Abstract
Defects in membrane trafficking are hallmarks of neurodegeneration. Rab GTPases are key regulators of membrane trafficking. Alterations of Rab GTPases, or the membrane compartments they regulate, are associated with virtually all neuronal activities in health and disease. The observation that many Rab GTPases are associated with neurodegeneration has proven a challenge in the quest for cause and effect. Neurodegeneration can be a direct consequence of a defect in membrane trafficking. Alternatively, changes in membrane trafficking may be secondary consequences or cellular responses. The secondary consequences and cellular responses, in turn, may protect, represent inconsequential correlates or function as drivers of pathology. Here, we attempt to disentangle the different roles of membrane trafficking in neurodegeneration by focusing on selected associations with Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and selected neuropathies. We provide an overview of current knowledge on Rab GTPase functions in neurons and review the associations of Rab GTPases with neurodegeneration with respect to the following classifications: primary cause, secondary cause driving pathology or secondary correlate. This analysis is devised to aid the interpretation of frequently observed membrane trafficking defects in neurodegeneration and facilitate the identification of true causes of pathology.
Introduction
The endomembrane system is a corollary of compartmentalization in eukaryotic cells. Most intracellular compartments, including the nucleus, mitochondria and a plethora of endolysosomal compartments, are separated by membranes. Hence, all eukaryotic cells must have mechanisms that ensure trafficking between these organelles based on recognition of organelle identities [1]. In multicellular organisms, different cell types with highly divergent functions and morphologies exacerbate the challenges and opportunities that come with coordinated membrane trafficking.
Neurons vary greatly in morphology and function but share special requirements in membrane trafficking. This is because neurons are long-lived cells with complicated shapes that require membrane trafficking between distant axonal and dendritic extensions in order to maintain function. Neuron-specific roles of membrane trafficking include the regulation of protein and organelle compositions in dendrites and axons, synaptic transmission, and correct distribution of countless cell surface receptors [2–7]. Not surprisingly, membrane trafficking has been implicated in virtually every aspect of neuronal function and, in particular, neuronal maintenance and degeneration [8].
Rab GTPases, the largest branch of the Ras superfamily, are key organizers of intracellular membrane trafficking. Rab GTPases were initially discovered in brain tissue, where their abundance, diversity and functional adaptations reflect neuronal challenges for membrane trafficking [9]. However, Rabs are found in all eukaryotic cells, where they mediate fundamental processes of vesicle sorting and transport between target membranes[10, 11]. Consequently, Rab GTPases are commonly used as markers and identifiers of various organelles and vesicles in the endocytic and secretory systems. Similar to other small GTPases, Rab proteins switch between GTP-bound active and GDP-bound inactive states. The activity state and membrane association of Rab GTPases are controlled by accessory proteins [10, 12, 13]. Furthermore, the precise regulation of membrane trafficking processes by Rab GTPases is dependent on interactions with effector proteins, such as coat proteins (COPI, COPII and clathrin), motor proteins (kinesins and dyneins), tethering complexes (EEA1, Golgins, the exocyst complex and HOPS complex) and SNAREs [14, 15]. In neurons, such interactions are essential to regulate trafficking of proteins and lipids for the maintenance of cell morphology and synaptic function.
More than 60 Rab GTPases are encoded by the human genome, up to 31 in Drosophila melanogaster and 11 in yeast [16, 17]. Half of all Drosophila Rab proteins are strongly enriched or exclusively expressed in neurons [18, 19]. In humans, 24 Rab proteins are specific to or enriched in the central nervous system [20]. Yet, only few of these neuronal Rabs, including Rab3, Rab26 and Rab27, have been functionally characterized. In addition, ubiquitously expressed Rab proteins often execute specialized functions in neurons [18, 19, 21–24]. Hence, Rab GTPases provide a window into understanding how membrane trafficking is regulated in neurons. Many Rab GTPases have been directly and indirectly linked to neurodegenerative diseases. In some cases, mutations in rab genes or genes encoding Rab-associated proteins have been directly implicated [25–28]. In other cases, upregulation of Rab proteins can partially rescue degenerative phenotypes [29–31]. And in most cases, progression of neurodegeneration is associated with alterations to Rab-mediated membrane trafficking [32–35]. The cause and effect relationships underlying the associations of many neurodegenerative diseases with Rab GTPases have often remained unclear.
Rab GTPases in Wild-Type Neurons
For the maintenance of normal neuronal function both specialized ubiquitous membrane trafficking machinery as well as neuron-specific mechanisms are used [7, 36, 37]. Here, we only provide a brief outline of membrane trafficking in neurons from the perspective of ubiquitous and neuron-specific Rab GTPase functions (Figure 1).
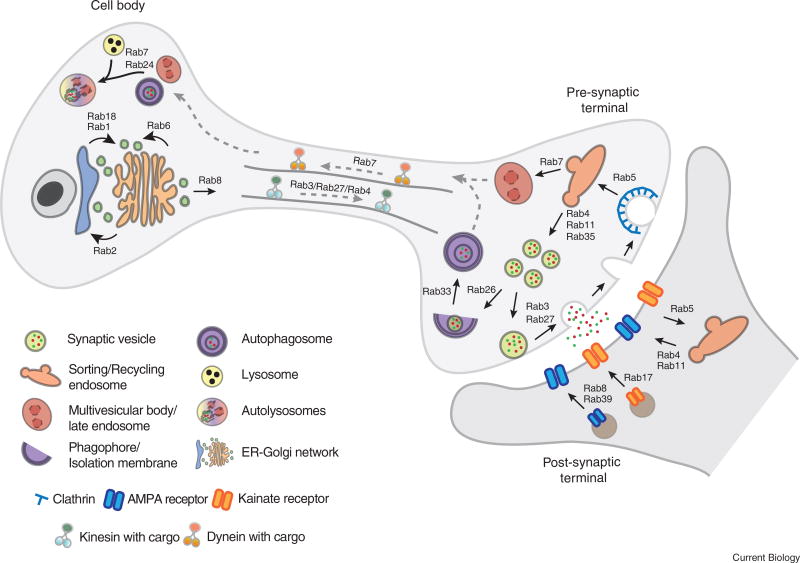
Figure 1. Rab GTPases in wild-type neurons.
Shown are a schematic cell body (left), axon (middle) and synaptic terminal (right). The post-synaptic terminal is marked in green. Rab GTPases have been depicted with arrows between membrane compartments for which their role has been studied in wild-type neurons.
Rab GTPases at the Synapse
Synaptic function requires a continuous flow of membrane and membrane proteins at synapses, largely because synaptic vesicles undergo continuous cycles of exocytosis and endocytosis [5, 6]. Hence, synaptic vesicle recycling presents a major challenge for neuronal maintenance [38, 39]. A systematic analysis of Rab GTPase expression and localization in Drosophila revealed that all neuron-specific Rabs localize to synapses, where the majority co-localize with endosomal markers [18, 19]. The precise synaptic function of the majority of these Rab GTPases remains unknown.
The two best characterized Rab GTPases in synaptic vesicle exocytosis are Rab3 and Rab27 (Figure 1). In vertebrates, the Rab3 subfamily (Rab3A, Rab3B, Rab3C and Rab3D) and Rab27B are expressed exclusively in neurons and share effector and regulator proteins [40]. Rab3A is a structural component of the synapse’s active zone that forms a tripartite complex together with other core active zone proteins RIM1 and MUNC13. Together, these regulate priming and docking of synaptic vesicles for neurotransmitter release [41]. Single and double knockout mice for any two of the four Rab3 genes are viable and fertile. Triple and quadruple mutants are lethal only if Rab3A is deleted. The quadruple mutants exhibit no significant changes in synaptic organization and only a 30% decrease in transmitter release [42]. This surprising finding is largely attributed to the redundant function of Rab27B in synaptic vesicle exocytosis [40]. In Drosophila, loss of the solitary Rab3 gene is viable, but leads to defects in active zone organization and mild functional defects [43]. Similarly, the rab27 mutant is viable and exhibits circadian rhythm defects [18]. Inhibition of Rab27 activity in presynaptic terminals of the squid giant neuron resulted in fewer docked synaptic vesicles and increased number of non-docked synaptic vesicles away from the active zones, demonstrating the role of Rab27 in synaptic vesicle exocytosis [44].
Clathrin-mediated endocytosis is a major pathway for synaptic vesicle retrieval from the plasma membrane. Rab5 is the key early endosomal Rab GTPase in clathrin-mediated endocytosis and endosomal maturation, upstream of synaptic vesicle retrieval [45, 46]. At the Drosophila larval neuromuscular junction, Rab5 is required for synaptic endosomal integrity, synaptic vesicle exo-/endocytosis rates and neurotransmitter release probability [47]. Rab5-dependent endosomal sorting may further regulate uniformity of synaptic vesicle size [48]. Other early or recycling endosomal Rabs, including Rab4 and Rab11, are also candidates for the synaptic vesicle recycling process, as these are also found on endocytic intermediates [49]. The expression of dominant-negative Rab4 (Rab4DN) impairs the formation of synaptic vesicle-like vesicles from early endosomes in PC12 cells [50]. The precise endocytic regulatory functions of these Rabs in different neurons remain largely unknown.
Endocytic intermediates downstream of synaptic vesicle retrieval may also function as sorting stations for synaptic vesicle proteins. Rab35 and its GAP Skywalker (Sky) are regulators of synaptic vesicle rejuvenation in Drosophila and vertebrate neuronal culture [51–53]. In sky mutants, Rab35 is over-activated and both the turnover of vesicles from the readily releasable pool and neurotransmission are increased. In parallel, the degradation rate of ubiquitinated (dysfunctional) synaptic vesicle protein neuronal Synaptobrevin (n-Syb) is increased, suggesting that Rab35/Sky pathway functions at the core of an interplay between synaptic vesicle recycling and degradation [51, 52]. Activity-dependent differential sorting and degradation of synaptic vesicle proteins through the Rab35/Sky pathway have previously been demonstrated [53]. Rab35/Sky, in concert with the ESCRT pathway, selectively degrades synaptic vesicle proteins n-Syb and SV2, but not Synaptotagmin1 (Syt1) and SNAP25. However, how exactly different synaptic vesicle proteins are separately sorted for degradation remains unknown.
