Supplemental Digital Content is available in the text.
Keywords: Itch, Histaminergic itch, Human surrogate models of itch, Topography, Pruriception, Cutaneous flare
Abstract
Introduction:
Little is known about the topographical distribution of pruriception (in particular for nonhistaminergic itch), although conditions with chronic itch frequently occur in distinct anatomic and often bilateral patterns. This study aimed to investigate regional differences in the sensitivity to itch stimuli by assessing the intensity of itch, pain, and cutaneous neurogenic flare evoked by histamine and cowhage in different anatomic regions in 20 healthy volunteers.
Methods:
Itch was induced by 1% histamine applied with a prick lancet or by insertion of 25±5 cowhage spicules in 4 regions: volar/dorsal forearm, lower back, and chin. The duration and intensity of itch and pain following each pruritic stimulus were measured by a continuous visual analogue scale (VAS0-100). Sensitivity to touch-evoked itch was assessed by von Frey filaments and cutaneous flare was quantified by full-field laser perfusion imaging.
Results:
Peak itch intensity was lower at the chin (19.4±3.6) compared with other areas (mean of 3 locations; 41.3±4.4), independently of whether histamine or cowhage was applied (P<0.01). Baseline sensitivity to touch-evoked itch was higher on the chin (P<0.01), but here hyperknesis did not develop in contrast to other areas (P<0.05). Cutaneous flare was more intense but had a smaller dispersion at the chin, compared with other areas (P<0.01).
Discussion:
In conclusion, sensitivity to histaminergic and non-histaminergic itch diverges considerably between body regions. Lower density of pruriceptive CMH and CMI-neurons or distinct neuronal substrates for itch in the mandibular part of the trigeminal area may explain the observed reduced itch and vasomotor responses.
1. Introduction
Itch is a cardinal symptom for a range of dermatologic conditions, such as atopic dermatitis (AD), urticaria, psoriasis as well as several neurological, hematological, and nephrologic conditions1,2. To date, little fundamental evidence exists regarding potential anatomic differences in sensitivity to, and processing of various itching stimuli. This is despite the fact that the first assessments of the topographical distribution and spatial acuity for touch across the body were published more than a century ago3 and that topography and regional acuity of the nociceptive system have been explored in the recent decades4,5. The interest in the topography of itch sensation was recently rekindled in a study showing notable differences between mechanically induced itch for spinal versus trigeminal innervated areas6, and is of principal relevance for 3 primary reasons. (1) Lesions in dermatological itch conditions often occur in distinct anatomic patterns. Psoriasis lesions are typically bilateral and occur very rarely in the trigeminal region, as opposed to neuropathic pruritus conditions7,8, whereas prurigo nodularis and dermatitis herpetiformis occur mainly on extensor surfaces of the extremities9,10. AD lesions are overrepresented in the elbow and knee creases while urticaria manifests frequently on the trunk and proximal extremities11. Such spatial patterns of skin lesions and associated sensations of itch and pain in various conditions have typically been attributed to differences in skin biology, and/or proneness to low intensity damage at different body sites as well as immunologic factors12–16. However, potential neuroanatomic differences in the receptiveness or coding of itch at different body sites have only been marginally investigated. (2) Knowledge on the basic outline of and differences related to the functional and molecular properties of pruriceptive system across the body is important to develop and optimize antipruritic treatment17,18. (3) The majority of human experimental studies applying itch provocations in healthy volunteers or patients with itch have done so only on the forearms17 (contrasting rodent studies on itch, where pruritogens are frequently applied to the back or to the cheek in order to achieve distinct behavioral responses19).
Itch is transmitted by 2 distinct pathways; a subset of nociceptive mechano-insensitive C-fibers (CMI) convey histaminergic itch and a subset of nociceptive C-mechano-heat C-fibers (CMH) transmit nonhistaminergic itch20,21. Compelling evidence suggests that chronic itch conditions may rely preferentially on activity in one of these pathways; for example, the histaminergic pathway seems to play a major role in chronic urticaria, whereas the nonhistaminergic pathway has been suggested to be implicated in AD, thus also explaining why these conditions are often responsive and intractable to antihistamines, respectively18,22,23.
The aim of this study was to investigate the topographical distribution of itch and nociceptive sensations induced by histaminergic and nonhistaminergic itch provocations at 4 different spinal and trigeminal anatomic regions. In addition, cutaneous flare responses, wheal reactions, and sensitivity to touch-evoked itch (STI) at baseline and after each itch provocations were assessed.
2. Methods
2.1. Study design and subjects
A total of 20 healthy volunteers (10 females, 25.5±0.9 y) were included in the study. Exclusion criteria were any previous or current musculoskeletal, neurological, dermatologic, allergic, and addictive disorders in addition to any ongoing itch, pain, or discomfort at the day of the experiment. All subjects signed the informed consent before the experimental procedures. Of the 10 young male subjects participating in the study, 5 had no discernible beard growth in the assessed facial area, the remaining 5 subjects were instructed to shave the areas approximately 24 hours before the experimental session. The study protocol was approved by the regional Ethics Committee under the jurisdiction of the Danish Medicines Agency (approval no.: N-20150058) and was carried out with a balanced randomization of both the order of application of histamine versus cowhage, anatomic location, and dominant versus nondominant side. The following 4 locations were chosen (Figs. 1A–D) for itch provocations: (1) the middle of dorsal forearm (≈C7), (2) the chin, that is, the lower half of the territory of the mandibular branch of the trigeminal nerve (TN), (3) the middle of back (≈T12–L2), and (4) the middle of the volar forearm (C5–T1). These locations were based on prior publications6,24 and to achieve comparability between extensor and flexor-surface processing. All experimental procedures of the main study were conducted in one 3-hour session.
Figure 1.
