Abstract
Intramural esophageal dissection is an uncommon condition, involving the separation of the esophageal mucosa from the muscular layers. To our knowledge, the temporal evolution of intramural esophageal dissection on computed tomography has not been previously demonstrated. We present a case of a 51-year-old male who first presented to the emergency department with fever, odynophagia, and dysphagia. He was treated for acute tonsillitis and discharged, but presented again after 10 days with worsening symptoms. A series of radiographs and computed tomography studies, with 3D reconstruction and cinematic virtual fly-through, in these 2 admissions depicts the temporal evolution of intramural hematoma to subsequent intramural esophageal dissection. Recognizing its appearance on imaging is invaluable in distinguishing it from other important differential diagnoses. A complete description of the case, relevant radiologic imaging, and review of the relevant literature are provided.
Keywords: Computed Tomography, Conservative Treatment, Dissection, Esophageal Mucosa, Hematoma
CASE REPORT
We present a case of a 51-year-old male who first presented to the emergency department with fever, productive cough, odynophagia, and dysphagia. This was associated with multiple episodes of non-bilious, non-bloody vomiting. There was no history of foreign body ingestion. Nasoendoscopy demonstrated hyperemic and hypertrophied tonsils and pooling of saliva in the vallecula.
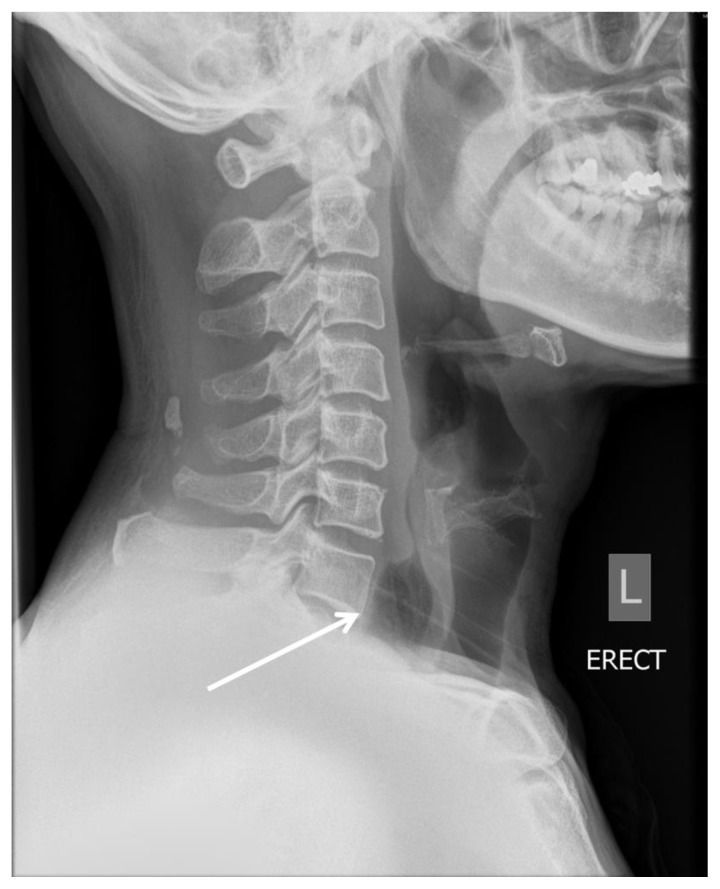
There was prevertebral soft tissue thickening in the lower neck on the lateral neck radiograph (Fig. 1). Due to the concern of a retropharyngeal abscess, computed tomography (CT) of the neck was obtained. This study demonstrated swollen tonsils as well as a thickened, distended esophagus (Fig. 2). The thickened esophagus was thought to represent esophagitis. The patient was treated for acute tonsillitis with intravenous (IV) antibiotics and he was discharged after 3 days upon showing considerable improvement.
Figure 1.
A 51-year-old male with intramural esophageal dissection.
Findings: Lateral neck radiograph on initial presentation shows thickened prevertebral soft tissue (arrow). No gas lucency is seen within the esophagus.
Technique: Radiography.
Figure 2.
A 51-year-old male with intramural esophageal dissection.
Findings: Axial contrast-enhanced CT of the neck (A), with coronal (B), and sagittal (C) reformats, on initial presentation demonstrates a thickened esophageal wall (arrows), displacing the esophageal lumen anteriorly, in keeping with an esophageal intramural hematoma.