Membrane protein degradation through the endolysosomal system requires delivery to multivesicular bodies and finally to lysosomes [54, 55]. Rab7 is a key regulator of multivesicular body maturation from early endosomes, as well as the fusion of multivesicular bodies with lysosomes [56]. Rab7 is ubiquitously expressed and is required to mediate lysosomal degradation in all cells. In Drosophila, Rab7 is expressed in neurons before other cell types, and its loss in photoreceptors leads to progressive degeneration starting at synapses. At axon terminals, Rab7 is required for sorting of plasma membrane proteins, but not SV membrane proteins, to degradative compartments [7]. These findings suggest that neurons, and synaptic terminals in particular, are sensitive to reduced endolysosomal degradation and employ various cargo-specific endolysosomal degradation mechanisms for neuronal maintenance [18, 22].
Autophagy is a major catabolic pathway for the degradation of cytosolic proteins, membrane proteins, organelles and protein aggregates [57]. In neurons, basal autophagy is necessary for neuronal function and maintenance, and loss-of-function leads to neurodegeneration [58, 59]. Autophagosomes form and capture their cargos at synaptic terminals independently of the cell body (Figure 1) [60]. Recent work has demonstrated compartmentalized regulation of autophagic activity by synaptic proteins at the synapse, linking autophagy to synaptic function and dysfunction in disease [61, 62]. Several Rab proteins play roles in the various stages of autophagic activity, yet only few have been characterized or validated in neurons, and most information comes from non-neuronal cell cultures. Rab1, Rab4 and Rab11 regulate membrane trafficking from various sources to the initial phagophore assembly site [63–65]. Rab5 interacts with the BECN1/PI3KC3 complex to regulate the nucleation of phagophore membrane [66]. Rab33 interacts with Atg16L1 to elongate phagophore membrane [67], and late-endosome Rab proteins, Rab7 and Rab24, mediate the fusion of autophagosomes with lysosomes [68, 69]. Rab2 functions in autophagosome and lysosome maturation in human breast cancer cells and in Drosophila fat cells and nephrocytes, but this has not yet been demonstrated in neurons [70]. Recently, the first link between synaptic vesicle recycling and autophagy was reported [71]. Synaptic vesicle-associated Rab26 binds to phagophore elongation factor Atg16L1 and directs synaptic vesicles into phagophores for bulk degradation. Accordingly, Rab26 overexpression leads to synaptic vesicle accumulation in autophagosomes.
Rab GTPases are also important regulators of the trafficking and turnover of neurotransmitter receptors at the postsynaptic site (Figure 1). The correct distribution and abundance of postsynaptic receptors are prerequisites for activity-induced synaptic plasticity during memory formation and learning [72]. Rab4, Rab8, Rab11, Rab17 and Rab39B are implicated in the postsynaptic trafficking of AMPA and Kainate receptors. Rab8 regulates the delivery of GluA1-AMPA receptors from ER-Golgi network to the postsynaptic membrane [73]. Rab39B functions at the interface of ER-Golgi network and is necessary for maturation of AMPA receptor GluA2 subunit [74]. Rab4 and Rab11 are well-known regulators of receptor recycling. Specifically, Rab4 and Rab11 regulate activity-dependent insertion and removal of AMPA receptors from the postsynaptic membrane during long-term potentiation and long-term depression. Expression of Rab11DN blocks recycling of AMPA receptors and eventually attenuates long-term potentiation [75]. Rab17 mediates the surface abundance of Kainate receptors, but not AMPA receptors, through its interaction with the t-SNARE syntaxin-4. Expression of constitutively active Rab17 results in accumulation of syntaxin-4 in dendrites, which eventually leads to increased insertion of GluK2-Kainate receptors to the postsynaptic membrane [76].
From Axons and Dendrites to the Cell Body
Neurons are highly polarized cells, in which many proteins functioning in axons, axon terminals and dendrites must be transported to and from the cell body for signaling and degradation. In axons and dendrites, microtubules serve as trafficking routes along which motor proteins carry their cargos bidirectionally. Kinesin superfamily motor proteins (KIFs) mediate anterograde transport from the cell body to axonal and dendritic terminals. Dynein motor proteins direct retrograde transport to the cell body [77]. Several Rab GTPases interact either directly with motor proteins or indirectly through adaptor molecules to regulate both anterograde and retrograde transport in axons and dendrites [78]. The transport of Rab3 to axon terminals is mediated by the Rab3 GEF DENN/MADD through its interaction with the stalk domain of kinesin motors, KIF1A and KIF1Bβ [41, 79]. Interestingly, both Rab3 and Rab27 are also reported to mediate transport of other proteins to the axon terminals. GTP-bound active Rab3 directs transport of amyloid precursor protein (APP) to the axon terminals through association with another kinesin superfamily member, KIF1C [80]. Rab27 interacts with another member of the kinesin superfamily, KIF5, via adaptor proteins Slp1 and CRMP-2 to mediate the axonal transport of neurotrophin receptor TrkB to axon terminals [81]. Recently, anterograde transport of Rab4-positive vesicles was proposed to contribute to synaptic organization and homeostasis [82], albeit a rab4 null mutant, to our knowledge, has not yet been analyzed in this context.
Retrograde transport of late endosomes and autophagosomes ensures removal of dysfunctional proteins from distal neurites and is important for neuronal survival and function [83, 84]. Cargos for degradation are packaged in late endosomes and autophagosomes locally at axon terminals, and have been shown to be transported to the cell body to fuse with lysosomes for degradation [60]. The interaction between Rab7 and its effector RILP (Rab7 interacting lysosomal protein) mediates the retrograde transport of late endosomes and lysosomes. Specifically, RILP binds to the carboxy-terminal region of dynactin subunit p150, which associates dynactin/dynamin complex to late endosomes and lysosomes for transport of these organelles [85]. Recent evidence suggests that retrograde transport of autophagosomes requires initial fusion with late endosomes to form amphisomes, which may be transported to the cell body by the same Rab7/RILP/dynactin complex [86].
Neurotrophins are a class of proteins that function in neuronal development and maintenance by binding to two different classes of receptors: tropomyosin-receptor kinases (Trks) and the neurotrophin receptor p75 (p75NTR) [23]. Intraventricular administration of neurotrophins results in the formation of vesicles containing receptor–ligand complexes that are actively transported along microtubules [87, 88]. Rab5 mediates the internalization of receptor–ligand complexes into early endosomes (‘signaling endosomes’) where signaling continues to induce neurite outgrowth and dendritic branching [89]. Signaling endosomes undergo Rab5-to-Rab7 conversion [90], and Rab7-associated vesicles undergo retrograde transport to the cell body to modulate gene expression in the nucleus to promote survival and maintenance (Figure 1) [88, 91].
The secretory system in all eukaryotic cells contains ER, Golgi apparatus, ER–Golgi intermediate compartment and the trans-Golgi network. The ER forms a continuous network in axons and dendrites and the Golgi has dendritic ‘outposts’ that are positive for the trans-Golgi network component syntaxin16 [2, 92–94]. Membrane trafficking within this large network of membrane-bound organelles is essential for protein synthesis, processing, sorting, turnover and targeting of the newly synthesized proteins to their acceptor membranes [95]. The secretory pathway in neurons is particularly important for the formation of secretory granules, including synaptic vesicles and dense-core vesicles, and their transport to the axon terminals [96]. Rab1 and Rab2 regulate transport of vesicles between ER and Golgi [97]. Rab2 is involved in the maturation of dense-core vesicles in Caenorhabditis elegans neurons. Its interaction with effector proteins RUND1, a RUN domain protein, and CCCP1, a coiled-coil protein at the Golgi, mediates sorting of soluble and transmembrane cargo into dense-core vesicles [98]. RUND1 also interacts with a Rab2 effector, RIC-19, for which the loss-of-function phenocopies dense-core vesicle maturation defect of Rab2 mutants [99]. In addition to Rab1 and Rab2, Rab18 has also been shown to regulate ER–Golgi trafficking in non-neuronal cells [100]. Enhanced activity of Rab18 inhibits secretion of secretory granule contents in response to stimulation in neuroendocrine cells [101].
The bidirectional movement of vesicles in the ER–Golgi network and from trans-Golgi to the acceptor membranes is dependent on dynein- and kinesin-mediated microtubule-based transport [102]. Rab6 has a key role in retrograde transport of vesicles from Golgi apparatus towards ER. Although the mammalian isoform Rab6A is ubiquitous, Rab6B is predominantly expressed in microglia and Purkinje cells and specifically localizes to Golgi apparatus and ERGIC-derived vesicles [103]. Both isoforms interact with a dynein light chain, DYNLRB1, to regulate retrograde transport from Golgi to ER [104]. In Drosophila, the expression of constitutively active Rab6 prevents the maturation of rhodopsins Rh1 and Rh3, possibly by increasing the recycling of rhodopsin-containing vesicles from Golgi to ER and thereby hindering their post-Golgi maturation [105]. The Rab6 GEF component rich regulates, together with Rab6, the localization of the cell adhesion receptor N-Cadherin during brain wiring in Drosophila [106]. Finally, Rab8 has been proposed to mediate anterograde transport of post-Golgi vesicles to the plasma membrane [107]. In Xenopus rod photoreceptors, Rab8 loss-of-function impairs the transport of post-Golgi rhodopsin-containing vesicles to the connecting cilium resulting in degeneration of photoreceptors [108]. Cilia are specialized membrane protrusions that regulate various processes like cell motility, sensation of environmental cues and signal transduction. Several Rabs, including Rab8, Rab11 and Rab23, have been implicated in ciliogenesis and cilia function [109]. How this plethora of wild-type neuronal functions relates to the associations of Rab GTPases with neurodegenerative diseases is often unclear and is the subject of the following section.