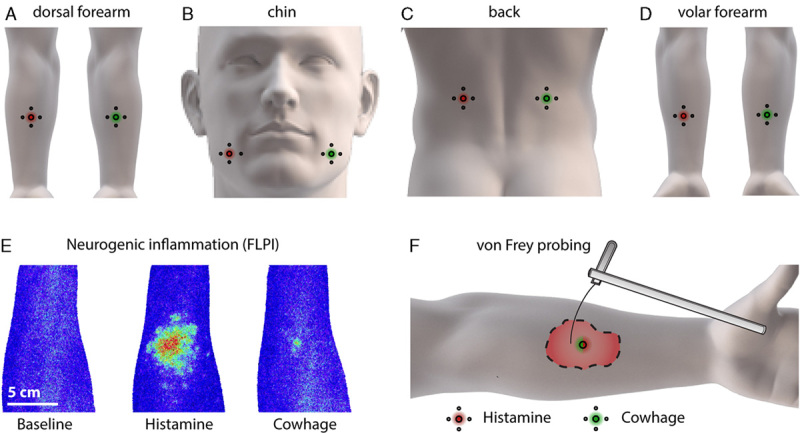
Applied body regions (A–D) and methodology (E and F). Approximate location of provocations with histamine and cowhage on the dorsal forearm (A), chin (B), back (C), and volar forearm (D). Typical cutaneous flare responses (F) measured by full-field laser imaging perfusion (FLPI) at baseline and in response to cowhage and histamine (subject no. 10). Using von Frey filaments in a specific force range (F) assessment of mechanically evoked itch sensitivity was conducted. Anatomic illustrations were modified from Navigate Pain© with permission by Aglance Solutions ApS (#NP02002).
2.2. Intraepidermal administration of histamine and cowhage spicules
A total of 8 areas (4 on each side of the body) were predefined and marked for subsequent study in accordance with Figures 1A–D. Itch provocations were conducted in 2 different ways: (1) histaminergic itch was evoked using intraepidermal punctures of 1% histamine with a standard 1 mm skin prick test (SPT) lancets (Allergopharma, Hamburg, Germany). A drop of histamine solution was placed on the predetermined area and an SPT lancet was pricked through the histamine using 120 g of weight for 1–2 seconds creating an epidermal puncture. (2) Nonhistaminergic itch was induced using cowhage spicules, which were prepared immediately before application under a stereomicroscope (Seben incognita microscope, Seben GmbH, Berlin, Germany) using a negative grip tweezer (Electron Microscopy Science, Dumont, Switzerland). Approximately 25 spicules were inserted into the center (∅0.5 cm) of the predefined skin area (∅2.5 cm) within a 15-second interval using light pressure as previously described25. The spicules were gently removed with tape after all assessments. Both of these itch provocation methods has previously been found to be reliable26.
2.3. Assessment of itch and pain
The duration and intensity of itch and pain (“pricking/stinging” or “warm/burning”) were assessed by a modified visual analogue scale (VAS) with 3 bars (1 for each sensory quality), following each itch provocation. A computerized 100-mm VAS ranging from 0 to 100 (eVAS Software, Aalborg University) installed on a Samsung Note 10.1 Tablet (Samsung, Seoul, South Korea) was used. The subjects were instructed to report the occurrence and intensity of the aforementioned sensations continuously throughout the 9-minute sampling, which were conducted at 0.2 Hz. On the VAS, 0 indicated “no itch” or “no pain” and 100 indicated “worst imaginable itch” or “worst imaginable pain.” The subjects were briefly provided with instructions on somatosensory rating. “Pricking” was introduced as the sensation induced from a needle/SPT lancet while “stinging” was described as, for example, being stung or bitten by an insect. “Warm/burning” was described the sensation associated with burns or exposure to chemical irritants. Itch was introduced as a sensation evoking a desire to scratch, for example, associated with a mosquito bite. The subjects were instructed that these sensations might or might not occur following any of the administered provocations and instructed only to rate itching/painful sensations, that is, not detection of innocuous associated sensations such as tingling. They were also instructed to disregard the mild initial pricking pain associated with insertion of spicules and the SPT puncture. From the VAS/time data temporal itch and pain intensity profiles were generated and area-under-the-curve (AUC), peak itch/pain intensity, and latency were calculated. AUC was calculated using the trapezoidal method.
2.4. Touch-evoked itch assessment
STI was probed in the 8 predetermined areas at baseline and approximately 15 minutes after itch provocations with 3 von Frey filaments; 9.8, 13.7, and 19.6 mN (North Coast Medical, Gilroy, CA) by stimulating with each filament 3×3 times, each time instructing the subject to report how much itch the stimulation elicited on a numerical rating scale (NRS) from 0 to 10 (with the same outer labels as the previously described VAS). Subjects were briefly instructed in reporting sensory qualities and informed before the onset of data collection that itch is defined by inducing a desire to scratch the area probed with von Frey filaments and hence this should be the hallmark of their rating. The subjects were also instructed that itch could occur during the von Frey stimulus itself or immediately after. All mechanical stimuli were delivered immediately next to the sites of itch provocations (0.5 to 2 cm distance) within the predefined ∅2.5 cm areas, but never directly within wheal reactions or the area of spiculae insertion. A pilot study was conducted with the entire set of 20 calibrated von Frey filaments in a subgroup of 11 subjects to select the 3 utilized von Frey filaments (see Supplemental Digital Content 1, http://links.lww.com/ITX/A0 for the applied definitions, methodology, and results). From the reported NRS-values the total mean was calculated. The applied mechanically induced itch assessment method did not allow accurate separation of alloknesis and hyperknesis (because of differences in individual baseline scores), but instead acted as a composite measure hereof. Hence, for baseline values the measure is referred to as STI, whereas the observed increases in the susceptibility to touch-evoked itch following itch provocations with histamine and cowhage are referred to as hyperknesis, as this was prevailing in the majority of subjects, that is, the incidence of reporting itch in response to the von Frey triplicate stimuli conducted at baseline with a NRS cutoff of >0.5 was: 487/720 or 66.9%.