Technique: Brilliance Philips 64-slice CT scanner, tube voltage 120 kV, tube current 150 mA, slice thickness 3 mm, 50 ml intravenous Omnipaque 350 (Iohexol).
He presented again after 10 days with a 2-day history of regurgitation and drooling, which was exacerbated in the supine position. He was unable to swallow solids 24 hours prior to the admission. He also reported a globus sensation which had not resolved since his previous admission. Nasoendoscopy again demonstrated enlarged, hyperemic tonsils with exudates. There was a large amount of saliva pooling in the pyriform sinuses.
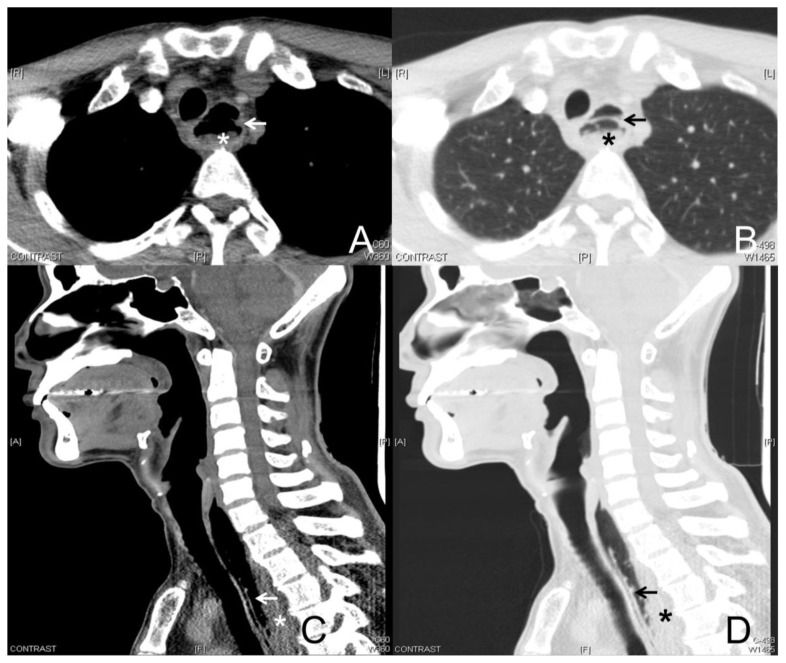
Lateral neck radiograph during re-admission showed a gas-distended upper esophagus (Fig. 3). In view of the previous concern of a retropharyngeal abscess, a CT neck was repeated on this admission. The CT neck demonstrated a gas-filled pocket paralleling the posterior wall of the visualized upper esophagus, giving the appearance of a double-barrel esophagus, typical of intramural esophageal dissection (IED) (Fig. 4). Dependent layering of fluid and debris was seen within the false lumen. 3D reconstruction (Fig. 5) and virtual fly-through (Video 1) of this CT study demonstrates the mucosal flap separating the true and false lumens.
Figure 3.
A 51-year-old male with intramural esophageal dissection.
Findings: Lateral neck radiograph, in the patient’s second admission 10 days later, demonstrates a gas-filled, distended upper esophagus at the level of C7 (arrow).
Technique: Radiography.
Figure 4.
A 51-year-old male with intramural esophageal dissection.
Findings: Axial contrast-enhanced CT of the neck in the soft tissue (A) and lung (B) windows, with sagittal (C, D) reformats, during the patient’s second admission 10 days later. This demonstrates a thin dissection flap in the esophagus (arrows). The true lumen is seen anterior to the flap and the posterior false lumen (*) contains secretions/food particles. This is the classic double-barrel appearance of intramural esophageal dissection.
Technique: Brilliance Philips 64-slice CT scanner, tube voltage 120 kV, tube current 150 mA, slice thickness 3 mm, 50 ml intravenous Omnipaque 350 (Iohexol).
Figure 5.
A 51-year-old male with intramural esophageal dissection.
Findings: CT 3D reconstruction with (A) and without (B) the secretion removal algorithm depicts the dissection flap (arrows). The esophageal true lumen is seen anterior to the flap and the false lumen (*) is posterior to the flap.
Technique: CT 3D reconstruction was performed using a Philips IntelliSpace workstation.