Rab GTPases in Neurodegeneration
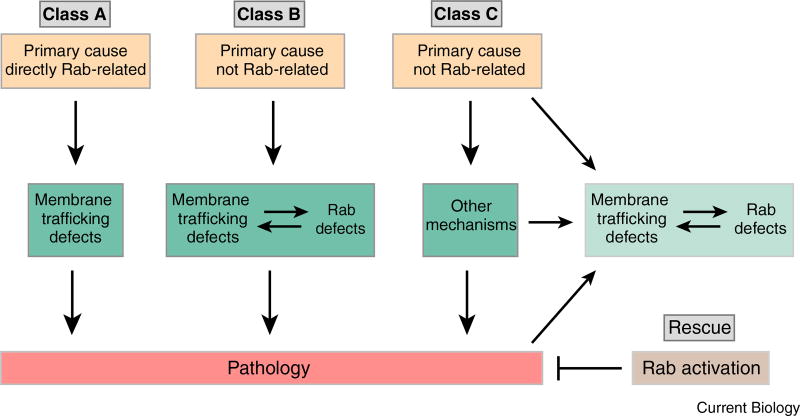
Rab GTPases have been associated with many neurodegenerative diseases, ranging from dementia to motor neuron degeneration. However, the types of association vary greatly. Mutations in Rab GTPases are a direct cause only in few cases, e.g. a rare case of familial Parkinson’s disease or the rare Charcot-Marie-Tooth type 2B disease. In contrast, the most common neurodegenerative diseases, including Alzheimer, are only indirectly linked to Rab GTPases and membrane trafficking dysfunction. Arguably the most important complication arising from indirect effects is the question of cause and effect: secondary effects can be either causative or ‘associated’ side effects with no causal relationship to pathology — or in fact a consequence of pathology. Yet, secondary effects can be critical, because these may indeed be the main cause of a pathology in instances where the primary trigger is not per se neurotoxic [8]. A summary of Rab GTPases associated with selected neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and Charcot–Marie–Tooth) is provided in Table 1. To facilitate understanding of the potential relevance of these associations, we classified them as follows (Figure 2): Class A: The primary cause of degeneration is a disease mutation in a Rab GTPase; Class B: The primary cause of degeneration is non-Rab-related, but a secondary effect on Rab GTPases, or the membrane trafficking these regulate, causes pathology; Class C: The primary cause of degeneration is non-Rab-related and causes pathology independent of a secondary effect on Rab GTPases or the membrane trafficking these regulate.
Table 1.
Summary of Rab GTPases and associated membrane trafficking processes implicated in Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amvotrophic lateral sclerosis (ALS) and Charcot–Marie–Tooth (CMT).
| Disease | Rab GTPases implicated |
Categories | Rescue | Key findings | Implicated membrane trafficking process |
References | ||
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease | Rabs 4, 5 | B | C | Upregulation of Rab4 and enlarged Rab5-positive endosomes in preclinical SAD | Early and recycling endosomal trafficking | [114] | ||
| Rabs 4, 5, 7, 27 | B | C | Upregulated levels in SAD postmortem brains | Early, late and recycling endosomal trafficking | [32, 116] | |||
| Rab4 | B | C | Increased protein level in PSEN1 mutant cells | Recycling endosomal trafficking | [125] | |||
| Rab6 | B | Membrane association of Rab6 in fibroblast cells is PSEN1 dependent | Intra-Golgi trafficking | [33] | ||||
| Rab6 | B | C | Upregulation of Rab6 in AD patient brains and PSEN1 mutant cells | Intra-Golgi trafficking | [124, 125] | |||
| Rab8 | B | C | Downregulation of Rab8 in mPSENI-treated PC12 cells | Post-Golgi trafficking | [34] | |||
| Parkinson’s disease | Rab39B | A | Loss of function mutations in Rab39B lead to intellectual disability and PD | Synaptic activity; ER-Golgi trafficking | [25–27] | |||
| Rab11 | B | x | Direct interaction with α-syn; colocalization with α-syn in intracellular inclusions; expression of Rab11 WT or Rab11 DN decreases the number of cells with intracellular α-syn inclusions and decreases α-syn toxicity | Recycling endosomal trafficking | [137] | |||
| Rab11 | x | Overexpression restores synaptic vesicle size and rescues impaired locomotor behavior induced by α-syn expression in Drosophila larvae and adults, normalizes synaptic activity in Drosophila larval NMJ, and reverses loss of dopaminergic neurons in adult Drosophila flies | Recycling endosomal trafficking | [139] | ||||
| Rabs 1, 3a, 8a | C | x | α-Syn accumulation inhibits ER to Golgi traffic; overexpression of Rab1, 3a or 8a rescues α-syn-induced toxicity in dopaminergic neurons | ER-Golgi trafficking | [136] | |||
| Rab3a | B | Direct interaction with α-syn; Rab3a recycling machinery regulates α-syn membrane binding | Synaptic activity | [138] | ||||
| Rabs 5a, 7, 11a | B | C | Colocalization with α-syn | Early, late and recycling endosomal trafficking | [134] | |||
| Rabs 3a/b/c/d, 8a/b, 10, 12, 35,43 | B | Phosphorylated by LRRK2 | (not discussed) | [151] | ||||
| Rab1 | C | x | α-syn accumulation impairs ER to Golgi traffic, resulting in ER stress and cell death; overexpression of Rab1 rescues dopaminergic neuron loss in PD animal models (D. melanogaster, C. elegans) | ER-Golgi trafficking | [135] | |||
| Rab8a | B | C | x | Interaction with α-syn in rat hippocampus and mouse cortical synaptosomes; expression of Rab8a decreases α-syn aggregation | Post-Golgi trafficking | [142] | ||
| Rab35 | B | C | Elevated protein level in PD patients’ serum and in the substantia nigra of PD mouse models; overexpression of Rab35 promotes the aggregation and secretion of α-syn in SH-SY5Y cells | Recycling endosomal trafficking | [140] | |||
| Rabs 8a, 8b, 13 | B | Phosphorylated by PINK1 | (not discussed) | [35] | ||||
| Rabs 8b, 11a, 13 | x | Overexpression of wild-type or constitutively active Rab8b, 11a or 13 reduces α-syn oligomerization | Post-Golgi and recycling endosomal trafficking | [141] | ||||
| Rab7 | B | Interaction with Lrrk; Lrrk LOF mutants disrupt Rab7-dependent lysosomal positioning | Endolysosomal degradation | [147] | ||||
| Rab7L1 | B | x | Interaction with LRRK2; overexpression of Rab7L1 rescues mutant phenotypes (lethality, dopaminergic neuron loss) | Endolysosomal sorting/degradation | [149] | |||
| Rabs 32, 38 | B | Interaction with LRRK2 | Late endosomal trafficking | [150] | ||||
| Huntington’s disease | Rab5 | C | Interaction with Htt-HAP40 complex; disrupted interaction leads to reduced endosome motility in HD cell lines | Early endosomal trafficking | [156] | |||
| Rab5 | x | Overexpression of Rab5WT or Rab5CA reduces mHtt aggregation and toxicity | Early endosomal trafficking | [30] | ||||
| Rab8 | B | Interaction with Htt-FIP2 complex | Post-Golgi trafficking | [154] | ||||
| Rab11 | B | x | Reduced activity (impaired GDP/GTP exchange) in Htt-null cells; elevated Rab11 activity (Rab11CA) decreases sensitivity of HD neurons to glutamate-induced cell death | Recycling endosomal trafficking | [157] | |||
| Rab11 | x | Overexpression of Rab11 rescues synaptic dysfunction and behavioral deficits in Drosophila model of HD | Recycling endosomal trafficking | [31] | ||||
| Amyotrophic lateral sclerosis | Rab1 | C | x | Colocalization with mSOD1, mFUS and mTDP-43; inhibited ER-Golgi transport by mSOD1, mFUSand mTDP-43; overexpression of Rab1 rescues the inhibited ER-Golgi transport | ER-Golgi trafficking | [29] | ||
| Rab1a | B | Interaction of C9orf72 with Rabla and ULK1 autophagy initiation complex; C9orf72 is an effector of Rabla | Autophagic flux | [166] | ||||
| Rabs 1,5, 7, 11 | B | C | Colocalization with C9orf72; increased colocalization of Rab7 and Rab11 with C9orf72 in patient postmortem brains; C9orf72 associates with autophagosome-like structures | Endolysosomal trafficking and autophagic flux | [162] | |||
| Rabs 8a, 39b | B | Regulated by C9orf72-SMCR8-WDR41 complex (RabGEF) | Autophagic flux | [164] | ||||
| Rab 11 | x | Expression of Rab11DN rescues TDP-43-induced disruption of BMP signaling, synaptic growth and larval crawling defects | Recycling endosomal trafficking | [167] | ||||
| Charcot-Marie-Tooth | Rab7 | A | Mutations in Rab7 cause CMT2B | Endolysosomal degradation | [16, 22, 28, 170–173] | |||
| Rab28 | A | B | MTMR13 and MTMR5 are putative RabGEFs for Rab28 and mutations in MTMR13 and MTMR5 cause CMT4B2 and CMT4B3, respectively | (not discussed) | [177] | |||
| Rab11 | A | B | SH3TC2 is a Rab11 effector and mutations in SH3TC2 cause CMT4C | Recycling endosomal trafficking | [178] | |||
Figure 2. Classifications of causal and correlative relationships between Rab GTPases, Rab-mediated membrane trafficking and pathology in neurodegeneration.
Class A: The primary cause of neurodegeneration is a mutation in a Rab GTPase that causes impaired Rab function, membrane trafficking defects, and pathology. Class B: The primary cause is unrelated to Rab GTPases, but leads either directly to a defect in Rab function or indirectly through membrane trafficking defects. These secondary defects cause pathology. Class C: Neither a Rab GTPase nor membrane trafficking defects have been established as a cause for pathology; however, defects in Rabs or membrane trafficking defects may be observed and could be upstream or downstream of pathology. Rescue: Overexpression of Rab GTPases has a protective effect.
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia. It is characterized by progressive loss of neurons, resulting in decline in memory, thinking skills and eventually the ability to carry out simple daily tasks [110, 111]. More than 90–95% of AD cases are sporadic (SAD) and are not associated with known disease mutations. Around 5–10% of cases are categorized as familial AD (FAD) and are linked to mutations in amyloid precursor protein (APP), Presenilin 1 (PSEN1) and Presenilin 2 (PSEN2). Mutations in these three genes are directly linked to the altered production of amyloid-β (Aβ), which is the cardinal component of the disease’s hallmark: Aβ plaques [112, 113]. Although SAD constitutes the majority of cases, it is still unclear to what extent Aβ deposition is a cause or an effect of pathology progression. Similarly, to what extent membrane trafficking defects constitute a cause or effect in AD has been difficult to ascertain.