2.5. Microvascular reactivity to histamine and cowhage
The longest wheal diameter was assessed 9.5 minutes after each itch provocation in millimeter using a ruler. Cutaneous neurogenic flare (assessed by superficial blood perfusion) was monitored in terms of dispersion and flux intensity by the use of full-field laser perfusion imaging (FLPI). For this purpose a MoorFLPI-1 (Moor Instruments, Axminster, Devon, UK) was used with a 40 cm distance between the area and the lens, a 5 Hz display rate, a 8.3 ms exposure time, and 160 units of gain. An image was obtained at baseline and 10 minutes following each itch provocation and analyzed using proprietary software (MoorFLPI Review Version 4.0; Moor Instruments). The FLPI data were analyzed by 2 methods: (1) using a region of interest (ROI) approach with a ROI equivalent to the predefined ∅2.5 cm area giving rise to an arithmetic mean and a peak perfusion within this area (referred to as “cutaneous flare”) and (2) a flare size approach wherein the area associated with the itch provocation and with an ≥50% increase compared with the surrounding background perfusion was quantified in cm2 using the ∅2.5 cm area as a reference (referred to as flare area). These quantification methods are previously described in detail in Olsen et al27.
2.6. Control experiment to adjust for baseline superficial blood perfusion
As superficial skin perfusion rates varies between body regions a control experiment was conducted to rule out the possibility that higher local clearance of histamine and mucunain (the active pruritogen in cowhage) were causing differences in perceived itch intensity; that is, higher blood perfusion levels in the facial region could cause a rapid elimination of the used pruritogens. This subexperiment was conducted separately from the main experiment in 10 random subjects from the main study cohort and provocations were performed in 4 areas; on each volar forearm (being the most commonly investigated site) and on each chin (being the site with the highest baseline perfusion levels). Histamine application, VAS recording, and assessment of superficial blood perfusion were conducted as previously described with the exception that the VAS recording was extended to 20 minutes (due to the possibility that itch and pain sensations would last longer). To decrease superficial blood perfusion a topical α2-adrenergic-agonist brimonidine (0.33% gel; Mirvaso, La Défense, France) or vehicle (inert gel) was applied 20 to 30 minutes before each itch provocation.
2.7. Statistical analysis
Statistical analyses were performed using SPSS (version 23, IBM Corporation, Armonk, NY). Sample size calculations were conducted based on previously obtained data applying similar itch induction models in a test-retest study26 and using the approach outlined in Mørch et al28 for crossover designs. The obtained data are presented as arithmetic means±SE of the mean (SEM). Data from all assessments were tested for normality using visual inspection and if unclear, the Shapiro-Wilk normality test with and without log-transformation. Peak (primary outcome), and AUC of itch/pain profiles were calculated and compared from VAS recordings. The primary statistical analyses for all outcome measures of the primary experiment were performed with repeated measures analysis of variance using Sidak post hoc tests. For itch and pain outcomes the test was constructed with exposure (2 levels) and location (4 levels), whereas for STI and FLPI data an additional baseline level was added to the exposure factor. Mauchly’s test of sphericity was utilized and in cases where sphericity was violated the Greenhouse-Geisser correction was applied. For the control experiment the Student t test was used to compare VAS and FLPI data. For an overview of the differences characterizing STI across the 4 sites Z-score transformations were made. For STI the relative increases after histamine and cowhage provocations were analyzed. The Pearson correlation analyses were conducted between parameters of particular interest, that is, peak itch intensities for histamine and cowhage at various locations and between peak itch intensities for histamine versus cutaneous flare areas in line with previously suggested associations29. A P-value of ≤0.05 was considered significant for all analyses.
3. Results
All subjects recruited for the experiment completed the study without experiencing any adverse reactions during or after the completion of the study. Refer to supplementary material 1 (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/ITX/A0) for results of the initial study performed to optimize von Frey force range used to assess STI.
3.1. Itch sensations evoked by histamine and cowhage
Temporal profiles of itch and pain sensations following provocations with histamine and cowhage are depicted in Figures 2A–D. An effect of location was observed for both itch peak intensity (F3,57=20.5, P<0.001) and itch AUC, and post hoc comparisons showed that the itch peak intensity in the chin was highly reduced compared with all other areas (P<0.001). The lowest effect size was found between the peak itch intensity of the dorsal forearm (VAS=39.9±4.7) and the chin (VAS=19.34±2.6), P<0.001 (Fig. 2E) and the lowest effect size for AUC was found between the itch AUC of the lower back (VAS×min=20.2±2.6) and the chin (VAS×time=8.1±1.3) (P<0.001; Fig. 1F). No differences were observed between the remaining 3 areas (P<0.97) for peak itch intensity. There was a nonsignificant main effect trend for exposure (F1,19=4.024, P=0.059), signifying that cowhage produced insignificantly higher itch peak intensity scores than histamine. As for peak itch intensity, itch AUC was dependent on location (F3,57=20.5, P<0.001), and post hoc comparisons showed that the itch AUC in the chin was highly reduced compared with all other areas (P<0.05). There was no main effect of exposure for itch AUC (F1, 19=0.3, P=0.571) and the exposure×location interaction was nonsignificant (F3,57=2.47, P=0.071), indicating that the observed differences for location were independent of whether histamine or cowhage were applied (although a trend was evident).
Figure 2.