Upon re-visiting the history of his initial presentation, no triggering event for his repeated episodes of vomiting was identified. There was no history of foreign body ingestion and no coagulopathy was identified.
The patient was kept nil by mouth and treated conservatively with IV antibiotics and IV hydration. His symptoms gradually improved and he was escalated to a soft diet and switched to oral antibiotics. He was discharged 5 days later.
Outpatient gastroscopy, a week after discharge, demonstrated an upper esophageal mucosal defect with sizeable granulation tissue at the defect, confirming the diagnosis of IED (Fig. 6A). Separate lesions were also noted in the mid and upper esophagus.
Figure 6.
A 51-year-old male with intramural esophageal dissection.
Findings: Gastroscopy a week after he was discharged (A) shows the dissection flap (arrows), with the true lumen anteriorly and the false lumen posteriorly (*). Follow up endoscopy 2 months later (B) shows a healed dissection flap (circled).
Technique: Endoscopy.
A repeat endoscopy 2 months later, following conservative therapy, demonstrated an almost healed dissection flap (Fig. 6B).
DISCUSSION
Etiology & Demographics
Intramural esophageal dissection is an uncommon condition. Although its exact incidence has not been determined, at least 150 cases have been reported [1]. It is seen most frequently in healthy females in the 7th and 8th decade.
IED involves the separation of the esophageal mucosa or submucosa from the muscular layers. There are two main theories of pathogenesis [2]. The first theory postulates that there is primary submucosal bleeding. This causes a secondary tear in the mucosa, which decompresses the intramural hematoma into the esophageal lumen [3]. This explains why anticoagulation remains a risk factor. The second theory is that the mucosa tears first, with secondary dissection of the submucosa. This theory is supported by the association of IED with ingestion of sharp foreign bodies and iatrogenic intervention such as endoscopy.
Repeated episodes of vomiting can cause an abrupt increase in intra-esophageal pressure. As in the case presented, this resulted in an intramural hematoma, which subsequently progressed to IED. The etiology and demographics are summarized in Table 1.
Table 1.
Summary table for intramural esophageal dissection.
| Etiology | The separation of the esophageal mucosa or submucosa from the muscular layers. Two main theories of pathogenesis:
|
| Incidence | Considered rare/uncommon. Although the exact incidence has not been reported, there are at least 150 reported cases in the literature. |
| Gender ratio | More common in elderly females. |
| Age predilection | Elderly (7th, 8th decade). |
| Risk factors |
|
| Treatment | Managed conservatively
|
| Prognosis | In majority of cases, perforation does not occur and thus there is no risk of contaminating the sterile mediastinal or peritoneal space. There is usually significant or complete resolution within 7–14 days with no reports of late sequelae. Severe bleeding or esophageal perforation is rare. These are usually seen in cases related to endoscopy and require urgent surgical intervention. |
| Findings on imaging | Endoscopic ultrasound: submucosal hematoma. Contrast swallow: double-barrel esophagus or elongated tubular filling defect. CT: presence of a mucosal flap with a submucosal distribution of gas (double-barrel appearance). The false lumen is usually larger and located posterior to the true lumen. |
Clinical & Imaging findings
Common presenting symptoms include acute dysphagia and/or odynophagia, chest pain and nausea.
IED is commonly diagnosed endoscopically. In its initial phase of an intramural hematoma, it can also be diagnosed on endoscopic ultrasound (EUS), in which the hematoma lies in the submucosa [4].
On contrast (barium or water soluble contrast) swallow, a double-barrel esophagus or elongated tubular filling defect is demonstrated [5,6].
Due to the nonspecific nature of the presenting symptoms, cross-sectional imaging such as CT or magnetic resonance imaging (MRI) can be useful in ruling out other differentials, including myocardial infarction [7]. Thus, as in this case, the initial diagnosis of IED may be made on cross-sectional imaging. Well-documented CT features include the presence of a mucosal flap with a submucosal distribution of gas, giving the double-barrel appearance of the esophagus. The false lumen is usually larger and located posterior to the true lumen [8], best illustrated on the sagittal view in this case (Fig. 4C–D). Addition of oral contrast and using 3D reconstruction or volume rendering images may increase diagnostic accuracy [6].
