Abstract
A two-step chemical methodology for the synthesis of β-nicotinamide riboside (NR) is described. NR has achieved wide use as an NAD+ precursor (vitamin B3) and can significantly increase central metabolite NAD+ concentrations in mammalian cells. β-NR can be prepared with an efficient two-step procedure. The synthesis is initiated via coupling of commercially available 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose with ethyl nicotinate in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf). 1H NMR showed that the product was formed with complete stereoselectivity to produce only the β-isomer in high yield (> 90% versus starting sugar). The clean stereochemical result suggests that the coupling proceeds via a cationic cis-1, 2-acyloxonium-sugar intermediate, which controls addition by nucleophiles to generate predominantly β-stereochemistry. The subsequent deprotection of esters in methanolic ammonia generates the desired product in 85% overall yield versus sugar.
Keywords: nicotinamide riboside, two-step methodology, nucleoside synthesis, stereoselective, NAD+
INTRODUCTION
Nicotinamide riboside is a nucleoside with a long history of scientific investigation, and has recently been established to be an effective means to significantly augment NAD+ levels in mammalian cells and tissues (Yang, Chan et al. 2007, Canto, Houtkooper et al. 2012, Brown, Maqsood et al. 2014, Ryu, Zhang et al. 2016, Zhang, Ryu et al. 2016). NAD+ is a coenzyme for cellular oxidation and reduction reactions, and also participates as a cosubstrate for a number of NAD+ consuming enzymes such as sirtuins (Imai, Armstrong et al. 2000, Haigis and Sinclair 2010, Houtkooper, Pirinen et al. 2012), poly (ADP-ribose) polymerases (PARPs) (Schreiber, Dantzer et al. 2006) and cyclic ADP-ribose synthases (CD38 and CD157) (De Flora, Zocchi et al. 2004, Chini 2009). The nucleoside NR provides a key pharmacologic tool to manipulate NAD+ levels and to study the effects of NAD+ concentration changes on cell and tissue physiology (Felici, Lapucci et al. 2015, Shi, Hegeman et al. 2017). Importantly, NR is found in milk (Trammell, Yu et al. 2016, Ummarino, Mozzon et al. 2017), constituting a dietary source of NR for NAD+ production. However, the relatively small quantities of NR in food and relative difficulty in obtaining large amounts in purified form from natural sources limited evaluation of its biological effects. The development of a reliable and efficient synthetic method for producing larger amounts of NR enabled a growing number of cell studies and animal feeding experiments to assess the effects of this compound in biological systems (Canto, Houtkooper et al. 2012, Gong, Pan et al. 2013, Khan, Auranen et al. 2014). Nicotinamide riboside is a nucleoside, which incorporates nicotinamide and ribose into a single chemical moiety. We describe here a simple and efficient method to stereoselectively synthesize β-NR using a two-step procedure. First, trimethylsilyl trifluoromethanesulfonate (TMSOTf) mediates completely stereoselective N-glycosylation of 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose and ethyl nicotinate. The resulting tetraester adduct is simultaneously deprotected and converted to the desired cationic amide by treatment with NH3/MeOH at 0 °C to furnish β-NR in excellent isolated yield (85% overall from starting sugar).
STRATEGIC PLANNING
Most of the important considerations of conducting this synthetic reaction rest in the planning stages and are related to the need to handle two reagents which are difficult to handle in a safe manner in large quantities. These are the TMSOTf and the ammonia reagents. We have found that the TMSOTf is quite reactive, even in air, fumes, and creates strongly acidic solutions with water. Individuals planning to use this reagent should be sure to take care for covering or protecting hands, body, face and especially eyes, since the reagent has a marked tendency to react instantaneously with fuming and splattering in air and will immediately cause an injury hazard to exposed tissue. All reactions should be performed in a well-ventilated fume hood. Amounts of TMSOTf in excess of 5 grams should be handled with special care and are not expected to be handled by inexperienced or novice-type investigators.
In addition, the dispensing of ammonia in methanol should be performed in a well-ventilated fume hood, with proper covering of eyes, hands and body. The final step of removing methanol and ammonia under reduced pressure (with corrosion and solvent resistant pump) requires a dry-ice cooled trap to capture the methanol and ammonia. Methanolic ammonia should be gradually neutralized by addition of aqueous hydrogen chloride for disposal in neutralized form. Again, large quantities of methanolic ammonia, above 500 mL should be reserved for experienced investigators.