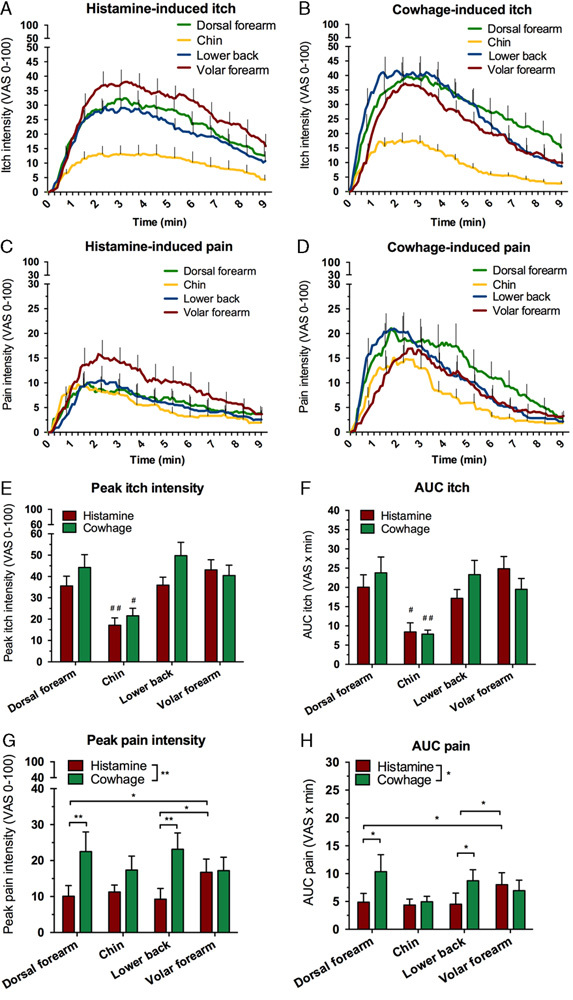
Temporal profiles of itch (A and B) and pain (C and D) across the 4 sites. Peak and AUC of evoked itch (E and F) and pain (G and H). */#P≤0.05, **/##P≤0.01. #Significance in comparison with all other conditions of the parameter. AUC indicates area-under-the-curve; VAS, visual analogue scale.
3.2. Pain sensations evoked by histamine and cowhage
Both histamine and cowhage gave rise to mild nociceptive sensations occurring alongside the itch (Figs. 2G, H). However, sensations rated as “warm/burning” pain never reached an average peak VAS above >5, and this outcome parameter was not subjected to further analysis. For “pricking/stinging” pain, peak intensities was found to be significantly higher for cowhage (VAS=21.1) than for histamine (VAS=11.8) across locations shown by a main effect for exposure (F1,19=14.01, P<0.001). No overall difference was found for location (F3,57=0.49, P=0.69); however, a significant interaction was found for location×exposure (F3,57=3.47, P=0.022; Fig. 2G). Cowhage induced higher peak pain levels than histamine specifically on the dorsal forearm (P<0.01) and on the lower back (P<0.01) and similar results were found for pain assessed by AUC. Cowhage induced higher pain AUC scores than histamine on the dorsal forearm (P<0.05) and on the lower back (P<0.05), and in the histamine condition higher pain AUC scores was found for volar forearm versus dorsal forearm and for the volar forearm versus the lower back (P<0.05).
3.3. Touch-evoked itch sensitivity
STI was quantified at baseline and after each itch provocation (Supplementary material 1, Supplemental Digital Content 1, http://links.lww.com/ITX/A0). At baseline a majority of stimulus triplicates were reported as itch evoking, that is, incidences were 66.1% for the dorsal forearm, 77.7% for the chin, 50.5% for the lower back, and 73.3% for the volar forearm (NRS cutoff of>0.5). An isolated analysis of the baseline data (Fig. 3A) showed significant differences in STI depending on location (F3,57=13.9, P<0.001). Specifically, STI was higher in the chin compared with the dorsal forearm and the lower back (P<0.01) and was reduced at the lower back compared with the volar forearm (P<0.01). Analysis of hyperknesis after itch provocations showed main effects of exposure (F2,38=21.7 P<0.001) and location (F3,57=3.03 P<0.037). An interaction was present between location and exposure (F6,114=2.922 P=0.044) and post hoc tests showed hyperknesis after itch provocations with both histamine and cowhage, for all areas except the chin (P<0.05); that is, cowhage and histamine conditions were associated with overall higher STI than baseline measurements (P<0.01) and before itch provocation the lower back was generally less sensitive than the chin and the volar forearm (P<0.05) (Figs. 3B, C).
Figure 3.
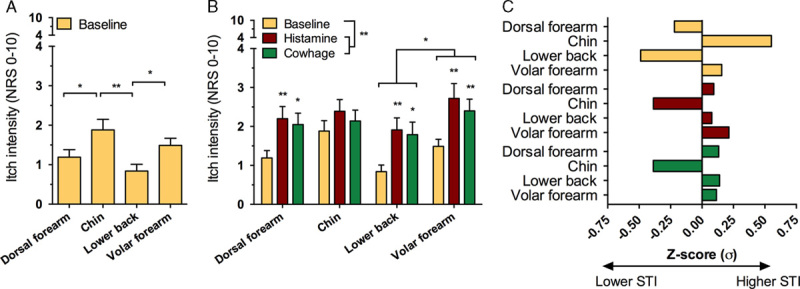
Differences between susceptibility to mechanical itch stimuli at baseline (A), degree of hyperknesis developed after each itch provocation (B), and Z-transformed results (C) at baseline (yellow) and increases signifying relative hyperknesis after itch provocations. For histamine (red) and cowhage (green) results in (C), relative increases in mechanically evoked itch sensitivity are compared with the dataset mean. *P≤0.05, **P≤0.01. Z-transformation of baseline STI scores and relative increases prompted by histamine and cowhage show the chin being more sensitive at baseline while the lower back is less sensitive and how hyperknesis did not occur at the chin after either histamine or cowhage provocations (C). NRS indicates numerical rating scale; STI, sensitivity to touch-evoked itch.
