Abstract
Only a few randomized clinical trials have been performed so far in heart transplant recipients, mainly because of the relatively small number of heart transplants performed worldwide each year. The main focus of the few controlled trials that have been completed has been the prevention and treatment of heart allograft rejection. In the area of pharmacologic immunosuppression, both biological agents and drugs have been the subject of investigation. Among the biological agents, chimeric monoclonal antibodies directed against the interleukin (IL)-2 receptor, which have been found to be safe and effective in renal transplant recipients, are now undergoing the test of controlled trials in heart transplant recipients. Immunosuppressive drugs that have been studied in controlled trials include calcineurin inhibitors (such as the microemulsion formulation of cyclosporine and tacrolimus) and inhibitors of purine synthesis, such as mycophenolate mofetil. Non-pharmacologic prophylactic immunosuppression with photopheresis has also been tested in a prospective, multicenter, randomized trial. New immunosuppressive regimens, such as mycophenolate mofetil combined with a monoclonal antibody against the IL-2 receptor, are being tested with the aim to reduce or eliminate calcineurin inhibitors or corticosteroids. Although clinical approaches to the induction of tolerance have undergone preliminary clinical evaluation, the ability to induce tolerance to an allograft in humans remains an elusive goal.
Keywords: heart transplantation, immunosuppression, monoclonal antibodies, rejection, tolerance
Introduction
Data from the International Society of Heart and Lung Transplantation (ISHLT) show that since 1995 the number of heart transplants (HTs) performed worldwide has gradually declined and was approximately 3000 in the year 2000 [1]. This small number of procedures is the main reason why only few randomized single-center, and even fewer multicenter, clinical trials have been performed so far in HT recipients. Other factors have hampered the performance of controlled studies in HT recipients. These include the following: the lack of a uniform system for measuring rejection severity until the publication of the ISHLT rejection grading system in 1991; the approximately 80% 1-year survival, which creates the requirement for patient populations larger than the total number of HTs performed worldwide to detect differences between the effects on outcome of two therapeutic interventions; the lack of reliable 'surrogate' endpoints, such as the incidence and severity of cardiac allograft vasculopathy, owing to the insensitivity of contrast coronary angiography for the detection of cardiac allograft vasculopathy; the lack of uniformity in maintenance immunosuppression and in the threshold to treat rejection; and the priority traditionally given to kidney transplant recipients for the randomized evaluation of new immunosuppressive therapies, which are then applied to HT recipients without the test of controlled trials.
Despite these serious limitations, some important controlled evaluations of new immunosuppressive therapies have been conducted in HT recipients during the past decade. The main focus of these trials has been the prevention and treatment of HT rejection (Fig. 1).
Figure 1.
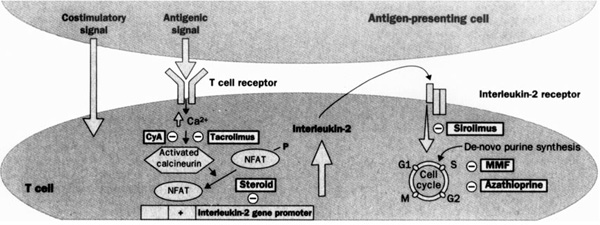
Stages of T cell activation: multiple targets for immunosuppressive agents. Signal 1: stimulation of T cell receptor (TCR) results in calcineurin activation, a process inhibited by cyclosporin (CyA) and tacrolimus. Calcineurin dephosphorylates nuclear factor of activated T cells (NFAT), enabling it to enter the nucleus and bind to interleukin-2 gene promoter. Corticosteroids inhibit cytokine gene transcription in lymphocytes and antigen-presenting cells by several mechanisms. Signal 2: co-stimulatory signals are necessary to optimize T cell interleukin-2 gene transcription, prevent T cell anergy, and inhibit T cell apoptosis. Experimental agents, but not current immunosuppressive agents, interrupt these intracellular signals. Signal 3: stimulation of interleukin-2 receptor induces the cell to enter the cell cycle and proliferate. Signal 3 can be blocked by interleukin-2 receptor antibodies or by sirolimus, which inhibits second messenger signals induced by the ligation of interleukin-2 receptor. After progression into the cell cycle, azathioprine and mycophenolate mofetil (MMF) interrupt DNA replication by inhibiting purine synthesis. Reproduced with permission from [29]..
Monoclonal antibodies
Antilymphocyte antibody preparations have been used as adjuncts to standard triple-drug immunosuppression [consisting of cyclosporine (CSA), corticosteroids, and azathioprine (AZA)] to prevent rejection in the early postoperative period after HT [2]. However, polyclonal antibody preparations, such as rabbit anti-thymocyte and horse anti-lymphocyte globulins, lack specificity and consistency of immunosuppressive action between batches, and require a central intravenous (IV) line for administration. These limitations have been overcome with the development of mouse monoclonal antibodies directed against specific lymphocyte targets (Table 1). The first monoclonal antibody to be used in HT recipients was OKT3, a murine preparation directed against the CD3 molecule on activated T cells [3].
Table 1.