Evidence for causality often focuses on the timing. For example, endolysosomal abnormalities precede clinically detectable Aβ deposition in SAD. Enlarged Rab5-positive early endosomes and accumulation of lysosomes have been observed as an early sign of SAD [114, 115]. In support of these studies, upregulation of Rab4, Rab5, Rab7 and Rab27 transcripts and protein levels were reported in post mortem cholinergic basal forebrain neurons and CA1 pyramidal neurons in SAD [32, 116]. These findings are interpreted as overactivation of endocytic mechanisms through upregulation of Rab GTPases, which might lead to imbalanced endosomal signaling and protein turnover. Autophagic vacuoles (autophagosomes, amphisomes and autolysosomes) are especially accumulated in dystrophic neurite swellings in diseased brains [117]. This accumulation could be caused by impaired fusion of autophagosomes with late endosomes and lysosomes, and/or a degradation defect in dysfunctional lysosomes [111]. A major genetic risk factor for AD is allelic variation in the apolipoprotein E (APOE) gene, which has been reported to promote Aβ degradation through the endolysosomal pathway [118–121]. Expression of human APOE causes increased colocalization of Aβ with Rab7 and Aβ degradation in primary microglial culture [118] and consistently, the expression of the ‘toxic’ variant APOE4 impairs Aβ degradation [119]. Clinical and epidemiological data indicate that 40–80% of AD patients are carriers of the APOE4 allele [122]. These studies leave open whether altered levels of Rab GTPases drive pathology (class B) or are a correlate of pathology (class C, Figure 2; Table 1).
Presenilins are proteases that function in various cellular processes (e.g. processing of APP, protein trafficking and turnover, calcium homeostasis and autophagosome-lysosome function) via interaction with substrates, including Rab GTPases [123]. PSEN1 has been suggested to be a membrane receptor for a RabGDI in the ER/Golgi network to regulate the amount of active Rab6 associated with the network. Loss-of-function of PSEN1 results in a two-fold decrease in the amount of RabGDI, causing decreased levels of membrane-associated, active Rab6 and consequently defective recycling of vesicles from Golgi to ER, suggesting a class B defect causative for pathology (Figure 2; Table 1) [33]. Additionally, the expression level of Rab6 is significantly increased by five-fold in brains of AD patients, and overall protein levels of Rab6 and Rab4 are increased in PSEN1 mutant cells, suggesting a compensatory response (class B or C) [124, 125]. Similarly, a significant reduction of Rab8 associated with membranes has been previously observed in PC12D cells transfected with the FAD mutant PSEN1 [34], but it remains unclear whether this reduction drives pathology (class B) or is a non-disease causing secondary effect of the PSEN1 mutation (class C). PSEN1 has also been implicated in autolysosome acidification and cathepsin activation. Effective autophagic clearance of dysfunctional proteins is of prime importance in neurons to prevent degeneration [58, 59]. Collectively, these results suggest membrane trafficking defects as a cause of pathology (class B), albeit only indirectly linked to Rab GTPases. PSEN2, unlike broadly distributed PSEN1, is restrictively located to late endosomes/lysosomes and it cleaves substrates localized to these compartments. FAD-associated mutations in PSEN2 increase the level of aggregation-prone Aβ42 in late endosomes and lysosomes, which may result in lysosomal dysfunction and cell death [126–128]. Interestingly, a subset of FAD-associated mutations in PSEN1 phenocopies PSEN2 and localizes Aβ42 to late endosomes and lysosomes, suggesting that mislocalization of APP processing enzymes to late endosomal compartments contributes to the pathogenesis in AD [129].
Parkinson’s Disease
Parkinson’s disease (PD) is the most prevalent movement disorder. It is characterized by accumulation of Lewy bodies consisting of α-synuclein and selective degeneration of dopaminergic neurons in the substantia nigra pars compacta. During the course of the disease, patients develop movement disabilities including resting tremor and muscle rigidity [130]. About 95% of PD cases are sporadic and the rest is familial. To date, mutations in at least 18 genes have been ‘associated’ with the etiology of PD, and a few of these mutations have been implicated in Rab function and membrane trafficking: Rab39B, α-synuclein (SNCA), PTEN-induced putative kinase 1 (PINK1) and leucine-rich repeat kinase 2 (LRRK2) [131, 132].
Several loss-of-function mutations in rab39B, including missense and nonsense mutations as well as the complete deletion, have been identified in inherited early-onset PD with Lewy body pathology, and are also linked to symptoms atypical to PD cases such as intellectual disability [25–27]. Rab39B is exclusively expressed in neurons, localizes to the Golgi, and functions in trafficking of AMPA receptor subunit GluA2 to postsynaptic membrane in hippocampal neurons (Figure 1). Rab39B mutant neurons show increased levels of immature GluA2, which leads to the formation of AMPA receptors lacking this subunit and has been associated with immature synapses and intellectual disability [74, 132]. This is a direct demonstration of how a defective Rab GTPase can underlie neurodegeneration (class A, Figure 2; Table 1), although the exact mechanism of how Rab39B loss-of-function leads to selective neurodegeneration in dopaminergic neurons remains elusive [25, 132].
The small neuronal protein α-synuclein is highly enriched at presynaptic terminals [133] and has been shown to colocalize and interact with several Rab GTPases (Rab1, Rab3a, Rab5, Rab7, Rab8a, Rab11, Rab13 and Rab35) in the regulation of membrane trafficking processes (Table 1) [131, 134–140]. Overexpression of specific Rab GTPases restores membrane trafficking defects ensuing from mutant α-synuclein. Rab11DN expression reduces α-synuclein secretion in HEK cells (class B) [134], and expression of wild-type or dominant-negative Rab11 decreases the number of cells with intracellular inclusions in H4 human neuroglioma cells [137]. Overexpression of Rab11 rescues several phenotypes caused by PD mutations in α-synuclein in Drosophila, including decreased locomotor activity, shortened lifespan and degeneration of dopaminergic neurons (Figures 2 and 3) [139]. Accumulation of α-synuclein disrupts ER/Golgi trafficking in a dose-dependent manner and results in dopaminergic neuron loss (class C), which can be rescued by overexpression of Rab1, Rab3a or Rab8a (Figures 2 and 3) [135, 136]. Rab8b, Rab11a and Rab13 were identified from a shRNA-based screen as modulators of α-synuclein, and overexpression of the wild-type or a constitutively active form of these Rabs significantly reduces α-synuclein oligomerization [141]. Rab8, a regulator of post-Golgi trafficking, directly binds the carboxy-terminal of α-synuclein and Rab8 overexpression reduces toxicity caused by mutant or overexpression of wild-type α-synuclein [136, 142]. Recently, increased level of Rab35 in serum was reported to correlate with the age of onset and disease duration of PD patients. Moreover, overexpression of Rab35 increases the aggregation of mutant α-synuclein in dopaminergic neurons [140]. Collectively, these results suggest that α-synuclein-related PD pathology may be, at least partially, attributed to the dysregulation of Rab GTPases and membrane trafficking.
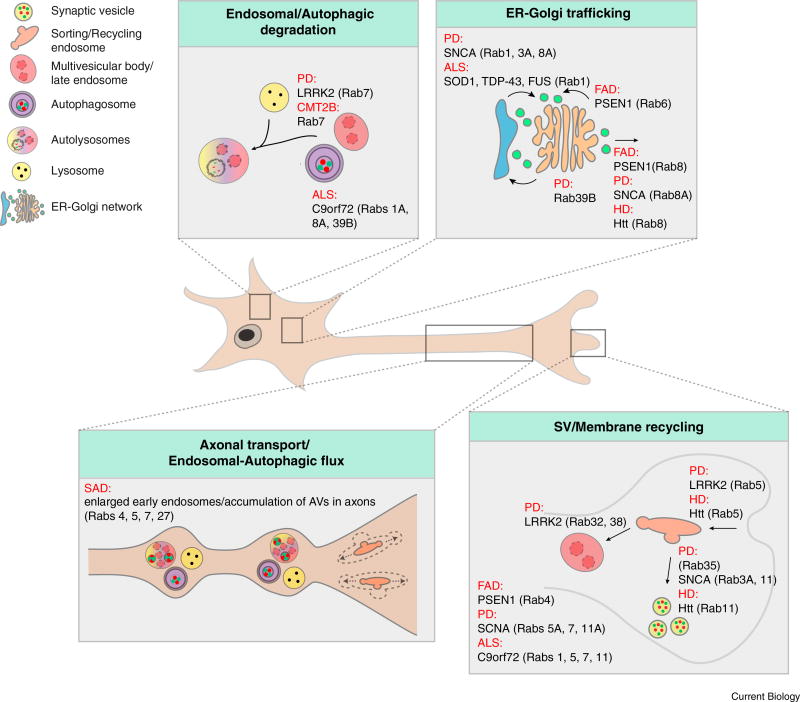
Figure 3. Rab GTPases in neurodegeneration.
Neurodegenerative disorders (in red) with their related disease-causing proteins and Rab GTPases are listed next to the implicated membrane trafficking steps: endosomal/autophagic degradation, ER-Golgi trafficking, axonal transport/endosomal-autophagic flux, or synaptic vesicle/membrane recycling. Rab GTPases in round brackets: Rabs with disrupted interaction with disease proteins, or Rabs that cause pathology as a secondary effect (class B). Rab GTPases without brackets: Disease causing mutations in these Rabs directly lead to pathology (class A). (ALS: amyotrophic lateral sclerosis; AV: autophagic vacuoles; CMT2B: Charcot-Marie-Tooth type 2B; FAD: familial Alzheimer’s disease; HD: Huntington’s disease; PD: Parkinson’s disease; SAD: sporadic Alzheimer’s disease; SV: synaptic vesicle).
PINK1 (kinase) and Parkin (E3 ubiquitin ligase) regulate mitochondrial quality control. Upon phosphorylation of Parkin’s Ser65 residue by PINK1, Parkin is activated and recruited to damaged mitochondria to ubiquitinate outer mitochondrial membrane proteins to trigger selective autophagy [143]. A SILAC-based phosphoproteomic screening of PINK1 substrates revealed that Rab8a, Rab8b and Rab13 are also phosphorylated by PINK1. PINK1 knockdown or mutated PINK1 abolish phosphorylation of Rab8a in HEK cells and patient-derived fibroblasts, respectively, suggesting that post-Golgi trafficking regulated by Rab8 may be impaired by PINK1 mutation (class B, Table 1) [35].