In addition to safety concerns, it is helpful to have C18-Reverse Phase HPLC available with 260 nm wavelength detection capability to assay reaction mixtures especially during and after the methanolic ammonia step. This is especially helpful for analytical quantitation of nicotinamide, NR and methyl nicotinate riboside, in order to define a stopping point for the deprotection.
BASIC PROTOCOL 1
PREPARATION OF β-NICOTINAMIDE RIBOSIDE
The synthesis of β-NR was initiated by preparation of ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate as an intermediate by coupling of 1,2,3,5-tetra-O-acetyl-β-D-ribofuranose with ethyl nicotinate (1.5 equivalents) in the presence of TMSOTf (1 equivalent). Treatment of this intermediate with 5.5 N ammonia in methanol at 0 °C for 15-18 hours provided simultaneous deprotection and conversion of the cationic nicotinate ester to the corresponding amide to furnish β-NR in excellent isolated yield (Figure 1). The ease of synthesis of β-NR from the intermediate at low temperatures highlights the reactivity of the pyridinium ester to substitution by amine to achieve ester to amide exchange under uncatalyzed conditions. In contrast, ethyl nicotinate itself was unreactive under these conditions. Nevertheless, reaction temperature control as well as reaction time are crucial for reducing side products. More specifically, it was determined that methyl nicotinate riboside is formed preferentially at -20 °C and the formation of NR is slow under these conditions. This indicates that methyl nicotinate riboside is an intermediate formed prior to NR, and that methoxide in the reaction mixture is inherently more reactive than ammonia with the ethyl pyridinium ester. On the other hand, extended incubations lead to formation of nicotinamide as a major product, due to subsequent decomposition of NR. Thus, HPLC is used to monitor the reaction process in order to achieve the optimal yield of NR as compared with the side products methyl nicotinate riboside and nicotinamide.
Figure 1.
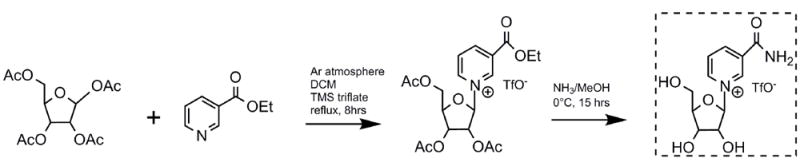
Synthesis of ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate and transformation to β-nicotinamide riboside triflate.
Materials
1,2,3,5-tetra-O-acetyl-β-d-ribofuranose, 98% (Sigma-Aldrich)
Ethyl nicotinate, 99% (Sigma-Aldrich)
Trimethylsilyl trifluoromethanesulfonate (TMSOTf), ≥98.0% (Sigma-Aldrich), used fresh when received or stored in a desiccator containing Drierite.
Anhydrous dichloromethane (DCM), ≥99.8%, contains 40-150 ppm amylene as stabilizer (Sigma-Aldrich)
Anhydrous ethyl acetate (EtOAc), 99.8% (Sigma-Aldrich)
Anhydrous methanol (MeOH), 99.8% (Sigma-Aldrich)
Anhydrous triethylamine, ≥99% (Sigma-Aldrich)
Anhydrous hexane, 95% (Sigma-Aldrich)
Sulfuric acid (H2SO4), 99.999% (Sigma-Aldrich)
Ammonia solution, 7 N in methanol (Sigma-Aldrich)
Distilled water (Millipore Water Systems)
Argon (Tech Air)
Octadecyl-functionalized silica gel, 16-18% carbon loading, 200-400 mesh (Sigma-Aldrich)
100 mL Single-neck round-bottom flasks (VWR Scientific)
100 mL Three-neck round-bottom flasks (VWR Scientific)
Glass condenser, 110 mm (VWR Scientific)
Magnetic stirrer with hot plate and magnetic stir bars (VWR Scientific)
Oil bath (VWR Scientific)
Thermometer, -10 to 110°C (VWR Scientific)
High vacuum oil pump (VWR Scientific)
TLC plates (VWR Scientific)
Separations funnel (VWR Scientific)
Glass chromatography column, 24/40 outer joint at top and a 2 mm bore glass stopcock at the bottom (VWR Scientific)
Glass rods (VWR Scientific)
Syringe, 1000 μL, chromatography, removable needle, Ace Glass (VWR Scientific)
Deuterium oxide (D2O), 99.9 atom % D (Sigma-Aldrich)
1H, 19F and 13C NMR spectra recorded on Bruker 300, 400 or 500 MHz NMR spectrometers. 1H and 13C chemical shifts are expressed in ppm with respect to the chemical shift of tetramethylsilane.