Induction immunosuppressive drugs
| Agent | Molecular target | Molecular effect | Specific side effects | Comments |
| ATG/ALG | Binds multiple antigens on lymphoid cells | Complement-mediated lysis | Serum sickness | Batch variability |
| Opsonization and clearance | Thrombocytopenia | |||
| Modification of cell surface receptor | Granulocytopenia | |||
| OKT3 | Binds T cell CD3 | Complement mediated lysis | Cytokine release syndrome | Tachyphylaxis due to anti-idiotypic antibodies |
| Opsonization and clearance | (eg fever, chills, headache, and pulmonary edema) | |||
| Modification of CD3 receptor | ||||
| Daclizumab | Binds α-subunit of interleukin-2 receptor | Down-regulation of receptor | No major side effects reported so far | Humanized antibody |
| ? CD4 T cell depletion | Long half-life (20 days) | |||
| Five-dose regimen | ||||
| Basiliximab | Binds α-subunit of interleukin-2 receptor | Down-regulation of receptor | No major side effects reported so far | Chimeric antibody |
| ? CD4 T cell depletion | Long half-life (10-14 days) | |||
| Two-dose regimen |
ALG, antilymphocyte globulin; ATG, antithymocyte globulin.
The CD3 molecule fulfills a critical role in the recognition of alloantigens by the T cell receptor. In HT recipients OKT3 has been associated with serious adverse effects, which include the following: (1) the development of a cytokine release syndrome, owing to the ability of OKT3 to activate T cells, ranging in severity from a mild febrile illness to a syndrome of severe volume overload and hemodynamic compromise [4]; (2) the appearance of human anti-mouse antibodies that decrease the efficacy or preclude the use of subsequent OKT3 courses and can mediate humoral rejection [5]; (3) the increased incidence of opportunistic infections, such as those caused by cytomegalovirus (CMV) [6]; and (4) the increased incidence of post-transplant lymphoproliferative disorders [7].
These limitations have spurred the development of monoclonal antibodies that have a greater human component and achieve a more selective suppression of the immune system. These monoclonal antibodies can be either 'chimeric', when the entire variable portion remains of murine origin, and 'humanized', when the only remaining murine component is the complementarity-determining region, the portion of the variable region that binds to the antigen. Among these, a chimeric antibody has been developed against the CD4 molecule, which is present on helper T cells and has a central role in alloantigen recognition and the early phases of allograft rejection. The chimeric anti-CD4 antibody consists of the variable region of the murine CD4 antibody cM-T412 and the constant region of a human IgG1κ immunoglobulin [8].
Before its use in HT recipients, this antibody had been shown to be safe in patients with autoimmune diseases. In one study the outcome of 11 HT recipients receiving the chimeric CD4 monoclonal antibody cM-T412 during the operation and on postoperative days 1-7, 9, 11, 13, 17, and 21 was compared with that of 11 patients receiving antithymocyte globulin (ATG) until the achievement of therapeutic CSA levels. Maintenance immunosuppression was similar in the two groups. Over a mean observation time of 600 days, the number of acute rejection episodes per 100 patient days was 0.26 in the CD4 group, in comparison with 0.41 in the control group, indicating a 40% reduction in rejection rates. The mean time to the first rejection episode was 43.7 days in the CD4 monoclonal antibody-treated group and 25.3 days in the control group. Furthermore, in comparison with the control group, the group treated with CD4 monoclonal antibody had fewer infections (0.49 compared with 0.91 per 100 patient days) and better survival (91% compared with 73%) at one year after HT. It should be noted that none of these differences between the two groups achieved statistical significance because of the small patient population [8].
In contrast with resting T cells, antigen-activated T cells express high-affinity interleukin-2 (IL-2) receptors. The use of monoclonal antibodies directed against epitopes of these receptors has been shown to prolong allograft survival in animal models. In a prospective randomized trial, BT53, a murine IgG1 anti-IL-2 receptor antibody, was compared with OKT3 for the prevention of early post-HT rejection [9]. Over a median follow-up period of 34 months, rejection tended to occur earlier in the BT563-treated group than in the OKT3-treated group, but 3-month and 12-month rejection and infection rates were similar. Notably immunohistochemistry showed that, during acute rejection, in the presence of circulating BT563, cells bearing IL-2 receptors were present in only 20% of rejection biopsies, whereas these cells were detected in 75% of the rejection biopsies in patients not treated with BT563 [10]. These findings indicate that, despite adequate blockade of the IL-2/IL-2 receptor pathway, patients can still develop acute rejection. This might be due to the redundancy of the cytokine network, in which other cytokines, such as IL-5, can fulfill the role of the blocked IL-2. A cytokine release syndrome occurred in most OKT3-treated patients, but not in any of the BT563-treated patients.
More recently, two chimeric monoclonal antibodies against IL-2 receptors [daclizumab (Zenapax; Hoffmann-La Roche, Nutley, New Jersey, USA) and basiliximab (Simulect; Novartis Pharma AG, Basel, Switzerland, and East Hanover, New Jersey, USA)] have been tested in renal transplant recipients in randomized clinical trials. In comparison with triple immunosuppression, both monoclonal antibodies against IL-2 receptors significantly reduced 6-month rejection rates and improved survival. In a single-center study, 55 nonsensitized patients undergoing their first HT were randomly assigned to receive either prophylactic immunosuppression with daclizumab (1.0 mg/kg) given IV within 24 hours of HT and every 2 weeks thereafter, for a total of five doses, or conventional perioperative immunosuppression [11]. In both groups, maintenance immunosuppression consisted of CSA, mycophenolate mofetil (MMF) and prednisone. During the perioperative period, acute rejection rates were significantly lower in the daclizumab group than in patients treated conventionally (0.19 compared with 0.64; P = 0.02). Acute rejection occurred in 18% of the daclizumab group and in 63% of the control group (relative risk 2.8; 95% confidence interval 1.1-7.4; P = 0.04). Throughout the follow-up period, ISHLT grade 3 rejection occurred in two daclizumab-treated and in nine controls (P = 0.003) and the first rejection episode was significantly delayed in the daclizumab group (P = 0.004). There were no adverse reactions to daclizumab, and infection and malignancy rates were similar between groups.