LRRK2 is involved in synaptic vesicle recycling, autophagy and mitochondrial function [144–146]. Lrrk, the Drosophila homolog of LRRK2, directly interacts with late endosomal/ lysosomal Rab GTPase Rab7 and mediates the subcellular localization of lysosomes. LrrkGS, the Drosophila analogue of PD-associated LRRK2 mutant (LRRK2G2019S) impairs the Lrrk–Rab7 interaction and subsequently the lysosomal positioning (class B, Table 1) [147]. Consistently, overexpression of Rab7-like protein 1 (Rab7L1) rescues neurodegeneration induced by LRRK2G2019S in Drosophila dopaminergic and rat primary cortical neurons (Figure 3; Table 1) [148, 149]. These findings suggest that degeneration in LRRK2 mutant neurons is linked to Rab7 function. LRRK2 also interacts with Rab32 and its homologue Rab38, both of which are closely related to Rab7L1. Rab32 regulates LRRK2-related late endosomal traffic, and both Rab32 and Rab38 are involved in trans-Golgi network organization and transport of key enzymes during melanogenesis [150]. However, their roles in neurons remain elusive. Furthermore, LRRK2 directly interacts with Rab5 to regulate synaptic vesicle endocytosis [144], and phosphorylates Rab3a/b/ c/d, Rab8a/b, Rab10, Rab12, Rab 35 and Rab43 [151]. Collectively, these results suggest that PD-causing mutant LRRK2 causes pathology by dysregulated Rab GTPase functions and hence membrane trafficking (class B, Table 1).
Huntington’s Disease/PolyQ
Huntington’s disease (HD) is the most common and well-studied form of polyglutamine (PolyQ) diseases, which is a group of progressive neurodegenerative disorders characterized by the expansion of a trinucleotide repeat cytosine-adenine-guanine (CAG). HD is caused by mutant variants of the huntingtin (htt) gene that contain at least 36–40 residues of elongated glutamine repeats [152]. Htt is a large, membrane-associated protein located on the Golgi as well as endocytic and exocytic vesicles [153]. The wild-type function of Htt remains to be fully characterized.
Mutations in Htt have been directly or indirectly linked to Rab5, Rab8 and Rab11 (Figure 3; Table 1). Htt interacts with Rab8 via the coiled-coil protein FIP2 [154] and also with Rab8/optineurin complex to function in the post-Golgi trafficking [155] (class B, Figure 2). Htt loss of function causes mislocalization of this Rab8/optineurin complex, impairing the vesicle trafficking between Golgi and lysosomes [155]. Htt also forms a complex with HAP40 (Htt-associated protein 40) to function as a Rab5 effector that regulates the motility of early endosomes. In HD, Htt-HAP40 complex formation is disrupted, resulting in an upregulation of HAP40 and a decline in early endosome motility [156]. Htt activates Rab11 by interaction with a Rab11 GDP-containing complex (class B). In a mouse model of HD, primary cortical neurons show reduced recycling of transferrin receptors back to the plasma membrane, suggesting that HD pathogenesis is at least partially linked to decreased Rab11 activity in recycling endosomes [157]. Furthermore, increase of Rab11 activity by expression of the constitutively active form decreases the sensitivity of HD neurons to glutamate-induced cell death [157]. Similarly, overexpression of Rab11 rescues synaptic dysfunction and behavioral deficits in a Drosophila model of HD (Figure 3) [31]. Additionally, overexpression of wild-type or constitutively active Rab5 significantly decreases the toxicity and aggregation of mutant Huntingtin (Table 1) [30].
Finally, HD is also associated with alterations in both anterograde and retrograde axonal transport through disrupted interaction of mutant Htt with the dynein/dynactin complex and Kinesin-1 [158, 159]. HD-associated alterations in axonal transport particularly affect the transport of neurotrophin receptors, and hence impaired neurotrophin signaling might also contribute to pathogenesis (class C, Figure 2) [160].
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that specifically affects motor neurons in the brain and spinal cord [161]. Similar to AD and PD, 90% of all ALS cases are sporadic; 10% of ALS cases are of familial origin, caused by mutations in genes encoding a variety of proteins, such as SOD1 (superoxide dismutase 1), TDP-43 (transactive response DNA-binding protein 43), FUS (fused in sarcoma), C9orf72 (Chromosome 9 open reading frame 72) and VAPB (VAMP (synaptobrevin)-associated protein B) [29, 162, 163]. Many cellular defects are associated with ALS, including ER stress, autophagy defects, protein aggregation and inhibition of axonal transport. Intracellular membrane trafficking is fundamental to all these cellular processes, and several Rab GTPases have been associated with ALS (Figure 3; Table 1).
The intronic expansion of a hexanucleotide GGGGCC repeat in c9orf72 gene is the major cause of familial ALS (~33%) and frontotemporal dementia (~25%), the second most prevalent form of presenile dementia after AD [162, 164]. C9ORF72 has been reported to colocalize with Rab1, Rab5, Rab7 and Rab11 in the endolysosomal system in cortical neurons [162, 165]. C9ORF72 was recently shown to form a stable complex with SMCR8 and WDR41 to function as a RabGEF for Rab8a and Rab39b and regulate autophagic flux (class B and C, Table 1) [164]. C9ORF72 was also identified as a Rab1 a effector that regulates the recruitment of Unc-51-like kinase 1 (ULK1) to the phagophore assembly site to initiate autophagy (Table 1) [166]. Consistently, reduction of C9orf72 expression in neurons leads to accumulation of p62-positive aggregates, similar to the p62 pathology observed in ALS as well as frontotemporal dementia patients, indicating autophagic defects [164, 166]. Compared to the healthy control group, motor neurons of patients’ postmortem brains showed increased colocalization of C90RF72 with Rab7 and Rab11, but not Rab5 [162], further suggesting dysregulated endosomal traffic (class B, Figure 2).
ALS-causing mutations in SOD1, TDP-43 or FUS cause mis-localization of Rab1, impaired protein transport in the ER-Golgi network, increased ER stress and formation of inclusions in neurons. It remains unclear to what extent these contribute to pathology (class B or C). Overexpression of Rab1 exerts a protective function against the ER stress induced by mSOD1, mTDP-43 and mFUS (Figures 2 and 3; Table 1) [29]. Recently, both gain- and loss-of-function of TDP-43 was reported to decrease bone morphogenic protein (BMP) signaling in Drosophila NMJ. BMP signaling controls growth and function of synapses, and misregulation of TDP-43 function disrupts endocytic transport of BMP receptors, resulting in mislocalization to recycling endosomes [167]. Expression of Rab11DN partially rescues the TDP-43-induced disruption of BMP signaling and synaptic growth as well as larval crawling defects (Table 1) [167].
Finally, a point mutation in the major sperm protein (MSP) domain of VAPB has been established as a cause for ALS. The MSP domain is cleaved, secreted and functions as a ligand for Eph receptors. The Drosophila homolog of VAPB, DVAP-33A, with the corresponding point mutation fails to be secreted, is ubiquitinated and forms inclusions in the ER, similar to those observed in ALS patients [163]. In addition, DVAP-33A plays a role in the organization of the microtubule cytoskeleton in pre-synaptic terminals [168]. The membrane trafficking defects in VAPB mutant neurons have not been directly linked to Rab GTPase function (class C).
Charcot-Marie-Tooth Disease
Charcot-Marie-Tooth disease (CMT) is a hereditary motor and sensory neuropathy characterized by progressive muscle atrophy that starts from feet and hands [169]. CMT is classified by abnormalities in either myelin formation and maintenance (demyelinating CMT) or distal axon degeneration of motor and sensory neurons (axonal CMT). Numerous mutations have been identified for both types, which are listed and discussed in detail elsewhere [169]. Here, we highlight only two directly Rab-related CMT disorders.
Charcot-Marie-Tooth type 2B (CMT2B) is a rare peripheral neuropathy that is caused independently by five different missense mutations in Rab7 (class A, Figure 2) [170, 171]. CMT2B mutant variants of Rab7 were originally characterized to cause the disease by dominant gain of function, based on the initial observation of reduced intrinsic GTP hydrolysis activity similar to the constitutively active form Rab7Q67L [28, 172, 173]. However, in Drosophila photoreceptors and motor neurons, overexpression of the mutant variants of Rab7 did not display neuron-specific dominant gain-of-function phenotype but reduced wild-type function. These findings led to the hypothesis that CMT2B may result from a reduced endolysosomal capacity to which neurons are sensitive [22]. In contrast, other studies found that overexpression of CMT2B-mutant rab7 in cultured cells and neurons led to functional defects [170, 174, 175]. Moreover, CMT2B-associated mutations lead to reduced localization of Rab7 to autophagic compartments and decreased autophagic flux [176]. It remains unclear whether pathology in humans is a consequence of a partial reduction in autophagic/endolysosomal degradation due to partial rab7 loss of function, or an extra function of Rab7 that was not seen in the Drosophila studies. In either case, CMT2B is a direct consequence of Rab7 dysfunction (class A). Since Rab7 is a ubiquitous Rab GTPase, this rare disease also suggests that neurons are particularly sensitive to alterations in membrane trafficking.
CMT type 4 (CMT4) is an autosomal recessive demyelinating motor and sensory neuropathy for which associations with several Rabs have been reported [169]. CMT4B2 and CMT4B3 are caused by mutations in myotubularin-related (MTMR) phospholipid phosphatases MTMR13 and MTMR5, respectively. Both MTMR13 and MTMR5 are reported to function as RabGEFs that may activate Rab28 [177]. CMT4C is caused by mutations in SH3TC2, a Rab11 effector [178]. Dysregulation of Rab11 may therefore contribute to CMT4C pathology. In all these cases, disease causing mutations lead to pathology via altered Rab GTPase function (class B, Table 1).