3.4. Cutaneous flare and wheal reactions
Histamine, but not cowhage, consistently produced wheal reactions with an average of the longest diameter being 4.8±0.3 mm. The repeated measures analysis of variance showed no effect of location (F1.9,33.5=2.59 P=0.091) signifying that wheal reactions did not differ between locations. Cutaneous flare was consistently induced by histamine while cowhage only occasionally gave rise to minor responses (Fig. 1E). Superficial blood perfusion measurements were shown to be dependent on the location (P<0.001), that is, higher levels of baseline perfusion measured by ROI mean and ROI peak were present in the chin compared with all other locations (Fig. 4A). Histamine (average, 7.24±0.71 cm2) produced more consistent and larger flare areas than cowhage (average, 0.85±0.36 cm2) (F1,19=131.9, P<0.001). In total, only 24 of 80 cowhage provocations resulted in discernable but small flare areas (Fig. 4D). The size of flare areas in response to histamine were significantly diminished at the chin (3.51±0.49 cm2) compared with all other areas (average, 8.50±0.73 cm2; P<0.01) and flare areas in response to histamine were larger on the volar arm (9.59±0.67 cm2) than on the lower back (7.10±0.81 cm2, P<0.05; Fig. 4D). A significant correlation between the area of cutaneous flare and the peak itch intensity across all areas was found (r2=0.43, 95% confidence interval, 0.23-0.64; P<0.001; n=80).
Figure 4.
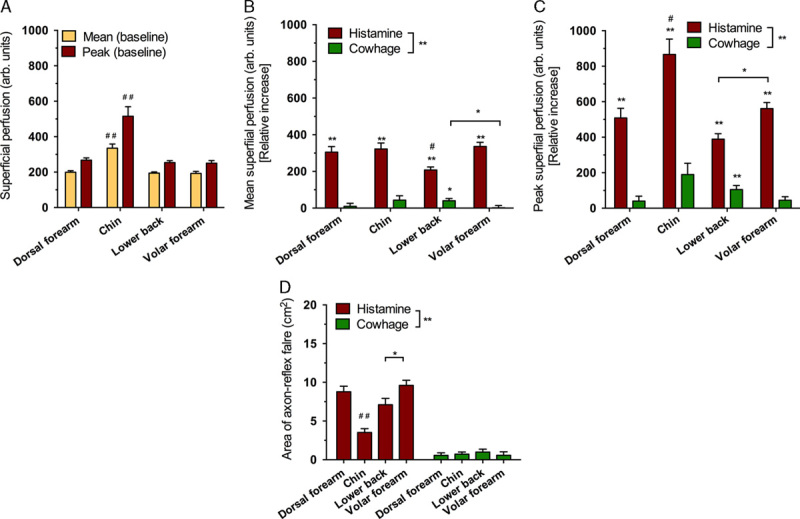
Baseline differences in perfusion (A), relative increases in mean perfusion across different sites (B), relative increases in peak perfusion across different sites (C), and the flare area (D). */#P≤0.05, **/##P≤0.01. #Significance in comparison with all other conditions of the parameter.
3.5. Control experiment to adjust for baseline superficial blood perfusion
Brimonindine reduced baseline superficial perfusion (Fig. 5D) particularly at the chin (P<0.01) where perfusion rates were reduced to levels equal to those of the volar forearm without brimonidine treatment (P>0.8). Peak itch intensity and peak latency were unchanged by the pretreatment of brimonidine versus vehicle (Figs. 5A–C). A larger AUC was observed for histamine on the volar forearm as a consequence of a slowed decline of itch (P<0.05).
Figure 5.
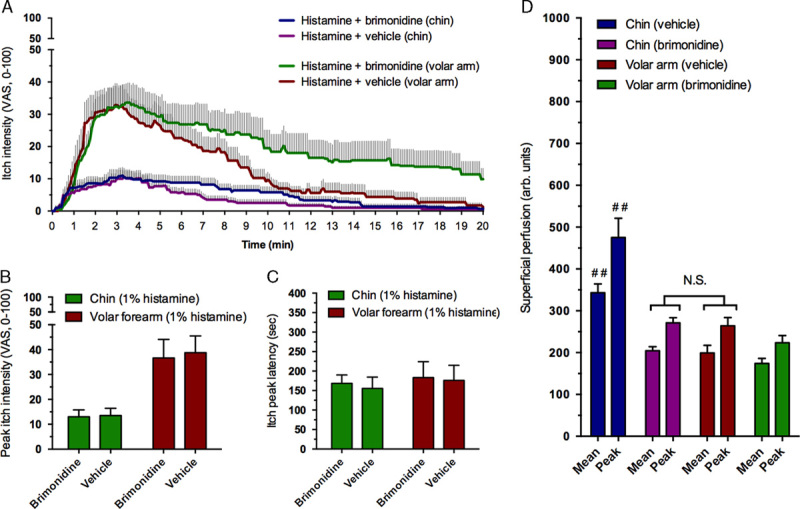
Itch intensity profiles and superficial blood perfusion induced by histamine before and after brimonidine treatment at the chin and volar forearm (n=10). Temporal itch profiles (A), peak itch intensity (B), peak itch latency (C), and superficial blood perfusion measured as mean and peak intensity (D). #P≤0.05, ##P≤0.01. #Significance in comparison with all other conditions of the parameter. Notice that no significant (NS, highlighted) difference is present for superficial perfusion between the chin after brimonidine pretreatment versus the volar forearm at baseline. Superficial blood perfusion at baseline did not affect the peak itch intensity scores obtained (P>0.8). VAS indicates visual analogue scale.
4. Discussion
The intensity of histaminergic and nonhistaminergic itch and vascular responses evoked by itch provocation models differs greatly between different anatomic areas. Most notably, itch sensitivity to both histaminergic and nonhistaminergic stimuli was highly reduced at the TN-innervated area. Here, both itch provocations resulted in lower peak and AUC itch scores compared with all other areas investigated and on average the reduction amounted to ≈55%. Itch-associated nociceptive sensations, that is, “pricking/stinging” were found not to depend on location for either histamine or cowhage, indicating that the observed topographical differences are specific to itch. Conversely, STI was increased at the chin, but hyperknesis after itch provocations did not develop despite the fact that ≈2-fold sensitivity increases were found for all other tested locations. Lastly, distinct patterns were observed for the cutaneous flare response; at the chin, reactions in response to histamine were more intense but highly localized, whereas in the other regions more widespread and less intensive inflammatory responses were observed.