A multicenter, prospective, randomized clinical trial of daclizumab against no antilymphocyte antibodies is continuing in HT recipients treated with CSA, MMF and prednisone.
Calcineurin inhibitors
The introduction of CSA has improved the survival of HT recipients owing to decreased mortality from infection and rejection (Table 2). One of the major limitations of the original oil-based CSA formulation [Sandimmune (SM); Novartis Pharma] is its variable and unpredictable bioavailability [12]. In contrast, the new microemulsion formulation [Neoral (NL); Novartis Pharma] might have more consistent bioavailability, which has been associated with lower rejection rates in kidney and liver recipients.
Table 2.
Maintenance immunosuppressive drugs
| Agent | Pharmacology | Molecular target | Molecular effect | Side effects |
| Corticosteroids | Increased bioavailability with hypoalbuminaemia and liver disease. | Cytosolic receptors. Heat shock proteins. | Blocks transcription of cytokine genes (eg IL-1, IL-2, IL-3, TNF-α, and IFN-γ). | Hypertension, glucose intolerance, dyslipidemia, osteoporosis. |
| Cyclosporine | Lipid soluble, poor/variable oral absorption. Neoral has improved and more predictable bioavailability. | Binds cyclophylin. Inhibits calcineurin. | Inhibits IL-2 production. Stimulates TGF-β production. | Nephrotoxic effects, hypertension, dyslipidemia, glucose intolerance. |
| Tacrolimus (FK506) | Better oral bioavailability than cyclosporin standard form | Binds FKBP-12. Inhibits calcineurin. | Inhibits IL-2 production. Antagonizes TGF-β. | Similar to cyclosporine but less hirsutism/gum enlargement. Up to 20% incidence of IDDM. |
| Hepatic metabolism. | ||||
| Azathioprine | Hepatic metabolism to active product. | Metabolites bind DNA. | Inhibits purine synthesis, Blocks DNA and RNA synthesis. | Marrow suppression. |
| MMF | Good bioavailability. Hepatic metabolism to form active product. | Inhibits inosine monophosphate dehydrogenase. | Blocks de novo pathway of purine synthesis (selective for lymphocytes). Blocks glycosylation. | Diarrhea/gastrointestinal upset. Cytomegalovirus. Increased but no reported. cases of PCP. |
| Sirolimus | Lipid soluble. Poor oral bioavailability. | Binds FKBP-12. Blocks p70 S6 kinase. | Blocks IL-2-induced cell cycle. progression. | Hyperlipidemia. Thrombocytopenia. |
IDDM, insulin-dependent diabetes mellitus; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor; FKBP, FK506 binding protein; PCP, pneumocystis carinii pneumonia.
A total of 380 HT recipients at 24 centers were enrolled in a double-blind randomized trial comparing the safety and efficacy of SM and NL. At 6 months after HT, allograft and patient survivals were the same for both groups. The frequencies of ISHLT grade ≥ 3A rejection episodes were identical in the two groups. In comparison with SM patients, fewer NL patients required rescue rejection therapy with antilymphocyte antibodies (ATG or OKT3) (5.9% compared with 14.1%; P = 0.01). Interestingly, female HT recipients in the NL arm who had ISHLT rejection grade ≥ 3A had a 46% lower rejection rates than SM-treated females (31.3% compared with 57.6%; P = 0.032). Fewer infections were seen in the NL group (Fig. 2). With the exception of the early postoperative period, in which creatinine levels were higher in the NL group, overall renal function was similar in the two groups [13].
Figure 2.
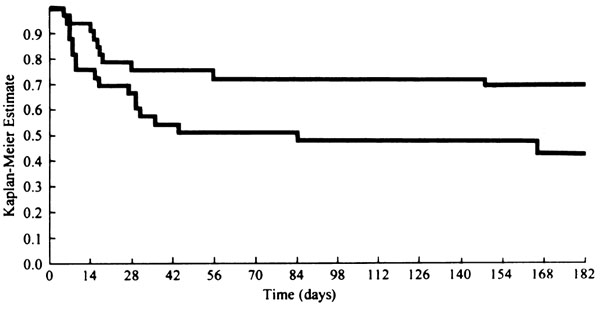
Freedom from ISHLT grade ≥ 3A cardiac allograft rejection (Kaplan-Meier method) in females receiving either cyclosporine-Neoral (upper line; n = 32) or cyclosporine-SM (lower line; n = 33). In the log-rank test, P = 0.032. Reproduced with permission from [13].
Tacrolimus (FK506; Fujisawa, Japan) has also been compared with SM in both a US trial and a European trial. Patients in the two treatment groups had similar rates of rejection, infection, hyperglycemia, and renal function. The tacrolimus-treated patients had lower rates of hypertension requiring pharmacologic therapy in both the US (48% compared with 71%; P = 0.05) and European (59.5% compared with 87.55%; P = 0.025) trials [14,15].