Hitachi EZChrom Elite HPLC system with a L2450 diode array as a detector
0.1% TFA as mobile phase on HPLC
NUCLEOSIL 100-5 C18 (5 μM, 250 × 4.6 mm) column (Macherey-Nagel)
HRMS spectra obtained by Dr. Cliff Soll of Hunter College Mass Spectrometry Facility
Prepare ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate
Thoroughly clean all glassware and flasks used for reactions (100 mL single-neck round-bottom flask and glass condenser). In order to remove the moisture and air absorbed on the glass, flame dry the glassware or heat with a heat gun. Upon completion of heating, allow the glassware to cool to room temperature.
Weigh 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose (1.4 g, 4.4 mmol) and ethyl nicotinate (0.9 mL, 6.6 mmol) into the flame dried 100 mL single-neck round-bottom flask. Add a clean and dry stirring bar to the flask and connect the flask with the glass condenser. Seal all the ground glass joints with Teflon tape.
Apply the high vacuum oil pump for 15 minutes by connecting the top of the condenser to remove the moisture and air in the reaction system. Apply argon atmosphere to the reaction system by connecting the top of the condenser immediately after the vacuum process.
Under an argon atmosphere, add 50 mL dry dichloromethane into the flask through the top of the condenser.
Carefully and slowly add TMSOTf (1.039 g, 846 μL, 4.4 mmol) using a syringe to a stirred mixture of ethyl nicotinate and 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose in 50 mL dry dichloromethane under argon atmosphere. Place the flask connected with the glass condenser on the oil bath at 45 °C. Heat the mixture at reflux for 8 hrs.
Stain the TLC (EtOAc/MeOH/triethylamine, 5/0.3/0.05) with 10% H2SO4/MeOH. The TLC should show complete disappearance of starting ribofuranose and appearance of a product in a single UV active spot at Rf = 0.2 in EtOAc/MeOH/trimethylamine 5:0.3:0.05.
-
After evaporation of volatiles via vacuum distillation, extract the crude product with MeOH/hexane (1:1) (5 × 40 mL) which forms a two-phase system. Discard the hexane extracts (5 × 20 mL) and evaporate the bottom MeOH layer to dryness.
NOTE: The material after this step can be used to start step 11 to complete a one-pot procedure. However, to obtain a purified and fully characterized intermediate, perform step 8.
Transfer the methanol derived residue with 30 mL of water washing to a 100 mL flask immersed in an ice bath, and add 1 mL of 0.1 M sodium hydroxide solution slowly with stirring. Finally, adjust the pH to 7.0 and extract the aqueous solution with 30 mL ethyl acetate/hexane (1:1). Evaporate the water layer containing purified product to dryness under high vacuum pump. The syrup-like product ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate is obtained. Yield: 100 % versus sugar.
-
Characterize the product by 1H NMR and 13C NMR.
1H NMR (400 MHz, D2O): δ = 9.47 (s, 1H, H-2), 9.27 (d, 1H, J = 6.3 Hz, H-6), 8.97 (d, 1H, J = 8.1 Hz, H-4), 8.28 (dd, 1H, J = 7.2 Hz, H-5), 6.50 (d, 1H, H-1’), 5.40 (t, 1H, J = 4.2 Hz, H-2’), 5.25 (t, 1H, J = 5.6 Hz, H-3’), 4.63 (m, 1H, H-4’), 4.41 (m, 4H, H-5’ and H-7), 2.05 (s, 3H, -O-CO-CH3), 2.04 (s, 3H, -O-CO-CH3), 2.01(s, 3H, -O-CO-CH3), 1.32 (t, J = 7.2 Hz, 3H, H-8).
13C NMR (300 MHz, D2O): δ = 170.84, 170.58, 170.07, 161.79, 147.96, 145.74, 143.31, 131.67, 129.59, 98.19, 83.24, 76.42, 69.53, 63.83, 62.45, 20.93, 20.62, 20.60, and 14.28.
-
Characterize the product by HRMS.
HRMS: m/z (%): 410.06 (14, [M] +), 259.0 (100), 138.9 (22).