4.1. Itch and pain induction by histamine and cowhage
The temporal itch intensity profiles (Figs. 2A–D), evoked in response to histamine and cowhage on all locations apart from the chin, are well aligned with previous research applying these human surrogate models of itch25,29–31. Earlier studies on cowhage and histamine-induced itch have reported that the sensation of itch frequently occurs together with pricking/stinging and warm/burning sensations30,31. The present study also showed pricking/stinging pain after both cowhage and histamine application, although not to the same extent as some previous publications25,30. In addition, very low levels of burning pain were observed and generally reported to be slightly lower than in previous studies30,31. These discrepancies may be caused by methodological differences regarding definitions of labels, delivery method of histamine and cowhage, sampling frequency, different VAS’, and the multiplicity of repeated provocations applied in the present study. Surprisingly, itch evoked by both histamine and cowhage was significantly decreased at the chin, whereas nociceptive sensations did not show a similar anatomic distribution. A lack of notable and consistent differences between extensor and flexor surfaces was observed for both cowhage and histamine provocations. Thus it is unlikely that itch fiber topography of histaminergic and mucunain-responsive fibers is etiologically involved in itch conditions characterized by manifesting preferentially on flexor or extensor surface skin.
4.2. Topography of itch induced by histamine, cowhage, or von Frey stimulation
Topographical sensitivity to itch stimuli is a sparsely investigated topic. To our knowledge only 4 previous studies have explored itch sensitivity in a territory innervated by the TN6,24,32,33. These studies generally find decreased itch scores in various TN-innervated areas in response to histamine most commonly applied by iontophoresis (a method more prone to differences in barrier integrity than SPT) compared with the volar forearm, in line with this study. Fukuoka and colleagues, notably reported that while itch scores in response to histamine iontophoresis were generally decreased by approximately 50% at the chin versus the forearm, touch-evoked itch as well as tickle scores were much more vigorous in face than on the volar forearm where almost no itch was elicited. The devised mechanical stimulation technique relied on vellus-hair vibration and is hence influenced by their regional distribution but nonetheless the observation supports the current findings of increased STI in the face which also aligns with the classical functional distribution of mechano-receptive fibers; face>arm>thorax3,4. It should be noted that sensations such as “tingling” or “crawling” in response to von Frey stimuli were not quantified in this study, but that these are likely much more readily evoked in the facial region6. The presented data on histaminergic itch sensitivity are aligned with a previous psychophysical study in which a dose-response design (intradermal histamine) was applied in various spinally innervated locations34 and a previous study showing decreased histaminergic responsiveness in skin of the scalp35. Lastly, the present data are also in agreement with a preclinical study in which intradermal injection of serotonin (5-HT) elicited significantly more hindlimb scratch bouts, and more prolonged scratching, in a spinal versus trigeminal area36 (although such behavioral data should be interpreted with caution).
In the present study we used von Frey filaments in a preoptimized force range to probe itch sensitivity at baseline and after each provocation. Using von Frey filaments to probe itch and pain sensitivity has been described earlier, for example, to document increased sensitivity to mechanically evoked itch and pain in patients with AD37–39, but it has not been studied systematically for different forces nor across different body regions before and after itch provocations. As the test-retest reliability of probing mechanical pain sensitivity with von Frey filaments is relatively poor, pain in response to the von Frey set of 20 filaments was only quantified in the pilot study (supplementary material 1, Supplemental Digital Content 1, http://links.lww.com/ITX/A0). In this context it should be noted that one previous study did not detect pain in normal skin to any of the 20 filaments40, contradicting the current and several previous studies37–39,41.
Hyperknesis developed in response to both cowhage and histamine in all areas apart from the chin (as previously shown on the volar forearm42). The lack of significant hyperknesis after itch provocations on the chin is likely a consequence of the limited pruriceptive drive or simply that this location is much more sensitive to touch-evoked itch at baseline causing a ceiling effect43. It remains unclear which neuronal structures that convey touch-evoked itch. Aδ-nociceptors are probable substrates given their involvement in pinprick hyperalgesia44, but tactile C-fibers have also been proposed6 and a very recent study broadly suggested low threshold mechano-receptors as a primary afferent candidate for mechanically evoked itch transduction in hairy skin and revealed that a dedicated spinal cord inhibitory pathway characterized by the expression of neuropeptide Y that gates the transmission of touch-evoked itch under normal conditions45,46. Although speculative, the fact that touch-evoked itch is more readily elicited in the face while chemically induced histaminergic or nonhistaminergic itch (as presently shown) is more readily induced on, for example, extremities could be evolutionarily driven6. While, skin contact with irritant or allergenic plants is likely to occur for extremities it is less likely to occur in the face. Conversely, poisonous insects (the detection of which requires sensitive processing of tactile stimuli) are potentially more damaging to structures in the facial region than on the extremities.
4.3. Cutaneous flare responses to histaminergic itch
The finding that the extent of the cutaneous flare in response to histamine is smaller, but of higher magnitude in a trigeminal area versus, for example, on the volar forearm has previously been observed for intradermal capsaicin injections and is thought to reflect the innervation density, receptive field sizes, and microvascular reactivity47,48. The cutaneous flare responses observed in response to histamine in the present study occur when peptidergic nerve fibers, upon activation and antidromic signaling, release vasoactive substances, most prominently calcitonin gene–related peptide, causing microvasculature dilation. Although both CMH and CMI fibers are capable of producing calcitonin gene–related peptide, the larger receptive field of CMI fibers causes much more pronounced responses particularly beyond the injections site49. As such our findings support the notion of increased density of peptidergic fibers, including Aδ fibers, characterized by significantly smaller receptive fields in the facial region compared with the forearms or thorax50,51 and indicate that the innervation density and territories of pruriceptive histaminergic fibers specifically are smaller at the chin. A significant positive correlation was observed between the size of the flare area and the peak itch intensity, conceivably because a subset of CMI-fibers mediates both histamine-induced itch and the extent of the flare reaction.