Conclusions
Associations of Rab GTPase-mediated membrane trafficking with different neurodegenerative diseases are diverse, but reveal a few common themes. In particular, early endosomal trafficking and endolysosomal degradation are commonly described as ‘drivers of pathology’ in AD, PD and CMT. A second group of associations encompass defects of the secretory pathway where post-Golgi trafficking defects have been described as causative for pathology in AD, PD and HD. These causative associations are further supported by the protective roles of increased Rab1, Rab5, Rab8 or Rab11 function in several models for PD, HD and ALS. Causative links for Rab-mediated membrane trafficking defects are clearer in PD and CMT than in AD, HD and ALS. We did not find ‘class A’ or ‘Rescue’ associations of Rab GTPases with AD (Table 1). Rare diseases, such as CMT2B, offer important insights into roles of membrane trafficking critical to neuronal maintenance. However, most associations of specific Rab GTPases with neurodegenerative diseases remain correlative and ‘class B’ and ‘class C categorizations were often difficult to distinguish. Ultimately, associations with diseases will make most sense when wild-type functions of Rabs, and the membrane trafficking these regulate, are understood. This is the flip-side of neurodegeneration research: understanding what keeps neurons alive in the first place remains a formidable challenge, and Rab-mediated membrane trafficking is sure to play a key role.
Acknowledgments
We thank all members of the Hiesinger lab for discussion. Due to citation limitations, we were unable to cite many of the primary publications. This work was supported by the Deutsche Forschungsgemeinschaft (SFB958 and SFB/TRR186), the NeuroCure Cluster Berlin, grants from the National Institute of Health (RO1EY018884, RO1EY023333), and the Muscular Dystrophy Association of the USA to P.R.H.
References
- 1.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez OA, Couve A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol. 2011;21:219–227. doi: 10.1016/j.tcb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Bentley M, Banker G. The cellular mechanisms that maintain neuronal polarity. Nat. Rev. Neurosci. 2016;17:611–622. doi: 10.1038/nrn.2016.100. [DOI] [PubMed] [Google Scholar]
- 4.Buckley KM, Melikian HE, Provoda CJ, Waring MT. Regulation of neuronal function by protein trafficking: a role for the endosomal pathway. J. Physiol. 2000;525:11–19. doi: 10.1111/j.1469-7793.2000.t01-2-00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 6.Rizzoli SO. Synaptic vesicle recycling: steps and principles. EMBO J. 2014;33:788–822. doi: 10.1002/embj.201386357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin EJ, Kiral FR, Ozel MN, Burchardt LS, Osterland M, Epstein D, Wolfenberg H, Prohaska S, Hiesinger PR. Live observation of two parallel membrane degradation pathways at axon terminals. Curr. Biol. 2018;28:1–12. doi: 10.1016/j.cub.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Chan CC, Cherry S, Hiesinger PR. Membrane trafficking in neuronal maintenance and degeneration. Cell. Mol. Life Sci. 2013;70:2919–2934. doi: 10.1007/s00018-012-1201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touchot N, Chardin P, Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc. Natl. Acad. Sci. USA. 1987;84:8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 2015;128:3171–3176. doi: 10.1242/jcs.166074. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer SR. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell. 2017;28:712–715. doi: 10.1091/mbc.E16-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barr F, Lambright DG. Rab GEFs and GAPs. Curr. Opin. Cell Biol. 2010;22:461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 14.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brighouse A, Dacks JB, Field MC. Rab protein evolution and the history of the eukaryotic endomembrane system. Cell. Mol. Life Sci. 2010;67:3449–3465. doi: 10.1007/s00018-010-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, et al. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Curr. Biol. 2011;21:1704–1715. doi: 10.1016/j.cub.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin EJ, Chan CC, Agi E, Cherry S, Hanacik E, Buszczak M, Hiesinger PR. Similarities of Drosophila rab GTPases based on expression profiling: completion and analysis of the rab-Gal4 kit. PLoS One. 2012;7:e40912. doi: 10.1371/journal.pone.0040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Adamo P, Masetti M, Bianchi V, More L, Mignogna ML, Giannandrea M, Gatti S. RAB GTPases and RAB-interacting proteins and their role in the control of cognitive functions. Neurosci. Bio-behav. Rev. 2014;46:302–314. doi: 10.1016/j.neubiorev.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain. Res. Rev. 2008;58:236–246. doi: 10.1016/j.brainresrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Cherry S, Jin EJ, Ozel MN, Lu Z, Agi E, Wang D, Jung WH, Epstein D, Meinertzhagen IA, Chan CO, et al. Charcot-Marie-Tooth 2B mutations in rab7 cause dosage-dependent neurodegeneration due to partial loss of function. eLife. 2013;2:e01064. doi: 10.7554/eLife.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucci C, Alifano P, Cogli L. The role of rab proteins in neuronal cells and in the trafficking of neurotrophin receptors. Membranes. 2014;4:642–677. doi: 10.3390/membranes4040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mignogna ML, D’Adamo P. Critical importance of RAB proteins for synaptic function. Small GTPases. 2017:1–13. doi: 10.1080/21541248.2016.1277001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Rise-ley JR, Stephenson SE, Fitzpatrick E, Haas SA, Pope K, et al. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am. J. Hum. Genet. 2014;95:729–735. doi: 10.1016/j.ajhg.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L, Guerreiro R, Majounie E, Federoff M, Heutink P, et al. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol. Genet. 2015;1:e9. doi: 10.1212/NXG.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mata IF, Jang Y, Kim CH, Hanna DS, Dorschner MO, Samii A, Agarwal P, Roberts JW, Klepitskaya O, Shprecher DR, et al. The RAB39B p.G192R mutation causes X-linked dominant Parkinson’s disease. Mol. Neurodegener. 2015;10:50. doi: 10.1186/s13024-015-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinosa MR, Progida C, De Luca A, Colucci AM, Alifano P, Bucci C. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J. Neurosci. 2008;28:1640–1648. doi: 10.1523/JNEUROSCI.3677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soo KY, Halloran M, Sundaramoorthy V, Parakh S, Toth RP, Southam KA, McLean CA, Lock P, King A, Farg MA, et al. Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta. Neuropathol. 2015;130:679–697. doi: 10.1007/s00401-015-1468-2. [DOI] [PubMed] [Google Scholar]
- 30.Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J. Cell Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinert JR, Campesan S, Richards P, Kyriacou CP, Forsythe ID, Giorgini F. Rab11 rescues synaptic dysfunction and behavioural deficits in a Drosophila model of Huntington’s disease. Hum. Mol. Genet. 2012;27:2912–2922. doi: 10.1093/hmg/dds117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S. Upregulation of select rab GTPases in cholin-ergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Chem. Neuroanat. 2011;42:102–110. doi: 10.1016/j.jchemneu.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheper W, Zwart R, Baas F. Rab6 membrane association is dependent of Presenilin 1 and cellular phosphorylation events. Brain Res. Mol. Brain Res. 2004;122:17–23. doi: 10.1016/j.molbrainres.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Kametani F, Usami M, Tanaka K, Kume H, Mori H. Mutant presenilin (A260V) affects Rab8 in PC12D cell. Neurochem. Int. 2004;44:313–320. doi: 10.1016/s0197-0186(03)00176-1. [DOI] [PubMed] [Google Scholar]
- 35.Lai YC, Kondapalli C, Lehneck R, Procter JB, Dill BD, Woodroof HI, Gourlay R, Peggie M, Macartney TJ, Corti O, et al. Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J. 2015;34:2840–2861. doi: 10.15252/embj.201591593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin EJ, Kiral FR, Hiesinger PR. The where, what, and when of membrane protein degradation in neurons. Dev. Neurobiol. 2017;78:283–297. doi: 10.1002/dneu.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons-focusing in on endosomes. Mol. Cell. Neurosci. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito G, Ana Clara F, Verstreken P. Synaptic vesicle trafficking and Parkinson’s disease. Dev. Neurobiol. 2012;72:134–144. doi: 10.1002/dneu.20916. [DOI] [PubMed] [Google Scholar]
- 39.Bezprozvanny I, Hiesinger PR. The synaptic maintenance problem: membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol. Neurodegener. 2013;8:23. doi: 10.1186/1750-1326-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlos NJ, Gronborg M, Riedel D, Chua JJ, Boyken J, Kloepper TH, Urlaub H, Rizzoli SO, Jahn R. Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis. J. Neurosci. 2010;30:13441–13453. doi: 10.1523/JNEUROSCI.0907-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluter CM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 dynamically controls protein composition at active zones. Neuron. 2009;64:663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu E, Kanno E, Choi S, Sugimori M, Moreira JE, Llinas RR, Fukuda M. Role of Rab27 in synaptic transmission at the squid giant synapse. Proc. Natl. Acad. Sci. USA. 2008;105:16003–16008. doi: 10.1073/pnas.0804825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J. Cell. Biol. 2008;783:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 47.Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell. Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu H, Kawamura S, Ozaki K. An essential role of Rab5 in uniformity of synaptic vesicle size. J. Cell Sci. 2003;116:3583–3590. doi: 10.1242/jcs.00676. [DOI] [PubMed] [Google Scholar]