To our knowledge, the temporal evolution of IED from an intramural hematoma on CT has not been previously demonstrated. In the case presented, the initial CT study showed a distended esophagus which appeared round in cross-section. There was thickening of the posterior wall of the esophagus, which appeared hyperdense. The true lumen was narrowed and gas within the lumen was displaced anteriorly (Fig. 2A). No gas was seen within the false lumen. This represents the initial stage of dissection, with the presence of an intramural hematoma. The diagnosis of IED was confirmed on the CT performed in the second admission, which demonstrated the classic double-barrel appearance (Fig. 4).
Differential Diagnoses
Recognizing the appearance of an intramural hematoma, as a precursor to IED, is helpful in ruling out other important differential diagnoses. This may prevent unnecessary investigations, such as in the work up for causes of esophagitis. It also ensures that appropriate conservative management takes place.
The clinical symptoms of IED may mimic those of acute myocardial infarction. In such cases, CT is invaluable in its ability to distinguish IED from acute cardiovascular disease [7]. This is particularly important as anticoagulation therapy in acute cardiovascular disease is contraindicated in the treatment of IED.
Another cause of esophageal mural thickening that can present with acute symptoms is esophagitis [9]. This can be due to a wide range of etiologies and is best diagnosed on contrast swallow or endoscopy. The appearance on contrast swallow is varied, depending on the etiology, and includes mucosal nodularity, ulcers, and strictures. On CT, the esophagus can appear thickened, with submucosal edema, and mucosal enhancement.
Three main types of acute esophageal injury have been described – mucosal tear (Mallory Weiss tear), IED, and full thickness rupture. The precipitating cause in each condition may be similar, such as with repeated vomiting (known as Boerhaave syndrome in full thickness rupture) or secondary to an iatrogenic cause.
However, appearances on imaging and endoscopy are usually sufficient in differentiating the type of esophageal injury [9]. Mallory Weiss tear is often occult on imaging. In esophageal perforation, pneumomediastinum or a pleural effusion (often left-sided) is usually seen on the chest radiograph. Contrast swallow may demonstrate extravasation of contrast medium at the site of perforation, which is most commonly located on the left, just above the gastroesophageal junction. On CT, transmural perforation of the esophagus may be diagnosed from the presence of extra-luminal gas and peri-esophageal collections.
The prognosis of IED is good, which is similar to a Mallory Weiss mucosal tear [5]. Conversely, full thickness rupture of the esophagus has a poor prognosis and if left untreated, has a high mortality rate. Esophagitis can occasionally progress to perforation. The imaging findings of these conditions are summarized in Table 2.
Table 2.
Differential diagnosis table for intramural esophageal dissection.
| Radiographic findings | Contrast swallow | CT | |
|---|---|---|---|
| Intramural esophageal dissection | Lateral neck radiograph may show thickened prevertebral soft tissue or a gas-distended esophagus. The classic double-barrel appearance may be appreciated. | Double-barrel esophagus or elongated tubular filling defect. | Presence of a mucosal flap with a submucosal distribution of gas (double-barrel appearance). The false lumen is usually larger and located posterior to the true lumen. |
| Mallory-Weiss tear | Usually normal. | Usually normal but mucosal irregularity may be demonstrated. | Usually normal. |
| Transmural esophageal perforation | Chest radiograph may show pneumomediastinum, a pleural effusion or hydropneumothorax (left > right). | Extravasation of contrast medium at the site of perforation. This is most commonly located on the left, just above the gastroesophageal junction. | Extra-luminal gas and peri-esophageal, pleural, and pericardial fluid collections. |
| Esophagitis | Usually normal. | Appearance can be varied, depending on the etiology. There may be mucosal nodularity, plaque-like defects, ulcers, and strictures. | Diffuse esophageal thickening, submucosal edema, and mucosal enhancement. |
Treatment & Prognosis
Repeat endoscopy in IED may reveal that the dissected mucosal layer sloughs away, leaving a large longitudinal ulcer [10]. Occasionally, severe bleeding or esophageal perforation may occur, requiring urgent surgical intervention [11]. These are usually seen in cases related to endoscopy [12]. In the majority of cases of IED, perforation does not occur and thus there is no risk of contaminating the sterile mediastinal or peritoneal space. There is usually significant or complete resolution within 7–14 days with no reports of late sequelae [13].
