12% Bis-Tris protein gel at a voltage of 190 V for 40 min and stained with CBB R250.
3. Results and discussion
Because R-PE is a water-soluble intracellular protein, higher cell fragmentation produced a higher yield. The present study applied the combination of hypotonic swelling and homogenization to get a high yield of extract [16]. The extract was precipitated with 65% saturated ammonium sulfate, and 8.3 g crude sample was obtained. The purity index of R-PE in the crude sample was 0.5, which was similar with reported results [17].
Optimization of various parameters was necessary for the successful separation. In the present study, the gradient in AS channel, the rotational speed and the sample loading were optimized.
The first experiment using 95% saturated AS solution in the linear gradient from 100% to 0% in 6 h as gradient showed that the R-PE cannot be eluted out in the gradient. However the protein pumped out in the contents of the rotor had the absobance ratio of 4.04 which is an improvement. In the reported study, R-PE can be precipitated by 50 % saturated AS solution[18], which suggests that initial concentration of AS in the experiment may be too high. Therefore, the initial AS concentration was lowered to 50% in the ensuing experiments.
The revolution speed is also very important parameter for CpC separation. The rotation speeds of 2000 rpm and 200 rpm were tested. The result showed that at 2000 rpm the proteins showed high retention and eluted out slowly from the column while there were not any components eluted out in first 10 collected fractions. In contrast, at 200 rpm the proteins were eluted out in proper time with a good separation. The optimum concentration of crude sample was 200 mg/ml to avoid blocking the column due to a large volume of precipitated proteins. Fig. 2 shows the CpC chromatogram of R-PE separation from Gracilaria lemaneiformis. In this separation, 0.5 ml (200mg/ml) crude sample was loaded into column, and then 50% saturated AS was used as a linear gradient elution in the upper channel from 100% to 0% in 21.5 h at 200 rpm. The collected fractions were measured at 615, 565, 280 nm using a UV-Visible spectrophotometer. Absorbance at these wavelengths indicated the concentration of major impurity R-phycocyanin (R-PC), R-PE, and the whole protein solution, respectively.
Fig. 2.
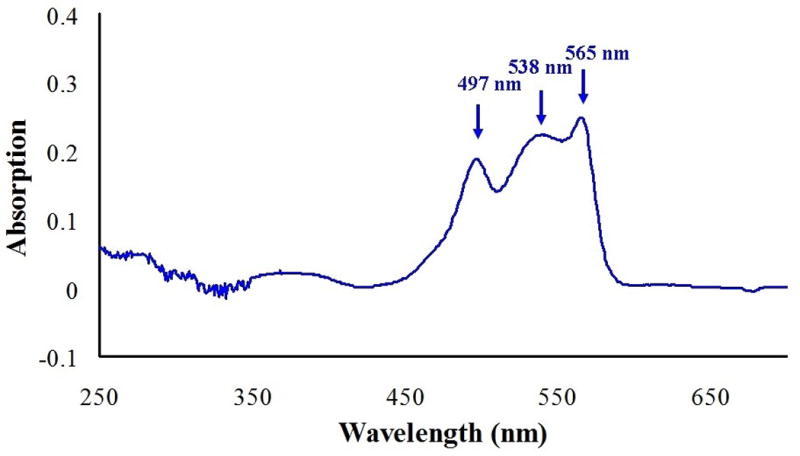
Absorption spectrum of the purified R-PE.
As shown in Fig. 1, peak 1 with very high absorbance at 280 nm was a light purple colored solution. Its absorbances at 565 nm and 615 nm were lower than that at 280 nm, indicating that it contains some impurities and pigments. Peak 2 and peak 3 were purple colored solution. Their absorbances at 280 nm and 565 nm were similar, and there were some absorbance at 615 nm, indicating that the solution probably contained some R-PC and R-PE. Peak 4 was red a solution and the absorbance at 565 nm was higher than that at 280 nm. There was not any absorption at 615 nm, which indicated this peak contained purified R-PE. The purified R-PE fractions were combined and lyophilized, and 0.5 mg R-PE was obtained from 100 mg crude sample.