Prepare β-Nicotinamide riboside triflate
-
11
Thoroughly clean and flame dry the 100 mL three-neck round-bottom flask used for reaction. Upon completion of the drying, allow the flask to cool to room temperature.
-
12
Dissolve the syrup-like intermediate ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate (210 mg) in 8.4 mL of ice cold 5.5 N NH3/MeOH under argon atmosphere and maintain at 0 °C for 15-18 hours.
-
13
After 15 hours of the reaction, monitor the reaction hourly. Take 50 μL of reaction and place into a chilled test tube and rapidly dry by air stream, and then add 200 μL of 1% acetic acid for assay. Inject 10 μL onto HPLC fitted with a RP C18 column and 260 nm detector to assay products (β-nicotinamide riboside versus the side products methyl nicotinate riboside and nicotinamide, Figure 2). Perform chromatography using an eluent of 0.1% TFA at 1.0 mL/min on a Machery-Nagel NUCLEOSIL 100-5 C18 (5 μM, 250 × 4.6 mm) column. Immediately stop the reaction once the peak of β-nicotinamide riboside starts to decline.
-
14
Remove the methanol and ammonia under reduced pressure (with corrosion and solvent resistant pump) employing a dry-ice cooled trap to capture the methanol and ammonia. Gradually neutralize the methanolic ammonia by addition of aqueous hydrogen chloride for disposal in neutralized form. Use pH paper to make sure the completion of ammonia evaporation. Evaporate again after addition of methanol (10 mL) to fully remove the ammonia.
-
15
Activate a 2.5 cm diameter glass column loaded with 20 gram octadecyl-functionalized silica gel with 100%, 75%, 50% and 25% MeOH/H2O elutions (20 mL each) and then wash with H2O (2 × 20 mL), respectively. Keep 5 mm residual solvent on the top of the bed prior to loading. Dissolve up to 250 mg of the crude residue from the previous step in water (1 mL) and load evenly to the top of the resin. This procedure can scale to up to 100 fold.
-
16
Elution proceeds with water and products elute, generally in order NR, nicotinamide and methyl nicotinate riboside. Fractions can be assayed by HPLC as described to identify those containing purified β-NR (See Figure 3 for a representative separation and HPLC analysis of fractions).
-
17
Evaporate the water fractions containing NR to dryness under high vacuum. Optimal overall yield of β-nicotinamide riboside triflate achieved 85%.
-
18
Characterize the product by 1H NMR, 13C NMR and 19F NMR.
1H NMR (D2O): δ= 9.60 (s, 1H, H-2), 9.24 (d, 1H, J = 6.0 Hz, H-6), 8.95 (d, 1H, J = 7.9 Hz, H-4), 8.23 (t, 1H, J = 7.1 Hz, H-5), 6.20 (d, 1H, J = 4.9 Hz, H-1’), 4.45 (m, 2H, H-2’ and H’-4’), 4.32 (t, 1H, J= 3.8 Hz, H-3’), 4.02 (dd, 1H, J = 2.7 and 12.3 Hz, H-5’a), 3.87 (dd, 1H, J = 2.4 and 11.1 Hz, H-5’b).
13C NMR (D2O): δ = 146.0, 143.0, 141.3, 128.7, 100.2, 88.1, 77.7, 70.2, 60.6.
19F NMR (D2O): δ = 78.81
-
19
Characterize the product by HRMS.
HRMS (ESI) m/z [M] + calcd for C11H15 N2O5+, 255.09755, found 255.09801.
Figure 2.
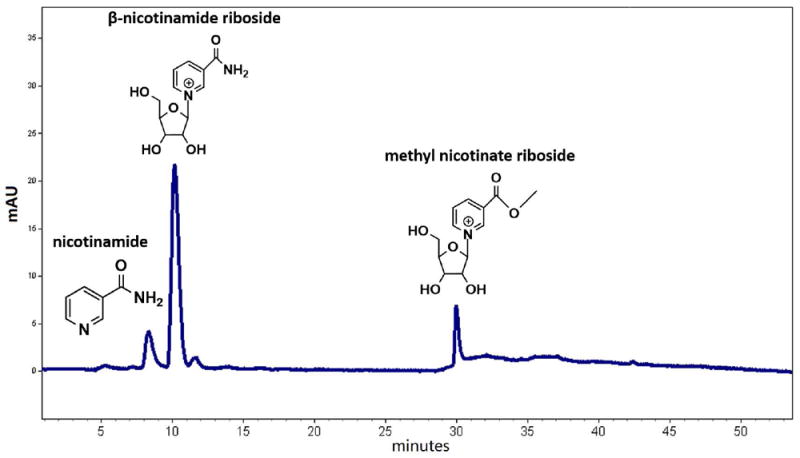
HPLC chromatogram (260 nm) of crude reaction mixture in methanol-ammonia step showing major products nicotinamide, nicotinamide riboside and methyl nicotinate riboside. Reaction is optimally terminated when NR production is maximized and the two co-products minimized, typically at 15-18 hours at 0 °C.