Compared with esophageal perforation, IED has a better morbidity and mortality rate as there is no contamination of the mediastinal space. As such, IED is usually managed conservatively, with nutritional support and IV hydration. This may include avoidance of oral medication, reversal of coagulopathy, and use of a proton pump inhibitor.
TEACHING POINT
Intramural esophageal dissection is an uncommon condition involving the separation of the esophageal mucosa from the muscular layers. Understanding the temporal evolution of this condition and recognizing an intramural esophageal hematoma and the classic double-barrel appearance of intramural esophageal dissection on imaging is invaluable in distinguishing it from other important differential diagnoses.
ABBREVIATIONS
- CT
Computed tomography
- EUS
Endoscopic ultrasound
- IED
Intramural esophageal dissection
- IV
Intravenous
- MRI
Magnetic resonance imaging
REFERENCES
- 1.Beumer JD, Devitt PG, Thompson SK. Intramural oesophageal dissection. ANZ J Surg. 2010;80(1–2):91–5. doi: 10.1111/j.1445-2197.2009.05179.x. [DOI] [PubMed] [Google Scholar]
- 2.Phan GQ, Heitmiller RF. Intramural esophageal dissection. Ann Thorac Surg. 1997;63(6):1785–6. doi: 10.1016/s0003-4975(97)83865-9. [DOI] [PubMed] [Google Scholar]
- 3.Soulellis CA, Hilzenrat N, Levental M. Intramucosal esophageal dissection leading to esophageal perforation: case report and review of the literature. Gastroenterol Hepatol. 2008;4(5):362–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Mion F, Bernard G, Valette PJ, Lambert R. Spontaneous esophageal hematoma: diagnostic contribution of echoendoscopy. Gastrointest Endosc. 1994;40(4):503–5. doi: 10.1016/s0016-5107(94)70224-1. [DOI] [PubMed] [Google Scholar]
- 5.Ho CL, Young TH, Yu CY, Chao YC. Intramural hematoma of the esophagus: ED diagnosis and treatment. Am J Emerg Med. 1997;15(3):322–3. doi: 10.1016/s0735-6757(97)90027-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Lee JM, Han JK, et al. Three-dimensional MDCT imaging and CT esophagography for evaluation of esophageal tumors: preliminary study. Eur Radiol. 2006;16(11):2418–26. doi: 10.1007/s00330-006-0337-8. [DOI] [PubMed] [Google Scholar]
- 7.Herbetko J, Delany D, Ogilvie BC, Blaquiere RM. Spontaneous intramural haematoma of the oesophagus: appearance on computed tomography. Clin Radiol. 1991;44(5):327–8. doi: 10.1016/s0009-9260(05)81268-1. [DOI] [PubMed] [Google Scholar]
- 8.Williams B. Case report; oesophageal laceration following remote trauma. Br J Radiol. 1957;30(360):666–8. doi: 10.1259/0007-1285-30-360-666. [DOI] [PubMed] [Google Scholar]
- 9.Young CA, Menias CO, Bhalla S, Prasad SR. CT features of esophageal emergencies. Radiographics. 2008;28(6):1541–53. doi: 10.1148/rg.286085520. [DOI] [PubMed] [Google Scholar]
- 10.Hsu MT, Lin XZ, Chang TT, Shin JS, Chen CY, Sheu BS. Sequential endoscopic findings in spontaneous intramural hematoma of the esophagus. Endoscopy. 1996;28(5):465. doi: 10.1055/s-2007-1005518. [DOI] [PubMed] [Google Scholar]
- 11.Cullen SN, McIntyre AS. Dissecting intramural haematoma of the oesophagus. Eur J Gastroenterol Hepatol. 2000;12(10):1151–62. doi: 10.1097/00042737-200012100-00014. [DOI] [PubMed] [Google Scholar]
- 12.Monu NC, Murphy BL. Intramural esophageal dissection associated with esophageal perforation. R I Med J. 2013;96(7):44–6. [PubMed] [Google Scholar]
- 13.Meulman N, Evans J, Watson A. Spontaneous intramural haematoma of the oesophagus: a report of three cases and review of the literature. Aust N Z J Surg. 1994;64(3):190–3. doi: 10.1111/j.1445-2197.1994.tb02176.x. [DOI] [PubMed] [Google Scholar]