The absorption spectrum of purified R-PE showed the characteristic three peaks at 497, 538, and 565 nm (Fig. 2), which was consistent with literature [9]. In the present study, the ratio A565/A280 of the purified R-PE reached 6.5, which exceeded the previous level of purification of phycobiliproteins [18]. Besides, the absorption ratio A615/A280 and A650/A280 were less than 0.001, which demonstrated the absence of R-PC and allophycocyanin (APC). To sum up, the above results clearly indicated that the R-PE of high purity was isolated by CpC using a linear gradient AS elution.
R-PE purity was confirmed by electrophoresis. Only one single pink band was shown in the Native-PAGE gel (Fig. 3A). SDS-PAGE gave four bands which was shown in Fig. 3B. The first upper band should correspond to a 50 kDa heterodimer of the α/β and γ subunits, and the second band at 40 kDa may correspond to non-denatured subunit (αβ), while the third band corresponded to γ subunit (30 kDa) and the fourth band corresponded to the overlapped α and β subunits (20 kDa). Therefore, it was illustrated that the purified R-PE had three subunits α, β and γ, this results were consistent with (αβ)6 γ subunits composition which had been reported in several references [4,19,20].
Fig. 3.


(A) Native-PAGE analysis of R-PE from Gracilaria lemaneiformis. (B) SDS-PAGE analysis of R-PE from Gracilaria lemaneiformis. Lane (left): purified R-PE; Lane (right): standard proteins
4. Conclusions
A simple and efficient CpC method was established for the separation of high purity R-PE from a complex extraction of the edible red algae, Gracilaria lemaneiformis, in the present study. The results show that centrifugal precipitation chromatography is a powerful technique for isolation of an active protein from a complex extract in one step.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 31300289), Program for Liaoning Excellent Talents in University (No. LJQ2015014) and China Scholarship Council (No. 201608210280).
References
- 1.Isailovic Dragan, Sultana Ishrat, Phillips Gregory J, Yeung Edward S. Formation of Xuorescent proteins by the attachment of phycoerythrobilin to R-phycoerythrin alpha and beta apo-subunits. Analytical Biochemistry. 2006;358:38–50. doi: 10.1016/j.ab.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronick Mel N. The use of phycobiliproteins as fluorescent labels in immunoassay. Journal of Immunological Methods. 1986;92:1–13. doi: 10.1016/0022-1759(86)90496-5. [DOI] [PubMed] [Google Scholar]
- 3.Oi Vernon T, Glazer Alexander N, Stryer Lubert. Fluorescent Phycobiliprotein Conjugates for Analyses of Cells and Molecules. The Journal of Cell Biology. 1982;93:981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Agnolo E, Rizzo R, Paoletti S, Murano E. R-Phycoerythrin from the red alga Gracilaria longa. Phyrochemistry. 1994;35(3):693–696. [Google Scholar]
- 5.Yang Yufeng, Chai Zhaoyang, Wang Qing, Chen Weizhou, He Zhili, Jiang Shijun. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Research. 2015;9:236–244. [Google Scholar]
- 6.Wen Xue, Peng Changlian, Zhou Houcheng, Lin Zhifang, Lin Guizhu, Chen Shaowei, Li Ping. Nutritional Composition and Assessment of Gracilaria lemaneiformis Bory. Journal of Integrative Plant Biology. 2006;48(9):1047–1053. [Google Scholar]
- 7.Guo Xinfeng, Gu Dongyu, Wang Miao, Huang Yu, Li Haoquan, Dong Yue, Tian Jing, Wang Yi, Yang Yi. Characterization of active compounds from Gracilaria lemaneiformis inhibiting the protein tyrosine phosphatase 1B activity. Food & Function. 2017;8(9):3271–3275. doi: 10.1039/c7fo00376e. [DOI] [PubMed] [Google Scholar]