Purine inhibitors
The largest study conducted so far in HT recipients is the 3-year double-blind randomized multicenter trial comparing the effects of MMF with those of AZA in 650 HT recipients treated with CSA and prednisone [16]. MMF inhibits purine synthesis de novo by blocking the enzyme inosine monophosphate dehydrogenase. Because lymphocytes lack the salvage pathway for purine synthesis, MMF selectively inhibits lymphocyte proliferation.
The protocol-specified primary endpoints were 6-month acute rejection with hemodynamic compromise and 12-month patient/allograft survival. In this double-blind active-control trial, 28 centers randomized 650 patients undergoing their first HT to receive either MMF (3000 mg/day) or AZA (1.5-3.0 mg/kg per day), in addition to CSA and prednisone. Rejection and survival data were obtained for 6 and 12 months, respectively. Because 11% of the patients withdrew before receiving study drug, data were analyzed on all randomized patients (enrolled patients), and on patients who received study medication (treated patients). Survival and rejection were similar in enrolled patients (MMF, n = 289; AZA, n = 323). In treated patients (MMF, n = 289; AZA, n = 289), the MMF-treated patients had a significant reduction in mortality at 1 year [18 (6.2%) compared with 33 deaths (11.4%); P = 0.031] and in the requirement for treatment for rejection (65.7% compared with 73.7%; P = 0.026) (Fig. 3). There was a trend for fewer MMF patients to have ≥ grade3A ISHLT rejection (45.0% compared with 52.9%; P = 0.055) or to require OKT3 or ATG (15.2% compared with 21.1%; P = 0.061).
Figure 3.
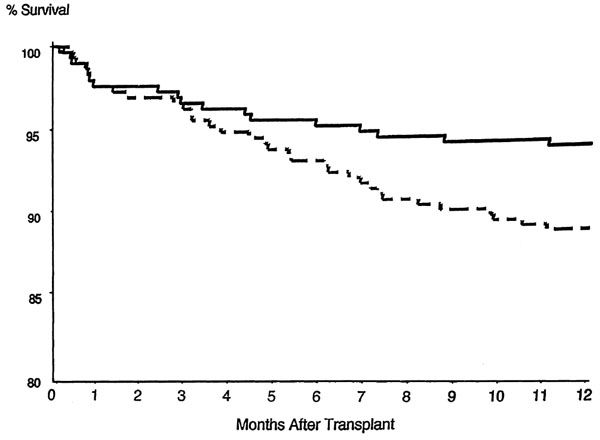
One-year survival of the mycophenolate mofetil (MMF; solid line) and azathioprine (AZA; broken line) groups in the treated patient population. Patients receiving mycophenolate mofetil had significantly greater survival. P = 0.031, MMF treatment compared with AZA. Reproduced with permission from [16].
Opportunistic infections, mostly herpes simplex, were more common in the MMF group (53.3% compared with 43.6%; P = 0.025). There were 102 MMF-treated patients and 94 AZA-treated patients who had baseline and 12 months intravascular ultrasound (IVUS) studies.
Although a post hoc endpoint, there was a significant benefit of MMF treatment compared with AZA in the mean change in lumen area from baseline to 12 months after HT. Lumen area increased by 0.33 mm2 in the MMF group compared with a decrease of 0.81 mm2 in the AZA group (P = 0.007). At 3 years, evaluation of the allograft's coronary arteries with IVUS revealed a trend for intimal thickening to involve a smaller portion of the vessel's circumference in MMF-treated patients than in AZA-treated patients (59° compared with 159°; P = 0.07). The 3-year survival was greater in MMF-treated patients than in AZA-treated patients (88.1% compared with 81.6%; P = 0.029), correlating with a 35% reduction in mortality. Most of the excess deaths in the AZA-treated patients were due to cardiovascular events, infection and allograft rejection. The average daily dose of study medication during the 12 months after HT was 2.72 g/day for MMF and 1.92 mg/kg per day for AZA patients. Among the treated patients, withdrawal from study drug occurred in 93 MMF patients (32.2%) and in 104 AZA patients (36%) and was for similar reasons in the two groups.
This study has received some criticism about the timing of randomization and the clinical/statistical analysis of significance [17]. Randomization of patients occurred before HT, but patients initiated study medication some time after HT. As stated above, 11% of randomized patients could not take the oral medication within 5 days of transplantation and were withdrawn from the study without receiving study medication. The decision to withdraw a patient was performed in a double-blind manner by investigators. Although demographic characteristics were balanced for the enrolled and treated populations, those patients randomized to MMF and excluded from the treated group had a higher death/retransplantation rate. Critics of the study also point out that MMF was not demonstrated to be superior to AZA in either the intent-to-treat or the as-treated analysis for the prevention of acute graft rejection with hemodynamic compromise at 6 months according to the original protocol definition of hemodynamic compromise.
The primary endpoint was changed from rejection with hemodynamic compromise to rejection with severe hemodynamic compromise because the 10-15% rate of the latter type of rejection was believed by the steering committee to be more clinically relevant than the 33% rate observed with the broader initial definition of hemodynamic compromise. The study was designed to demonstrate equivalence between groups for the 12-month patient and graft survival. In the analysis of the enrolled patients, the observed difference in survival rates for MMF minus AZA was 2.6% (87.2% compared with 84.8%; confidence interval -2.5% to 7.6%; P = 0.402). On the basis of this result, some believe that the study can only claim equivalence rather than superiority of MMF over AZA. Some even question the use of AZA as the active control, because the contribution of AZA to a prevention of acute rejection in the first 6 months after HT has not been clearly quantified [17]. Despite these potential limitations, this study's validity is supported by the double-blind design, the standardization of rejection diagnosis and treatment, and the analysis of 'treated patients', which provided valuable, clinically relevant information.