4.4. Limitations
Limitations concerning interpretation of the present results are foremost related to potential indirect non-neuronal involvement from factors such as baseline skin perfusion, epidermal thickness, and temperature all characterized by regional differences24,52,53. However, several observations indicate that these factors are not biasing the present results; for example, epidermal thickness is relatively uniform across the sites tested52,54, SPT in any of the sites rarely resulted in bleedings and wheal reactivity did not differ significantly (indicating that similar volumes of histamine were introduced at similar depths). Although increase in skin temperature is known to aggravate itch and is different between body regions53,55, this should, if anything, have increased itch responses at the chin and superficial skin perfusion is unlikely to be a major factor as brimonidine pretreatment significantly reduced perfusion at the chin without changing the itch or pain peak intensity to histamine application. With the proxy-based nature of the study in mind we provide compelling psychophysical and vasomotor evidence of altered pruriceptive innervation of the chin, but studies confirming this on a molecular level are needed. This study shows that elicitation of even moderate chemically induced itch, using the 2 most common human models of itch, is difficult to achieve in a TN-innervated area in humans. In this regard it should be noted that certain chronic itch patient populations, particularly those with neuropathic origins, frequently experience highly bothersome and treatment refractory itch in the facial region7 of which the pathophysiology remain poorly understood56 Lastly, it should be mentioned that the chin, innervated by the mandibular branch of the TN, might not be representative for itch sensitivity in the entire TN, although several previous studies also point toward reduced responses to histamine for both the scalp and forehead32,35.
In conclusion, itch sensitivity and vascular reactivity to histamine and cowhage provocations differ between body regions and particularly itch severity in response to both histamine and cowhage is greatly diminished at the chin while sensitivity to von Frey filament-induced, touch-evoked itch is increased. It is hypothesized that the observed differences are caused by lower density of pruriceptive CMH and CMI-neurons (peripheral) or by altered neuronal coding (central) of itch in the trigeminal region, for example, more reliant on mechano-receptive input. Our study suggests that itch in different anatomic regions have different neuronal substrates and could hence respond differently to various therapeutic interventions. Comparative analysis of itch processing and pruriceptive receptor expression in the dorsal root ganglion versus trigeminal neurons might yield targets for future antipruritic therapy.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.itchjournal.com.
Conflict of interest statement
HHA received support from the EliteForsk 2016 travel grant of the Danish Ministry of Higher Education and Science.
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 24 March 2017
References
- 1.Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007;87:291–294. [DOI] [PubMed] [Google Scholar]
- 2.Weisshaar E, Gieler U, Kupfer J, et al. Questionnaires to assess chronic itch: a consensus paper of the special interest group of the International Forum on the Study of Itch. Acta Derm Venereol 2012;92:493–496. [DOI] [PubMed] [Google Scholar]
- 3.Weber E. Ross H, Murray D. Tactile acuity in different parts of the body. De Subtilitate Tactus, 2nd ed London: Academic Press, UK; 1834:112–118. [Google Scholar]
- 4.Mancini F, Bauleo A, Cole J, et al. Whole-body mapping of spatial acuity for pain and touch. Ann Neurol 2014;75:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlereth T, Magerl W, Treede RD. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain 2001;92:187–194. [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka M, Miyachi Y, Ikoma A. Mechanically evoked itch in humans. Pain 2013;154:897–904. [DOI] [PubMed] [Google Scholar]
- 7.Oaklander AL. Common neuropathic itch syndromes. Acta Derm Venereol 2012;92:118–125. [DOI] [PubMed] [Google Scholar]
- 8.Oaklander AL, Cohen SP, Raju SVY. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain 2002;96:9–12. [DOI] [PubMed] [Google Scholar]
- 9.Vaidya DC, Schwartz RA. Prurigo nodularis: a benign dermatosis derived from a persistent pruritus. Acta Dermatovenerol Croat 2008;16:38–44. [PubMed] [Google Scholar]
- 10.Dieterich W, Laag E, Bruckner-Tuderman L, et al. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol 1999;113:133–136. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. Demographic and clinical profiles in patients with acute urticaria. Allergol Immunopathol (Madr) 2015;43:409–415. [DOI] [PubMed] [Google Scholar]
- 12.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014;134:792–799. [DOI] [PubMed] [Google Scholar]
- 13.Bos JD, De Rie MA. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today 1999;20:40–46. [DOI] [PubMed] [Google Scholar]
- 14.Bos JD, de Rie MA, Teunissen MBM, et al. Psoriasis: dysregulation of innate immunity. Br J Dermatol 2005;152:1098–1107. [DOI] [PubMed] [Google Scholar]
- 15.Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine 2015;73:311–318. [DOI] [PubMed] [Google Scholar]
- 16.Fostini AC, Girolomoni G, Tessari G. Prurigo nodularis: an update on etiopathogenesis and therapy. J Dermatolog Treat 2013;24:458–462. [DOI] [PubMed] [Google Scholar]
- 17.Andersen HH, Elberling J, Arendt-Nielsen L. Human surrogate models of histaminergic and non-histaminergic itch. Acta Derm Venereol 2015;95:771–777. [DOI] [PubMed] [Google Scholar]