- 8.Senthilkumara Namasivayam, Sureshc Veeraperumal, Thangam Ramar, Kurinjimalar Chidambaram, Kavitha Ganapathy, Murugan Pitchai, Kannan Soundarapandian, Rengasamy Ramasamy. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. International Journal of Biological Macromolecules. 2013;55:150–160. doi: 10.1016/j.ijbiomac.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Wang Guangce. Isolation and Purification of Phycoerythrin from Red Alga Gracilaria verrucosa by Expanded-Bed-Adsorption and Ion-Exchange Chromatogaphy. Chromatographia. 2002;56:509–513. [Google Scholar]
- 10.Galland-Irmouli AV, Pons L, Luc¸on M, Villaume C, Mrabet NT, Gue´ant JL, Fleurence J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. Journal of Chromatography B. 2000;739:117–123. doi: 10.1016/s0378-4347(99)00433-8. [DOI] [PubMed] [Google Scholar]
- 11.Rossano R, Ungaro N, D’Ambrosio A, Liuzzi GM, Riccio P. Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongata Ellis & Solander. Journal of Biotechnology. 2003;101:289–293. doi: 10.1016/s0168-1656(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Niu Jianfeng, Xu Meiling, Wang Guangce, Zhang Kaiyue, Peng Guang. Comprehensive extraction of agar and R-phycoerythrin from Gracilaria lemaneiformis (Bangiales, Rhodophyta) Indian Journal of Geo-Marine Science. 2013;42(1):21–28. [Google Scholar]
- 13.Ito Yoichiro. Centrifugal Precipitation Chromatography: Principle, Apparatus, and Optimization of Key Parameters for Protein Fractionation by Ammonium Sulfate Precipitation. Analytical Biochemistry. 2000;277:143–153. doi: 10.1006/abio.1999.4365. [DOI] [PubMed] [Google Scholar]
- 14.Baldermann Susanne, Mulyadi Andriati N, Yang Ziyin, Murata Ariaka, Fleischmann Peter, Winterhalter Peter, Knight Martha, Finn Thomas M, Watanabe Naoharu. Application of centrifugal precipitation chromatography and high-speed countercurrent chromatography equipped with a spiral tubing support rotor for the isolation and partial characterization of carotenoid cleavage-like enzymes in Enteromorpha compressa (L.) Nees. Journal of Separation Science. 2011;34:2759–2764. doi: 10.1002/jssc.201100508. [DOI] [PubMed] [Google Scholar]
- 15.Baldermanna Susanne, Fleischmann Peter, Bolten Mareike, Watanabe Naoharu, Winterhalter Peter, Ito Yoichiro. Centrifugal precipitation chromatography, a powerful technique for the isolation of active enzymes from tea leaves (Camellia sinensis) Journal of Chromatography A. 2009;1216:4263–4267. doi: 10.1016/j.chroma.2009.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Meizhen, Ge Anshan, Yu Jie, Zhang Yongyu, Liang Manjian. Influence of Different Treatment Factors on Antioxidative Activities of the Phycoerythrin from the Gracilaria Lemaneiformis. Journal of Shantou University (Natural Science) 2006;21(3):6–11. [Google Scholar]
- 17.Liu LuNing, Chen XiuLan, Zhanga XiYing, Zhang YuZhong, Zhou BaiCheng. One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia urceolata. Journal of Biotechnology. 2005;116:91–100. doi: 10.1016/j.jbiotec.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Siegelman HW, Kycia JH. Algal biliproteins: handbook of phycological method. Combridge University press; 1978. pp. 71–79. [Google Scholar]
- 19.Niu Jianfeng, Chen Zhangfan, Wang Guangce, Zhou Baicheng. Purification of phycoerythrin from Porphyra yezoensis Ueda (Bangiales, Rhodophyta) using expanded bed absorption. Journal of Applied Phycology. 2010;22:25–31. [Google Scholar]
- 20.Sun Li, Wang Shumei, Gong Xueqin, Zhao Mingri, Fu Xuejun, Wang Lu. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expression and Purification. 2009;64:146–154. doi: 10.1016/j.pep.2008.09.013. [DOI] [PubMed] [Google Scholar]