Photopheresis
Currently available rejection therapies improve graft survival by nonspecific immunosuppression, leaving the host at increased risk of opportunistic infections, malignancies and other serious adverse effects. Moreover, considerable morbidity and mortality persist as a result of acute episodes of acute rejection and cardiac allograft vasculopathy. Treatment directed at suppressing donor-specific T cell clones in the recipient have the potential to decrease graft rejection without further increasing the toxicity of immunosuppressive drugs. In photopheresis the patient's peripheral blood is removed and separated into leukocyte-depleted blood, which is returned to the patients, and leukocyte-enriched blood, which is exposed to ultraviolet radiation in the presence of extracorporeally administered liquid methoxalen [18]. Methoxalen, which is photoactive, covalently binds to DNA pyrimidine bases, cell-surface molecules, and cytoplasmic components in the exposed white cells, causing a lethal defect. These cells are then reinfused into the patient and die over 1-2 weeks, but during that interval they stimulate an autologous suppressor response, in part mediated by T cells, that targets nonirradiated T cells of similar clones. Cross-links in the DNA do not fully explain the immunomodulatory effects of photopheresis, because only 2-5% of the patient's mononuclear cells are affected with each treatment.
It has been shown that photopheresis-treated lymphocytes secrete inflammatory mediators, such as IL-6 and tumor necrosis factor α, that affect the entire immune cell population [19]. Other studies have suggested that photopheresis-treated mononuclear cells stimulate the generation of clone-specific suppressor T cells [20]. Apoptosis has been observed in photopheresis-treated peripheral blood lymphocytes and might be important in immunoregulation [21]. Another possibility is that photopheresis alters the unique T cell receptor associated with the expanded T cell clone, making it more susceptible to clearance by the immune system. In one study, 60 consecutive eligible recipients of primary HTs were randomly assigned to standard triple-drug immunosuppression with CSA, AZA and prednisone alone or in conjunction with photopheresis [22]. The photopheresis group received a total of 24 photopheresis treatments, each pair of treatments given on two consecutive days, during the first 6 months after HT. The regimen for maintenance immunosuppression, the definition and treatment of rejection episodes, the use of prophylactic antibiotics, and the schedule for heart biopsies were standardized in all 12 centers. All the cardiac biopsy samples were graded in a blinded manner at a central pathology laboratory. Plasma from the subgoup of 34 patients (57%) who were enrolled at the 9 US centers was analyzed by amplification by polymerase chain reaction for CMV DNA.
After 6 months of follow-up, the mean number of episodes of acute rejection per patient was lower in the photopheresis group than in the group undergoing standard therapy (0.91 ± 1.0 compared with 1.44 ± 1.0; P = 0.04). Significantly more patients in the former group had only one or no rejection episodes than in the latter group (81% compared with 48%), and significantly fewer patients in the former group had at least two rejection episodes (18% compared with 48%; P = 0.02). A rebound increase in rejection rates did not occur after completion of the photopheresis treatments during the second 6 months of follow-up. There was no significant difference in the time to a first episode of rejection, rejection associated with hemodynamic compromise, or survival at 6 and 12 months.
Although there were no significant differences in the rates and types of infection, CMV DNA was detected significantly less frequently in the photopheresis group than in the group undergoing standard therapy (P = 0.04) (Fig. 4). Multivariate analysis revealed that this decrease was independent of the donor's and recipient's CMV status and treatment of rejection, and that use of photopheresis was the only variable independently associated with the decrease [22]. The main limitations of this study include its small population, and the lack of a placebo group and of investigators' blinding. However, immunosuppression, the diagnosis and treatment of rejection episodes, the use of prophylactic antibiotics, and the cardiac biopsies' schedule were strictly standardized between all centers. Furthermore, acute rejection, the primary endpoint of the study, was assessed at a central pathology laboratory in a blinded manner.
Figure 4.
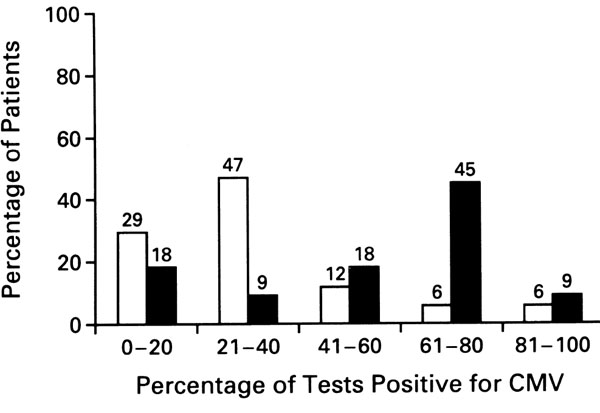
Frequency distribution of positive cytomegalovirus (CMV) test by polymerase chain reaction analysis in the 34 patients at US centers. Open columns, photopheresis plus standard therapy; filled columns, standard therapy. P = 0.04 by the Wilcoxon rank-sum test for the comparison between groups of the percentage of each patient's tests that were positive. (Adapted from [22].)