- 18.Patel T, Yosipovitch G. Therapy of pruritus. Expert Opin Pharmacother 2010;11:1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 2011;106:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 2014;15:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmelz M, Schmidt R, Bickel A, et al. Specific C-receptors for itch in human skin. J Neurosci 1997;17:8003–8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papoiu ADP, Tey HL, Coghill RC, et al. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One 2011;6:e17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003;23:6176–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magerl W, Westerman RA, Möhner B, et al. Properties of transdermal histamine iontophoresis: differential effects of season, gender, and body region. J Invest Dermatol 1990;94:347–352. [DOI] [PubMed] [Google Scholar]
- 25.LaMotte RH, Shimada SG, Green BG, et al. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 2009;101:1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen HH, Sørensen AKR, Nielsen GAR, et al. A test-retest reliability study of human experimental models of histaminergic and non-histaminergic itch. Acta Derm Venereol 2016. [In press]. [DOI] [PubMed] [Google Scholar]
- 27.Olsen RV, Andersen HH, Møller HG, et al. Somatosensory and vasomotor manifestations of individual and combined stimulation of TRPM8 and TRPA1 using topical L-menthol and trans -cinnamaldehyde in healthy volunteers. Eur J Pain 2014;18:1333–1342. [DOI] [PubMed] [Google Scholar]
- 28.Mørch CD, Gazerani P, Nielsen TA, et al. The UVB cutaneous inflammatory pain model: a reproducibility study in healthy volunteers. Int J Physiol Pathophysiol Pharmacol 2013;5:203–215. [PMC free article] [PubMed] [Google Scholar]
- 29.Johanek LM, Meyer Ra, Hartke T, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci 2007;27:7490–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann EM, Handwerker HO, Forster C. Gender differences in itch and pain-related sensations provoked by histamine, cowhage and capsaicin. Acta Derm Venereol 2015;95:25–30. [DOI] [PubMed] [Google Scholar]
- 31.Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 2009;144:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truini A, Leone C, Di Stefano G, et al. Topographical distribution of warmth, burning and itch sensations in healthy humans. Neurosci Lett 2011;494:165–168. [DOI] [PubMed] [Google Scholar]
- 33.Bin Saif GA, Alajroush A, McMichael A, et al. Aberrant C nerve fibre function of the healthy scalp. Br J Dermatol 2012;167:485–489. [DOI] [PubMed] [Google Scholar]
- 34.Wahlgren CF, Ekblom A. Perception of histamine-induced itch elicited in three different skin regions. Acta Derm Venereol 1991;71:205–208. [PubMed] [Google Scholar]
- 35.Rukwied R, Zeck S, Schmelz M, et al. Sensitivity of human scalp skin to pruritic stimuli investigated by intradermal microdialysis in vivo. J Am Acad Dermatol 2002;47:245–250. [DOI] [PubMed] [Google Scholar]
- 36.Spradley JM, Davoodi A, Gee LB, et al. Differences in peripheral endocannabinoid modulation of scratching behavior in facial vs. spinally-innervated skin. Neuropharmacology 2012;63:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Laarhoven AIM, Kraaimaat FW, Wilder-Smith OH, et al. Generalized and symptom-specific sensitization of chronic itch and pain. J Eur Acad Dermatology Venereol 2007;21:1187–1192. [DOI] [PubMed] [Google Scholar]
- 38.van Laarhoven AIM, Kraaimaat FW, Wilder-Smith OH, et al. Sensitivity to itch and pain in patients with psoriasis and rheumatoid arthritis. Exp Dermatol 2013;22:530–534. [DOI] [PubMed] [Google Scholar]
- 39.Van Laarhoven AIM, Vogelaar ML, Wilder-Smith OH, et al. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 2011;152:1486–1494. [DOI] [PubMed] [Google Scholar]
- 40.Keizer D, van Wijhe M, Post WJ, et al. Quantifying allodynia in patients suffering from unilateral neuropathic pain using von frey monofilaments. Clin J Pain 2007;23:85–90. [DOI] [PubMed] [Google Scholar]
- 41.Tena B, Escobar B, Arguis MJ, et al. Reproducibility of electronic von Frey and von Frey monofilaments testing. Clin J Pain 2012;28:318–323. [DOI] [PubMed] [Google Scholar]
- 42.Atanassoff PG, Brull SJ, Zhang J, et al. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res 1999;16:291–298. [DOI] [PubMed] [Google Scholar]
- 43.LaMotte RH. Gebhart GF, Schmidt RF. Alloknesis and Allodynia. Encyclopedia of Pain - Allodynia and Alloknesis. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013:52–55. [Google Scholar]
- 44.Ziegler EA, Magerl W, Meyer RA, et al. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain 1999;122:2245–2257. [DOI] [PubMed] [Google Scholar]
- 45.Davidson S. A spinal circuit for mechanically-evoked itch. Trends Neurosci 2016;39:1–2. [DOI] [PubMed] [Google Scholar]
- 46.Bourane S, Duan B, Koch SC, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science (80-) 2015;350:550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmelz M, Michael K, Weidner C, et al. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 2000;11:645–648. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R, Schmelz M, Weidner C, et al. Innervation territories of mechano-insensitive C nociceptors in human skin. J Neurophysiol 2002;88:1859–1866. [DOI] [PubMed] [Google Scholar]
- 49.Schmelz M, Schmid R, Handwerker HO, et al. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000;123:560–571. [DOI] [PubMed] [Google Scholar]
- 50.Young RF, King RB. Fiber spectrum of the trigeminal sensory root of the baboon determined by electron microscopy. J Neurosurg 1973;38:65–72. [DOI] [PubMed] [Google Scholar]
- 51.Arthur R, Shelley W. The innervation of human epidermis. J Invest Dermatol 1959;32:397–411. [DOI] [PubMed] [Google Scholar]
- 52.Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 2003;83:410–413. [DOI] [PubMed] [Google Scholar]
- 53.Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain 1986;24:259–269. [DOI] [PubMed] [Google Scholar]
- 54.Whitton JT, Everall JD. The thickness of the epidermis. Br J Dermatol 1973;89:467–476. [DOI] [PubMed] [Google Scholar]
- 55.Yosipovitch G, Fast K, Bernhard JD. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol 2005;125:1268–1272. [DOI] [PubMed] [Google Scholar]
- 56.Oaklander AL. Mechanisms of pain and itch caused by Herpes Zoster (Shingles). J Pain 2008;9(suppl):10–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.itchjournal.com.