Figure 3.
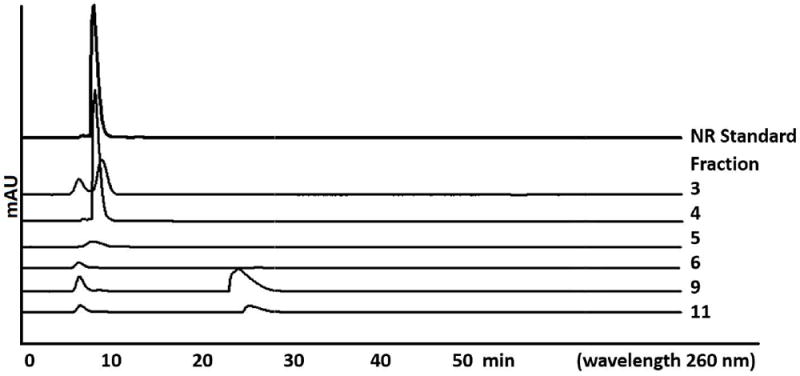
Representative HPLC chromatograms for eluted fractions of C18 purification of crude NR. Chromatograms are run on Machery-Nagel C-18 column, as described in text. NR standard is shown at top. Different eluting fractions first elute NR and then subsequent fractions elute NAM and methyl nicotinate riboside in the elution order as written.
COMMENTARY
Background Information
This work describes a relatively simple and efficient synthesis of nicotinamide riboside. Nicotinamide riboside has found increased attention as a pharmacophore with the ability to increase NAD+ levels in cells and tissues of mammals. It has been long appreciated that NAD+ is a cofactor in numerous enzyme-catalyzed redox reactions in living organisms and plays a fundamental role in cellular metabolic processes (Sauve 2008, Verdin 2015, Yang and Sauve 2016). NAD+ also participates as a cosubstrate in protein modifications catalyzed by NAD+-consuming proteins such as PARPs and sirtuins (Canto, Sauve et al. 2013, Schiedel, Robaa et al. 2017). Recent findings that cellular NAD+ concentration could represent a crucial parameter for the health of cells, tissues and organisms have stimulated evaluation of NAD+ precursors as a means to modulate mammalian health status (Klaidman, Morales et al. 2003, Canto, Houtkooper et al. 2012, Kulikova, Shabalin et al. 2015, Guan, Wang et al. 2017, Weidele, Beneke et al. 2017).
NR is considered a Vitamin B3 (Chi and Sauve 2013, Conze, Crespo-Barreto et al. 2016). Cells convert NR to NAD+ via pathways identified in mammals, yeast as well as bacteria (Belenky, Racette et al. 2007). NR has been found to be present naturally in yeast, bacteria, and mammals (Lu, Kato et al. 2009, Conze, Crespo-Barreto et al. 2016). The foods most enriched in NR are not well identified, although cow milk typically contained 12 μM NAD+ precursor vitamin/L, of which 40% is present as NR (Trammell, Yu et al. 2016). Assimilation of NR in mammalian cells is initiated by transport followed by phosphorylation to form NR 5’-phosphate (NMN) by NR kinases (Nrks) (Tempel, Rabeh et al. 2007). Alternatively, phosphorolysis of NR which forms nicotinamide (Rowen and Kornberg 1951) enables a fragment of NR to be converted to NAD+ via the mammalian nicotinamide salvage pathway. The mechanisms by which NR is produced in the biological setting are still not clear.
Prior methods described for stereoselective synthesis of β-NR, included modestly effective chemical syntheses (Tanimori, Ohta et al. 2002, Franchetti, Pasqualini et al. 2004) and an enzymatic synthesis (Rowen and Kornberg 1951). The enzymatic synthesis is unproven for multi-gram synthesis. The previously reported chemical synthesis of Tanimori and co-workers contained up to 13% of the α-anomer as determined by 1H NMR spectroscopy (Tanimori, Ohta et al. 2002). The α-anomer was removed by chromatography on activated charcoal and crystallization to give β-NR in 58% isolated yield. The Franchetti synthesis was not readily executed in our hands, and neither found wide adoption by other laboratories (Franchetti, Pasqualini et al. 2004).