Pravastatin
Pravastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, has been used in HT recipients because it is safe and effective in lowering cholesterol levels after HT. However, a randomized trial in HT recipients has shown that pravastatin can affect HT outcome not only by its lipid-lowering effects, but also because of previously unknown immunosuppressive actions [23]. Early after HT, patients were randomly assigned to receive either pravastatin (47 patients) or no HMG-CoA reductase inhibitor (50 patients). At 12 months after HT, compared with the control group, pravastatin-treated patients had lower cholesterol levels (193 ± compared with 248 ± 49 mg/dl; P < 0.001), less frequent cardiac rejection complicated by hemodynamic compromise (3 compared with 14 patients; P = 0.005), better survival (94% compared with 78%, P = 0.025), and a lower incidence of cardiac allograft vasculopathy as determined by angiography and at autopsy (3 compared with 10 patients; P = 0.049). In a subgroup of patients, IVUS measurement at baseline and 1 year after HT showed less progression in the pravastatin group than in the no HMG-CoA group in maximal intimal thickness (0.11 ± 0.09 mm compared with 0.23 ± 0.16; P = 0.002) and in the intimal index (0.05 ± 0.03 compared with 0.10 ± 0.10; P = 0.031).
In a subgroup of patients, the cytotoxicity of natural killer cells was lower in the pravastatin than in the control group (9.8% compared with 22.2% specific lysis; P = 0.014). Although there was no consistent relationship between high values of cytotoxicity of natural killer cells and acute rejection episodes, several patients in the control group did exhibit elevations of cytotoxicity that preceded or coincided with the onset of acute rejection. Pravastatin and CSA alone inhibited cyototoxic T lymphocyte cytotoxicity, although not significantly. The combination of pravastatin and CSA acted synergistically to inhibit cytotoxic T lymphocyte function (20.3% compared with 41.4% in the group receiving CSA alone; P < 0.01). These findings suggest that the combination of pravastatin and CSA enhances the overall level of immunosuppression in HT recipients [24].
Possible future studies
Owing to the observation that the nephrotoxicity of certain immunosuppressive drugs can negatively affect long-term HT function and survival, new regimens are being designed that consist of MMF with or without the anti-CD25 agent daclizumab (Table 3). The common denominator of these new immunosuppressive regimens is the reduction or elimination of calcineurin inhibitors or corticosteroids [25].
Table 3.
Evolving low-toxicity maintenance immunosuppression regimens
| Regimen | Comment | |||
| Cyclosporine + azathioprine/MMF + steroids | Standard regimen. MMF preferred because it reduces incidence of acute rejection; significantly more expensive. Neoral replacing. Sandimmune in many centers. | |||
| Tacrolimus + azathioprine + steroids | Alternative regimen to above | |||
| Daclizumab/basiliximab + MMF + steroids | IL-2 receptor blockers used only in early post-transplant period but may obviate need for calcineurin inhibitor. | |||
| Tacrolimus + MMF + steroids | Preliminary studies suggest very low incidence of acute rejection; gastrointestinal side effects are common but minimal steroid should reduce risk of diabetes mellitus. | |||
| Sirolimus + cyclosporine + steroids | Sirolimus may allow greater reduction in cyclosporin or steroid doses. |
IL, interleukin; MMF, mycophenolate mofetil.
Researchers in transplantation immunobiology have identified several T cell accessory molecules that participate in the alloimmune response through three possible methods: (1) stabilization of the interaction between cytotoxic T cells and the target cell; (2) provision of the antigen-dependent second signal necessary for facilitating signal transduction in T cells; (3) enhancement of the interaction between T cell receptors and major histocompatibility complex (MHC)-bearing antigen (Table 4). For instance, lymphocyte function associated antigen-1 (LFA-1) and intracellular adhesion molecule-1 (ICAM-1) are accessory molecules on T cells involved in adhesion reactions and co-stimulatory signals. Monoclonal antibodies against these accessory molecules are now in clinical trials. The use of antibodies against both these adhesion molecules, and the related strategy of combining ICAM-1 antisense oligonucleotides with anti-LFA-1, might prove to be even more promising. CD40 is a recently described molecule found on antigen-presenting cells, whereas CD40 ligand is expressed on T cells after stimulation with antigen. The interaction of CD40 with CD40 ligand is thought to be involved in T cell co-stimulation. Monoclonal antibodies against CD40 ligand have been effective in inducing tolerance in experimental transplant models.
Table 4.
Novel immunosuppressive strategies
| Site of action | Agent | Human studies | ||
| Interruption of TCR/MHC | ||||
| binding | ||||
| CD4/MHC class II | Anti-CD4 mAbs | Yes | ||
| Interruption of T cell | ||||
| co-stimulation | ||||
| CD28/B7 | CTLA4-Ig | Yes (psoriasis) | ||
| CD40/CD154 | Anti-ICAM-1 mAbs | Yes (ITP) | ||
| Interruption of cell adhesion | ||||
| LFA-1/ICAM-1 | Anti-LFA-1 mAbs | Yes | ||
| Anti-ICAM-1 mAbs | Yes | |||
| Interruption of accessory | ||||
| molecule interactions | ||||
| CD2/LFA-3 | Anti-LFA-3 mAbs | No | ||
| Anti-CD2 mAbs | Yes | |||
| CD45 | Anti-CD45 | Yes |
ICAM-1, intracellular adhesion molecule-1; ITP, idiopathic thrombocytopenic purpura; LFA, lymphocyte function associated antigen; mAb, monoclonal antibody; MHC, major histocompatibility complex; TCR, T cell receptor.