- 49.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell. Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Wit H, Lichtenstein Y, Kelly RB, Geuze HJ, Klumperman J, van derSluijs P. Rab4 regulates formation of synaptic-like micro-vesicles from early endosomes in PC12 cells. Mol. Biol. Cell. 2001;12:3703–3715. doi: 10.1091/mbc.12.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell. 2011;145:117–132. doi: 10.1016/j.cell.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes AC, Uytterhoeven V, Kuenen S, Wang YC, Slabbaert JR, Swerts J, Kasprowicz J, Aerts S, Verstreken P. Reduced synaptic vesicle protein degradation at lysosomes curbs TBC1 D24/sky-induced neurodegeneration. J. Cell. Biol. 2014;207:453–462. doi: 10.1083/jcb.201406026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheehan P, Zhu M, Beskow A, Vollmer C, Waites CL. Activity-dependent degradation of synaptic vesicle proteins requires Rab35 and the ESCRT Pathway. J. Neurosci. 2016;36:8668–8686. doi: 10.1523/JNEUROSCI.0725-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piper RC, Katzmann DJ. Biogenesis and function of multi-vesicular bodies. Annu. Rev. Cell. Dev. Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat. Rev. Mol. Cell. Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 56.Guerra F, Bucci C. Multiple roles of the small GTPase Rab7. Cells. 2016;5:E34. doi: 10.3390/cells5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 59.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 60.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell. Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijayan V, Verstreken P. Autophagy in the presynaptic compartment in health and disease. J. Cell. Biol. 2017;216:1895–1906. doi: 10.1083/jcb.201611113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okerlund ND, Schneider K, Leal-Ortiz S, Montenegro-Venegas C, Kim SA, Garner LC, Gundelfinger ED, Reimer RJ, Garner CC. Bassoon Controls Presynaptic Autophagy through Atg5. Neuron. 2017;93:897–913. e897. doi: 10.1016/j.neuron.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 63.Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic. 2010;11:1246–1261. doi: 10.1111/j.1600-0854.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 64.Talaber G, Miklossy G, Oaks Z, Liu Y, Tooze SA, Chudakov DM, Banki K, Perl A. HRES-1/Rab4 promotes the formation of LC3(+) autophagosomes and the accumulation of mitochondria during autophagy. PLoS One. 2014;9:e84392. doi: 10.1371/journal.pone.0084392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA. TBC1D14 regulates autophagosome formation via Rab11-and ULK1-positive recycling endosomes. J. Cell Biol. 2012;197:659–675. doi: 10.1083/jcb.201111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris DH, Yip CK, Shi Y, Chait BT, Wang QJ. Beclin 1 -Vps34 complex architecture: understanding the nuts and bolts of therapeutic targets. Front. Biol. 2015;10:398–426. doi: 10.1007/s11515-015-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol. Biol. Cell. 2008;79:2916–2925. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 69.Yla-Anttila P, Mikkonen E, Happonen KE, Holland P, Ueno T, Simonsen A, Eskelinen EL. RAB24 facilitates clearance of autophagic compartments during basal conditions. Autophagy. 2015;11:1833–1848. doi: 10.1080/15548627.2015.1086522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorincz P, Toth S, Benko P, Lakatos Z, Boda A, Glatz G, Zobel M, Bisi S, Hegedus K, Takats S, et al. Rab2 promotes autophagic and endocytic lysosomal degradation. J. Cell. Biol. 2017;216:1937–1947. doi: 10.1083/jcb.201611027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbruggen G, Schalk AM, Kuhnel K, Boyken J, Erck C, Martens H, et al. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. eLife. 2015;4:e05597. doi: 10.7554/eLife.05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 73.Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J. Biol. Chem. 2004;279:43870–43878. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- 74.Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat. Commun. 2015;6:6504. doi: 10.1038/ncomms7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 76.Mori Y, Fukuda M, Henley JM. Small GTPase Rab17 regulates the surface expression of kainate receptors but not alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors n hippocampal neurons via dendritic trafficking of Syntaxin-4 protein. J. Biol. Chem. 2014;289:20773–20787. doi: 10.1074/jbc.M114.550632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 78.Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem. Soc. Trans. 2011;39:1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- 79.Niwa S, Tanaka Y, Hirokawa N. KIFIBbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat. Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- 80.Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Muller U, Beyreuther K, et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J. Neurosci. 2009;29:14534–14544. doi: 10.1523/JNEUROSCI.1546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, et al. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev. Cell. 2009;16:675–686. doi: 10.1016/j.devcel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Dey S, Banker G, Ray K. Anterograde transport of Rab4-associated vesicles regulates synapse organization in Drosophila. Cel Rep. 2017;18:2452–2463. doi: 10.1016/j.celrep.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- 85.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 86.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J. Cell. Biol. 2015;209:377–386. doi: 10.1083/jcb.201412046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weissmiller AM, Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012;1:14. doi: 10.1186/2047-9158-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J. Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Lamb D, Chou MM, Liu YJ, Li GP. Nerve growth factor-mediated neurite outgrowth via regulation of Rab5. Mol. Biol. Cell. 2007;18:1375–1384. doi: 10.1091/mbc.E06-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 91.Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J. Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, De Camilli P. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. USA. 2017;114:E4859–E4867. doi: 10.1073/pnas.1701078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chua CE, Tang BL. Syntaxin 16 is enriched in neuronal dendrites and may have a role in neurite outgrowth. Mol. Membr. Biol. 2008;25:35–45. doi: 10.1080/09687680701504649. [DOI] [PubMed] [Google Scholar]
- 95.Gomez-Navarro N, Miller E. Protein sorting at the ER-Golgi interface. J. Cell. Biol. 2016;215:769–778. doi: 10.1083/jcb.201610031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park JJ, Koshimizu H, Loh YP. Biogenesis and transport of secretory granules to release site in neuroendocrine cells. J. Mol. Neurosci. 2009;37:151–159. doi: 10.1007/s12031-008-9098-y. [DOI] [PubMed] [Google Scholar]
- 97.Saraste J. Spatial and functional aspects of ER-Golgi Rabs and tethers. Front. Cell. Dev. Biol. 2016;4:28. doi: 10.3389/fcell.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ailion M, Hannemann M, Dalton S, Pappas A, Watanabe S, Hegermann J, Liu Q, Han HF, Gu M, Goulding MQ, et al. Two Rab2 interactors regulate dense-core vesicle maturation. Neuron. 2014;82:167–180. doi: 10.1016/j.neuron.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sumakovic M, Hegermann J, Luo L, Husson SJ, Schwarze K, Olendrowitz C, Schoofs L, Richmond J, Eimer S. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J. Cell. Biol. 2009;186:897–914. doi: 10.1083/jcb.200902096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, Handley MT, Barr FA. Rab18 and a Rab18 GEF complex are required for normal ER structure. J. Cell. Biol. 2014;205:707–720. doi: 10.1083/jcb.201403026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vazquez-Martinez R, Cruz-Garcia D, Duran-Prado M, Peinado JR, Castano JP, Malagon MM. Rab18 inhibits secretory activity in neuroendocrine cells by interacting with secretory granules. Traffic. 2007;8:867–882. doi: 10.1111/j.1600-0854.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 102.Vaughan KT. Microtubule plus ends, motors, and traffic of Golgi membranes. Biochim. Biophys. Acta. 2005;1744:316–324. doi: 10.1016/j.bbamcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 103.Opdam FJ, Echard A, Croes HJ, van den Hurk JA, van deVorsten-bosch RA, Ginsel LA, Goud B, Fransen JA. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J. Cell Sci. 2000;113:2725–2735. doi: 10.1242/jcs.113.15.2725. [DOI] [PubMed] [Google Scholar]
- 104.Wanschers B, van de Vorstenbosch R, Wijers M, Wieringa B, King SM, Fransen J. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell. Motil. Cytoskeleton. 2008;65:183–196. doi: 10.1002/cm.20254. [DOI] [PubMed] [Google Scholar]
- 105.Shetty KM, Kurada P, O’Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J. Biol. Chem. 1998;273:20425–20430. doi: 10.1074/jbc.273.32.20425. [DOI] [PubMed] [Google Scholar]
- 106.Tong C, Ohyama T, Tien AC, Rajan A, Haueter CM, Bellen HJ. Rich regulates target specificity of photoreceptor cells and N-cadherin trafficking in the Drosophila visual system via Rab6. Neuron. 2011;71:447–459. doi: 10.1016/j.neuron.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moritz OL, Tarn BM, Hurd LL, Peranen J, Deretic D, Paper-master DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol. Biol. Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blacque OE, Scheidel N, Kuhns S. Rab GTPases in cilium formation and function. Small GTPases. 2017:1–19. doi: 10.1080/21541248.2017.1353847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peric A, Annaert W. Early etiology of Alzheimer’s disease: tipping the balance toward autophagy or endosomal dysfunction? Acta. Neuropathol. 2015;129:363–381. doi: 10.1007/s00401-014-1379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajendran L, Annaert W. Membrane trafficking pathways in Alzheimer’s disease. Traffic. 2012;13:759–770. doi: 10.1111/j.1600-0854.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- 114.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-lsla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nixon RA, Mathews PM, Cataldo AM. The neuronal endosomal-lysosomal system in Alzheimer’s disease. J. Alzheimers Dis. 2001;3:97–107. doi: 10.3233/jad-2001-3114. [DOI] [PubMed] [Google Scholar]
- 116.Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, et al. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol. Psychiatry. 2010;68:885–893. doi: 10.1016/j.biopsych.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff O, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neural. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 118.Lee CY, Tse W, Smith JD, Landreth GE. Apolipoprotein E promotes beta-amyloid trafficking and degradation by modulating microglial cholesterol levels. J. Biol. Chem. 2012;287:2032–2044. doi: 10.1074/jbc.M111.295451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Kanekiyo T, Shinohara M, Zhang Y, LaDu MJ, Xu H, Bu G. Differential regulation of amyloid-beta endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms. J. Biol. Chem. 2012;287:44593–44601. doi: 10.1074/jbc.M112.420224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lane-Donovan C, Herz J. ApoE, ApoE receptors, and the synapse in Alzheimer’s disease. Trends Endocrinol. Metab. 2017;28:273–284. doi: 10.1016/j.tem.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83:771–787. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- 123.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scheper W, Hoozemans JJ, Hoogenraad CC, Rozemuller AJ, Eikelenboom P, Baas F. Rab6 is increased in Alzheimer’s disease brain and correlates with endoplasmic reticulum stress. Neuropathol. Appl. Neurobiol. 2007;33:523–532. doi: 10.1111/j.1365-2990.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- 125.Soejima N, Ohyagi Y, Nakamura N, Himeno E, Iinuma KM, Sakae N, Yamasaki R, Tabira T, Murakami K, Irie K, et al. Intracellular accumulation of toxic turn amyloid-beta is associated with endoplasmic reticulum stress in Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:11–20. [PubMed] [Google Scholar]