The synthetic method we describe here is initiated by coupling of 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose with ethyl nicotinate instead of nicotinamide in the presence of TMSOTf in dichloromethane followed by the addition of NH3/MeOH. 1H NMR showed that the product is formed stereoselectively to produce only the β-isomer in a high yield (>90% versus starting sugar). The clean stereochemical result suggests that the coupling proceeds via a cationic cis-1, 2-acyloxonium-sugar intermediate, which controls addition by nucleophiles to generate predominantly β-stereochemistry. We speculate that the less polar solvent is able to form a less dissociated transition state, and leads to improved stereocontrol. The subsequent ester to amide conversion is novel, as ester to amide exchange reactions generally require a catalyst. We postulate that the cationic nature of the pyridinium activates the ester to ammonia addition at extremely mild temperature (0°C), thereby explaining the efficient amide substitution at the carbonyl group. This methodology constitutes a simple and efficient two-step methodology for stereoselective chemical synthesis of β-NR.
Critical Parameters and Troubleshooting
Step 1: Preparation of ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate
TMSOTf is water sensitive, so the coupling of ethyl nicotinate and 1,2,3,5-tetra-O-acetyl-β-D-ribofuranose must be performed under an inert atmosphere. The apparatus in which the reaction is to be conducted must be scrupulously dry, so all glassware used for reactions should be thoroughly cleaned and dried. The solvent dichloromethane must be anhydrous. The initial coupling reaction demands an argon atmosphere. TMSOTf must be added slowly via syringe to avoid the formation of side products in an unheated reaction mixture containing sugar, dichloromethane and ethyl nicotinate. Only after Lewis acid is added should the reaction be heated to reflux.
To purify the product ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate, the crude post-coupling product is dissolved in water which is neutralized to pH 7.0 with 0.1 M NaOH solution prior to a subsequent extraction with ethyl acetate/hexane (1:1). The neutralization step is necessary to enable deprotonation of excess uncoupled ethyl nicotinate which is removed by the organic extraction. However, the addition of 0.1 M NaOH solution has to be very careful with stirring and the flask containing the crude product has to be chilled on ice, otherwise ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate can be degraded. Over addition of base is counter-indicated since it will hydrolyze the desired product.
The purified ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate that is obtained as colorless syrup after evaporation should not be stored at room temperature as it is subject to decomposition. It has to be stored in the freezer at -20 °C.
Step 2: Preparation of β-nicotinate riboside
During ammonia deprotection step, it is quite important to keep reaction at no higher than 0 °C. Removal of ammonia-methanol must also be performed cold. Allowing warming of the reaction can seriously degrade product outcome. HPLC is a very convenient and almost essential means to monitor the deprotection to ensure it is well-executed.
The column for NR purification loaded with octadecyl-functionalized silica gel has to be properly activated. We recommend elution with 100%, 75%, 50% and 25% MeOH/H2O (20 mL each) and then washing with H2O (20 mL), respectively for a 10 gram C18 silica column. In this way a more efficient separation can be obtained among NR, nicotinamide and methyl nicotinate riboside.
Purified β-nicotinamide riboside should not be stored at room temperature as it is subject to decomposition. It has to be stored in the freezer at -20 °C.
Time Considerations
Synthesis of ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate starting from commercially available 1,2,3,5-tetra-O-acetyl-β-d-ribofuranose and ethyl nicotinate in the presence of TMSOTf can be accomplished within 10 hours. An additional 4 hours is required to remove water phase under high vacuum oil pump to obtain purified ethyl nicotinate 2’,3’,5’-tri-O-acetylriboside triflate. The synthesis of β-nicotinamide riboside from 2’, 3’, 5’-tri-O-acetyl ethyl nicotinate riboside in the presence of 5.5 N NH3/MeOH at 0 °C in cool room requires 15-18 hours. It takes approximate 4 hours to remove methanol and ammonia. An additional 8 hours is required to purify the product with octadecyl-functionalized silica gel and remove water phase under high vacuum to obtain purified β-nicotinamide riboside.