CD28 is another accessory molecule involved in T cell activation. The binding of ligands B7-1 and B7-2 to CD28 promotes T cell activation, proliferation, and differentiation. Another monoclonal antibody being developed targets the co-stimulatory ligand, B7. CTLA4Ig is a chimeric protein formed from the fusion of the CTLA-4 gene (closely related to the gene that codes for CD28) and human IgG. It has greater affinity than CD28 for the ligands B7-1 and B7-2, and thus helps to thwart T cell activation.
Tolerance
The ability to induce tolerance to an allograft in humans remains a highly desirable but elusive goal [26,27,28]. Clinical approaches to the induction of tolerance have included the following: (1) donor-specific blood transfusions; (2) one MHC-haplotype/DR matched blood transfusion and (3) donor bone-marrow infusion after host conditioning.
Several strategies to induce tolerance are also being tested in experimental studies. Mixed chimeric infusions, involving a mixture of the recipient's and the donor's bone marrow cells, are given with nonmyeloablative radiation to avoid the development of graft-versus-host disease. Peptides derived from class I and class II MHC molecules have been found to induce unresponsiveness to an allograft. Finally, the induction of expression of Fas ligand on donor cells, which can bind to the Fas molecule expressed on activated T cells, can lead to the apoptosis of the T cells specifically activated against the allograft [28].
Conclusion
Immunosuppression is rapidly changing because of the increasing number of drugs and biological agents making the transition from the laboratory to clinical trials. The results of randomized studies in HT recipients support the belief that MMF and antibodies against IL-2R decrease the incidence and severity of acute rejection. However, many important questions about immunosuppression in HTs remain unanswered. These include whether new agents provide more specific or simply more potent immunosuppression, which combination of drugs can achieve maximal efficacy with minimal adverse effects, and whether the new agents will prevent chronic rejection and improve long-term survival after HT.
The hope for the future is that specific inhibition of antigen recognition, T cell co-stimulation, and function of accessory molecules will induce long-term acceptance of a transplanted organ without the complications of 'broad spectrum' immunosuppression.
References
- Hosenpud JD, Bennett LE, Keck BM, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: Seventeenth Official Report - 2000. J Heart Lung Transplant. 2000;19:909–931. doi: 10.1016/S1053-2498(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Szentpetery S, Mohanakumar T, Barnhart GR, Lower RR. Effects of prophylactic rabbit antithymocyte globulin in cardiac allograft recipients treated with cyclosporine. J Heart Transplant. 1987;6:214–217. [PubMed] [Google Scholar]
- Hegewald MG, O'Connell JB, Renlund DG, Lee HR, Burton NA, Karwande SV, Jones KW, Lassetter JE, Bristow MR. OKT3 monoclonal antibody given for ten versus fourteen days as immunosuppressive prophylaxis in heart transplantation. J Heart Transplant. 1989;8:303–310. [PubMed] [Google Scholar]
- Costanzo-Nordin MR. Cardiopulmonary effects of OKT3: determinants of hypotension pulmonary edema, and cardiac dysfunction. Transplant Proc. 1993;25 (Suppl 1):21–24. [PubMed] [Google Scholar]
- Hammond EA, Wittwer CT, Greenwood J, Knape WA, Yowell RL, Menlove RL, Craven C, Renlund DG, Bristow MR, DeWitt CW, O'Connell JB. Relationship of OKT3 sensitization and vascular rejection in cardiac transplant patients receiving OKT3 rejection prophylaxis. Transplantation. 1990;50:776–782. doi: 10.1097/00007890-199011000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Mullen GM, O'Sullivan EJ, Liao Y, Heroux AL, Kao WG, Pifarre R, Costanzo MR. Risk/benefit ratio of perioperative OKT3 in cardiac transplantation. Am J Cardiol. 1994;74:261–266. doi: 10.1016/0002-9149(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR, Heroux AL, Pifarre R, Fisher RI. Increased incidence of lymphoproliferative disorders following immunosuppression with OKT3 in cardiac transplantation. N Engl J Med. 1991;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- Meiser BM, Reiter C, Reichenspurner , Uberfuhr P, Kreuzer E, Rieber EP, Riethmuller G, Reichart B. Chimeric monoclonal CD4 antibody - a novel immunosuppressant for clinical heart transplantation. Transplantation. 1994;58:419–423. doi: 10.1097/00007890-199408270-00005. [DOI] [PubMed] [Google Scholar]
- Van Gelder T, Balk AHMM, Jonkman FAM, Zietse R, Zondervan P, Hesse CJ, Vaessen LMB, Mochtar B, Weimar W. A randomized trial comparing safety and efficacy of OKT3 and a monoclonal anti-interleukin-2 receptor antibody (BT263) in the prevention of acute rejection after heart transplantation. Transplantation. 1996;62:51–55. doi: 10.1097/00007890-199607150-00011. [DOI] [PubMed] [Google Scholar]