- 126.Esbjorner EK, Chan F, Rees E, Erdelyi M, Luheshi LM, Bertoncini CW, Kaminski OF, Dobson CM, Kaminski Schierle GS. Direct observations of amyloid beta self-assembly in live cells provide insights into differences in the kinetics of Abeta(1-40) and Abeta(1-42) aggregation. Chem. Biol. 2014;21:732–742. doi: 10.1016/j.chembiol.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedrich RP, Tepper K, Ronicke R, Soom M, Westermann M, Reymann K, Kaether C, Fandrich M. Mechanism of amyloid plaque formation suggests an intracellular basis of Abeta pathogenicity. Proc. Natl. Acad. Sci. USA. 2010;107:1942–1947. doi: 10.1073/pnas.0904532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc. Natl. Acad. Sci. USA. 2009;106:20324–20329. doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sannerud R, Esselens C, Ejsmont P, Mattera R, Rochin L, Thar-keshwar AK, De Baets G, De Wever V, Habets R, Baert V, et al. Restricted location of PSEN2/gamma-secretase determines substrate specificity and generates an intracellular Abeta pool. Cell. 2016;166:193–208. doi: 10.1016/j.cell.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 131.Shi MM, Shi OH, Xu YM. Rab GTPases: the key players in the molecular pathway of Parkinson’s disease. Front. Cell. Neurosci. 2017;11:81. doi: 10.3389/fncel.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang BL. Rabs, membrane dynamics, and Parkinson’s disease. J. Cell. Physiol. 2017;232:1626–1633. doi: 10.1002/jcp.25713. [DOI] [PubMed] [Google Scholar]
- 133.Burre J, Sharma M, Sudhof TC. Definition of a molecular pathway mediating alpha-synuclein neurotoxicity. J. Neurosci. 2015;35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hasegawa T, Konno M, Baba T, Sugeno N, Kikuchi A, Kobayashi M, Miura E, Tanaka N, Tamai K, Furukawa K, et al. The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of alpha-synuclein. PLoS One. 2011;6:e29460. doi: 10.1371/journal.pone.0029460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chutna O, Goncalves S, Villar-Pique A, Guerreiro P, Marijanovic Z, Mendes T, Ramalho J, Emmanouilidou E, Ventura S, Klucken J, et al. The small GTPase Rab11 co-localizes with alpha-synuclein in intracellular inclusions and modulates its aggregation, secretion and toxicity. Hum. Mol. Genet. 2014;23:6732–6745. doi: 10.1093/hmg/ddu391. [DOI] [PubMed] [Google Scholar]
- 138.Chen RH, Wislet-Gendebien S, Samuel F, Visanji NP, Zhang G, Marsilio D, Langman T, Fraser PE, Tandon A. alpha-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity. J. Biol. Chem. 2013;288:7438–7449. doi: 10.1074/jbc.M112.439497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Breda C, Nugent ML, Estranero JG, Kyriacou CP, Outeiro TF, Steinert JR, Giorgini F. Rab11 modulates alpha-synuclein-mediated defects in synaptic transmission and behaviour. Hum. Mol. Genet. 2015;24:1077–1091. doi: 10.1093/hmg/ddu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu CC, Yeh TH, Lai SC, Weng YH, Huang YC, Cheng YC, Chen RS, Huang YZ, Hung J, Chen CC, et al. Increased Rab35 expression is a potential biomarker and implicated in the pathogenesis of Parkinson’s disease. Oncotarget. 2016;7:54215–54227. doi: 10.18632/oncotarget.11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goncalves SA, Macedo D, Raquel H, Simoes PD, Giorgini F, Ramalho JS, Barral DC, Ferreira Moita L, Outeiro TF. shRNA-based screen identifies endocytic recycling pathway components that act as genetic modifiers of alpha-synuclein aggregation, secretion and toxicity. PLoS Genet. 2016;12:e1005995. doi: 10.1371/journal.pgen.1005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yin G, Lopes da Fonseca T, Eisbach SE, Anduaga AM, Breda C, Orcellet ML, Szego EM, Guerreiro P, Lazaro DF, Braus GH, et al. alpha-Synuclein interacts with the switch region of Rab8a in a Ser129 phosphorylation-dependent manner. Neurobiol. Dis. 2014;70:149–161. doi: 10.1016/j.nbd.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 143.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim CH, Han BS, Tong Y, Shen J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 145.Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernandez-Diaz S, Swerts J, Schoovaerts N, Vilain S, Gounko NV, Vints K, et al. A LRRK2-dependent EndophilinA phosphoswitch is critical for macroautophagy at presynaptic terminals. Neuron. 2016;92:829–844. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 146.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum. Mol. Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gan-Or Z, Bar-Shira A, Dahary D, Mirelman A, Kedmi M, Gurevich T, Giladi N, Orr-Urtreger A. Association of sequence alterations in the putative promoter of RAB7L1 with a reduced parkinson disease risk. Arch. Neurol. 2012;69:105–110. doi: 10.1001/archneurol.2011.924. [DOI] [PubMed] [Google Scholar]
- 149.MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Waschbusch D, Michels H, Strassheim S, Ossendorf E, Kessler D, Gloeckner CJ, Barnekow A. LRRK2 transport is regulated by its novel interacting partner Rab32. PLoS One. 2014;9:e111632. doi: 10.1371/journal.pone.0111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O, Tonelli F, Martinez TN, Lorentzen E, Pfeffer SR, et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife. 2017;6:e31012. doi: 10.7554/eLife.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Labbadia J, Morimoto RI. Huntington’s disease: underlying molecular mechanisms and emerging concepts. Trends Biochem. Sci. 2013;38:378–385. doi: 10.1016/j.tibs.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brandstaetter H, Kruppa AJ, Buss F. Huntingtin is required for ER-to-Golgi transport and for secretory vesicle fusion at the plasma membrane. Dis. Model. Mech. 2014;7:1335–1340. doi: 10.1242/dmm.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hattula K, Peranen J. FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol. 2000;10:1603–1606. doi: 10.1016/s0960-9822(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 155.del Toro D, Alberch J, Lazaro-Dieguez F, Martin-Ibanez R, Xifro X, Egea G, Canals JM. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol. Biol. Cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pal A, Severin F, Lommer B, Shevchenko A, Zerial M. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington’s disease. J. Cell. Biol. 2006;772:605–618. doi: 10.1083/jcb.200509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li X, Sapp E, Chase K, Comer-Tierney LA, Masso N, Alexander J, Reeves P, Kegel KB, Valencia A, Esteves M, et al. Disruption of Rab11 activity in a knock-in mouse model of Huntington’s disease. Neurobiol. Dis. 2009;36:374–383. doi: 10.1016/j.nbd.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Her LS, Goldstein LS. Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J. Neurosci. 2008;28:13662–13672. doi: 10.1523/JNEUROSCI.4144-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 160.Liot G, Zala D, Pla P, Mottet G, Piel M, Saudou F. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. J. Neurosci. 2013;33:6298–6309. doi: 10.1523/JNEUROSCI.2033-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zarei S, Carr K, Reiley L, Diaz K, Guerra C, Altamirano PF, Pa-gani W, Lodin D, Orozco G, Chinea A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015;6:171. doi: 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Farg MA, Sundaramoorthy V, Sultana JM, Yang S, Atkinson RA, Levina V, Halloran MA, Gleeson PA, Blair IP, Soo KY, et al. C9ORF72, implicated in amytrophic lateral sclerosis and fronto-temporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014;23:3579–3595. doi: 10.1093/hmg/ddu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sellier C, Campanari ML, Julie Corbier C, Gaucherot A, Kolb-Cheynel I, Oulad-Abdelghani M, Ruffenach F, Page A, Ciura S, Kabashi E, et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016;35:1276–1297. doi: 10.15252/embj.201593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang BL. C9orf72’s interaction with Rab GTPases-modulation of membrane traffic and autophagy. Front. Cell. Neurosci. 2016;10:228. doi: 10.3389/fncel.2016.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Webster CP, Smith EF, Bauer CS, Moller A, Hautbergue GM, Ferraiuolo L, Myszczynska MA, Higginbottom A, Walsh MJ, Whit-worth AJ, et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 2016;35:1656–1676. doi: 10.15252/embj.201694401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deshpande M, Feiger Z, Shilton AK, Luo CC, Silverman E, Rodal AA. Role of BMP receptor traffic in synaptic growth defects in an ALS model. Mol. Biol. Cell. 2016;27:2898–2910. doi: 10.1091/mbc.E16-07-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 169.Pareyson D, Saveri P, Piscosquito G. Charcot-Marie-Tooth disease and related hereditary neuropathies: from gene function to associated phenotypes. Curr. Mol. Med. 2014;14:1009–1033. doi: 10.2174/1566524014666141010154205. [DOI] [PubMed] [Google Scholar]
- 170.Bucci C, De Luca M. Molecular basis of Charcot-Marie-Tooth type 2B disease. Biochem. Soc. Trans. 2012;40:1368–1372. doi: 10.1042/BST20120197. [DOI] [PubMed] [Google Scholar]
- 171.Wang X, Han C, Liu W, Wang P, Zhang X. A novel RAB7 mutation in a Chinese family with Charcot-Marie-Tooth type 2B disease. Gene. 2014;534:431–434. [PubMed] [Google Scholar]
- 172.Cogli L, Progida C, Lecci R, Bramato R, Kruttgen A, Bucci C. CMT2B–associated Rab7 mutants inhibit neurite outgrowth. Acta. Neuropathol. 2010;720:491–501. doi: 10.1007/s00401-010-0696-8. [DOI] [PubMed] [Google Scholar]
- 173.McCray BA, Skordalakes E, Taylor JP. Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum. Mol. Genet. 2010;19:1033–1047. doi: 10.1093/hmg/ddp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.BasuRay S, Mukherjee S, Romero E, Wilson MC, Wandinger-Ness A. Rab7 mutants associated with Charcot-Marie-Tooth disease exhibit enhanced NGF-stimulated signaling. PLoS One. 2010;5:e15351. doi: 10.1371/journal.pone.0015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.BasuRay S, Mukherjee S, Romero EG, Seaman MN, Wandinger-Ness A. Rab7 mutants associated with Charcot-Marie-Tooth disease cause delayed growth factor receptor transport and altered endosomal and nuclear signaling. J. Biol. Chem. 2013;288:1135–1149. doi: 10.1074/jbc.M112.417766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Colecchia D, Stasi M, Leonardi M, Manganelli F, Nolano M, Vene-ziani BM, Santoro L, Eskelinen EL, Chiariello M, Bucci C. Alterations of autophagy in the peripheral neuropathy Charcot-Marie-Tooth type 2B. Autophagy. 2017 doi: 10.1080/15548627.2017.1388475. https://doi.org/10.1080/15548627.2017.1388475. [DOI] [PMC free article] [PubMed]
- 177.Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J. Cell. Biol. 2010;191:367–381. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roberts RC, Peden AA, Buss F, Bright NA, Latouche M, Reilly MM, Kendrick-Jones J, Luzio JP. Mistargeting of SH3TC2 away from the recycling endosome causes Charcot-Marie-Tooth disease type 4C. Hum. Mol. Genet. 2010;19:1009–1018. doi: 10.1093/hmg/ddp565. [DOI] [PMC free article] [PubMed] [Google Scholar]