Acknowledgments
This work was partially supported by NIH grant GM R01 106072. Cornell University and Anthony Sauve receive royalties on commercial sales of nicotinamide riboside by Chromadex Inc. Anthony Sauve has intellectual property related to uses and derivatives of nicotinamide riboside. AS is a co-founder and equity holder in Metro MidAtlantic Biotech LLC.
LITERATURE CITED
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129(3):473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20(6):1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Sauve AA, Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med. 2013;34(6):1168–1201. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care. 2013;16(6):657–661. doi: 10.1097/MCO.0b013e32836510c0. [DOI] [PubMed] [Google Scholar]
- Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des. 2009;15(1):57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze DB, Crespo-Barreto J, Kruger CL. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol. 2016;2:1–12. doi: 10.1177/0960327115626254. [DOI] [PubMed] [Google Scholar]
- De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- Felici R, Lapucci A, Cavone L, Pratesi S, Berlinguer-Palmini R, Chiarugi A. Pharmacological NAD-Boosting Strategies Improve Mitochondrial Homeostasis in Human Complex I-Mutant Fibroblasts. Mol Pharmacol. 2015;87(6):965–971. doi: 10.1124/mol.114.097204. [DOI] [PubMed] [Google Scholar]
- Franchetti P, Pasqualini M, Petrelli R, Ricciutelli M, Vita P, Cappellacci L. Stereoselective synthesis of nicotinamide beta-riboside and nucleoside analogs. Bioorg Med Chem Lett. 2004;14(18):4655–4658. doi: 10.1016/j.bmcl.2004.06.093. [DOI] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34(6):1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 2017;28(8):2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6(6):721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69(3):150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- Kulikova V, Shabalin K, Nerinovski K, Dolle C, Niere M, Yakimov A, Redpath P, Khodorkovskiy M, Migaud ME, Ziegler M, Nikiforov A. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J Biol Chem. 2015;290(45):27124–27137. doi: 10.1074/jbc.M115.664458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SP, Kato M, Lin SJ. Assimilation of endogenous nicotinamide riboside is essential for calorie restriction-mediated life span extension in Saccharomyces cerevisiae. J Biol Chem. 2009;284(25):17110–17119. doi: 10.1074/jbc.M109.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen JW, Kornberg A. The phosphorolysis of nicotinamide riboside. J Biol Chem. 1951;193(2):497–507. [PubMed] [Google Scholar]
- Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, Bellemin S, Iyer SR, Wang X, Gariani K, Sauve AA, Canto C, Conley KE, Walter L, Lovering RM, Chin ER, Jasmin BJ, Marcinek DJ, Menzies KJ, Auwerx J. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8(361):361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324(3):883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Schiedel M, Robaa D, Rumpf T, Sippl W, Jung M. The Current State of NAD+ -Dependent Histone Deacetylases (Sirtuins) as Novel Therapeutic Targets. Med Res Rev. 2017;0:1–54. doi: 10.1002/med.21436. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Shi W, Hegeman MA, van Dartel DA, Tang J, Suarez M, Swarts H, van der Hee B, Arola L, Keijer J. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol Nutr Food Res. 2017;61(4):1–11. doi: 10.1002/mnfr.201600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimori S, Ohta T, Kirihata M. An efficient chemical synthesis of nicotinamide riboside (NAR) and analogues. Bioorg Med Chem Lett. 2002;12(8):1135–1137. doi: 10.1016/s0960-894x(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Tempel W, Rabeh WM, Bogan KL, Belenky P, Wojcik M, Seidle HF, Nedyalkova L, Yang T, Sauve AA, Park HW, Brenner C. Nicotinamide riboside kinase structures reveal new pathways to NAD+ PLoS Biol. 2007;5(10):e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk. J Nutr. 2016;146(5):957–963. doi: 10.3945/jn.116.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummarino S, Mozzon M, Zamporlini F, Amici A, Mazzola F, Orsomando G, Ruggieri S, Raffaelli N. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017;221:161–168. doi: 10.1016/j.foodchem.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- Weidele K, Beneke S, Burkle A. The NAD+ precursor nicotinic acid improves genomic integrity in human peripheral blood mononuclear cells after X-irradiation. DNA Repair (Amst) 2017;52:12–23. doi: 10.1016/j.dnarep.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Yang T, Chan NY, Sauve AA. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J Med Chem. 2007;50(26):6458–6461. doi: 10.1021/jm701001c. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sauve AA. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864(12):1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]