- van Gelder T, Baan CC, Balk AH, Knoop CJ, Holweg CT, van der Meer P, Mochtar B, Zondervan PE, Niesters HG, Weimar W. Blockade of the interleukin (IL)-2/IL-2 receptor pathway with a monoclonal anti-IL-2 receptor antibody (BT563) does not prevent the development of acute heart allograft rejection in humans. Transplantation. 1998;65:405–410. doi: 10.1097/00007890-199802150-00020. [DOI] [PubMed] [Google Scholar]
- Beniaminovitz A, Itescu S, Lietz K, Donovan M, Burk EM, Groff BD, Edward N, Mancini DM. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Engl J Med. 2000;342:613–619. doi: 10.1056/NEJM200003023420902. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Welsh M, Schoenberg L, Rutzky LP, Katz SM, Urbauer DL, Van Buren CT. Variable absorption of cyclosporine: a biological risk factor for chronic renal allograft rejection. Transplantation. 1996;62:599–606. doi: 10.1097/00007890-199609150-00010. [DOI] [PubMed] [Google Scholar]
- Eisen HJ, Hobbs RE, Davis SF, Laufer G, Mancini DM, Renlund DG, Valantine H, Ventura H, Vachiery J-L, Bourge RC, Canver CC, Carrier M, Costanzo MR, Copeland J, Dureau G, Frazier OH, Dorent R, Hauptman PJ, Kells C, Masters R, Michaud J-L, Paradis I, Smith A, Vanhaecke J, Feutren G, Turkin D, Mellein B, Mueller EA. Safety, tolerability and efficacy of cyclosporine microemulsion in heart transplant recipients: a randomized, multicenter, double-blind comparison with the oil-based formulation of cyclosporine - results at six months after tranpslantation. Transplantation. 1999;68:663–671. doi: 10.1097/00007890-199909150-00012. [DOI] [PubMed] [Google Scholar]
- Taylor DO, Barr ML, Radovancevic B, Renlund DG, Mentzer RM, Smart FW, Tolman DE, Frazier OH, Young JB, VanVeldhuisen P. A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J Heart Lung Tranpslant. 1999;18:336–345. doi: 10.1016/S1053-2498(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Reichart B, Meiser B, Vigano M, Rinaldi M, Martinelli L, Yacoub M, Banner NR, Gandjbakhch I, Dorent R, Hetzer R, Hummel M. European multicenter tacrolimus (FK506) heart pilot study: one-year results. European Tacrolimus Multicenter Heart Study Group. J Heart Lung Transplant. 1998;17:775–781. [PubMed] [Google Scholar]
- Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- Korvick JA, Elashoff MR, Cavalle-Col M. A commentary on a randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Transplantation. 1999;68:708–711. doi: 10.1097/00007890-199909150-00020. [DOI] [PubMed] [Google Scholar]
- Costanzo-Nordin MR, Hubbell EA, O'Sullivan EJ, Johnson MR, Mullen GM, Heroux AL, Kao WG, McManus BM, Pifarre R, Robin-son JA. Photopheresis versus corticosteroids in the therapy of heart transplant rejection: Preliminary clinical report. Circulation. 1992;86:II-242–II-250. [PubMed] [Google Scholar]
- Vowels BR, Cassin M, Boufal MH, Walsh LJ, Rook AH. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-α by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992;98:686–692. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- Khavari PA, Edelson RK, Lider O, Gasparro EP, Weiner HL, Cohen IR. Specific vaccination against photoinactivated cloned T cells [abstract]. Clin Res. 1998;36:62A. [Google Scholar]
- Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction by ultraviolet light A and photochemotherapy in cutaneous T cell lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107:235–242. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- Barr ML, Meiser BM, Eisen HJ, Roberts RF, Livi U, Dall'Amico R, Dorent R, Rogers JG, Radovancevic B, Taylor DO, Jeevanandam V, Marboe CC. Photopheresis for the prevention of rejection in cardiac transplantation. N Engl J Med. 1998;339:1744–1751. doi: 10.1056/NEJM199812103392404. [DOI] [PubMed] [Google Scholar]
- Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, Trosian K, Hamilton MA, Moriguchi JD, Kawata N, Hage A, Drinkiwater DC, Stevenson LW. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- Katznelson S, Wang XM, Chia D, Ozawa M, Zhong HP, Hirata M, Terasaki PI, Kobashigawa JA. The inhibitory effects of pravastatin on natural killer cell activity in vivo and on cytotoxic T lymphocyte activity in vitro. J Heart Lung Transplant. 1998;17:335–340. [PubMed] [Google Scholar]
- Strom TB, Ettenger RB. Investigational immunosuppressants: biologics. In Primer on Transplantation Edited by Norman DJ, Suki WN Thorofare, NJ: American Society of Transplant Physicians, 1988. pp. 113–122.
- Chandraker A, Sayegh MH. Mechanisms of tolerance. In Primer on Transplantation Edited by Norman DJ, Suki WN Thorofare, NJ: American Society of Transplant Physicians, 1988. pp. 43–50.
- Helderman JHH, Goral S. Transplantation immunobiology. In Handbook of Kidney Transplantation Edited by Danovitch GM Philadelphia: Lippincott-Raven, 1996. p. chapter 2.
- Sayegh MH, Carpenter CB. Tolerance and chronic rejection. Kidney Int. 1997;58 (suppl):S11–S14. [PubMed] [Google Scholar]
- Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet. 1999;353:1083–1091. doi: 10.1016/S0140-6736(98)07493-5. [DOI] [PubMed] [Google Scholar]


