Abstract
Background:
Cognitive changes during the preclinical phase of Alzheimer’s disease (AD) dementia have been characterized among European Americans (EAs), but studies of preclinical changes among African Americans (AAs) are notably absent.
Methods:
Preclinical changes in cognition before the development of AD dementia and mild cognitive impairment over a period of 18 years were examined using change points in a biracial sample of 2,125 older adults.
Results:
Of 2,125 participants, 442 (21%) developed AD dementia and 661 (31%) developed mild cognitive impairment. A cognitive change point occurred between 4 and 5 years before the clinical diagnosis of AD dementia. Differences between AAs and EAs were observed: EAs had a higher starting level of composite cognitive function, and a change point occurred 4.3 years before AD dementia among AAs and 4.7 years among EAs. The slope of cognitive decline after the change point among those developing clinical AD dementia was significantly greater among EAs (0.233 units/y) than among AAs (0.171 units/y; p < .001). This difference in slope of cognitive decline persisted after diagnosis of AD dementia so that at the conclusion of observation the difference in average cognitive level was reversed. AAs without cognitive impairment had a lower average baseline of cognition than EAs, but the slopes of cognitive decline were similar.
Conclusions:
A prominent change to a steeper slope of cognitive decline occurs between 4 and 5 years prior to the diagnosis of AD dementia. The slope of cognitive decline after the change point is steeper among EAs than AAs.
Keywords: Cognitive change, Alzheimer’s disease, Mild cognitive impairment
Preclinical changes in cognitive function preceding Alzheimer’s disease (AD) dementia occur over almost 2 decades (1). A better understanding of preclinical cognition preceding AD dementia may facilitate improved strategies for preventing or slowing development of AD dementia. African Americans (AAs) have been reported to have higher incidence of AD dementia (2) and lower average levels of cognitive function test scores relative to European Americans (EAs) (3). However, the findings on cognitive decline are conflicting with reports of similar change (4–8), slower decline among AAs (9–13), and faster decline among AAs (14–16), which emphasize the need for improved understanding of potential racial/ethnic differences in the development of AD dementia. Recent reports suggest the existence of a sudden cognitive downward turn before developing AD dementia and mild cognitive impairment (MCI) in EAs (17,18), but there is little relevant information on the timing of cognitive turning point and slopes of cognitive trajectories among AAs.
We examine preclinical change in cognitive function before and after developing AD dementia and MCI in a large cohort study of 2,125 AAs and EAs from the Chicago Health and Aging Project (CHAP) (19). Our primary goal is to test for the existence of a sudden cognitive turn during preclinical AD dementia and MCI among AAs and contrast to EAs, and test for racial differences of change in cognitive function before and after sudden cognitive turn in those developing AD dementia and MCI. A secondary goal is to contrast the preclinical change in cognitive function in those developing AD dementia and MCI to participants with no cognitive impairment and examine any differences prior to a cognitive turn at the earliest possible time before developing clinical AD dementia and MCI.
Methods
Study Design and Participants
The CHAP study is a population-based epidemiologic study of AD and related health conditions among 10,801 adults over the age of 65 years conducted from 1993 to 2012 in four urban Chicago communities with large AA populations—Morgan Park, Beverly, Washington Heights, and Mt. Greenwood. The CHAP study used a rolling enrollment scheme, where residents who reached 65 years were enrolled as successive cohorts to replenish the original study cohort. Population interviews were conducted in participants’ homes in approximately 3-year cycles with up to seven cognitive assessments per participant from population interviews and clinical assessments. Of the 2,450 participants, 2,149 were evaluated for incident AD, of whom 24 were diagnosed with non-AD dementia and excluded from our analysis. Of the remaining 2,149 participants evaluated for incident AD, 24 were diagnosed with non-AD dementia and excluded from our analysis. The analytical sample consists of the 2,125 participants who developed clinical AD dementia or MCI or remained free of a diagnosis of cognitive impairment over a period of 18 years. A detailed description of the sample selection process has been published previously (1).
Participants selected but deceased prior to clinical diagnosis (N = 120) had significantly lower cognitive function scores than participants selected and assessed (0.243 vs 0.493, p < .001). Participants selected for clinical diagnosis but did not participate (N = 181) in the clinical assessment had cognitive function scores somewhat similar to those selected and assessed (0.477 vs 0.493; p < .001). This data suggests that higher attrition may have been due to mortality rather than to nonparticipation. A higher degree of mortality was also observed among participants after developing AD dementia and MCI compared to those with no cognitive impairment (48% vs 32%; p < .001).
The Rush University Medical Center Institutional Review Board approved this study. All participants provided written informed consent.
Cognitive Function
Cognitive function was evaluated using a battery of four tests with two tests of episodic memory (immediate and delayed recall of the East Boston Story) (20,21), one test of perceptual speed, a component of executive function (Symbol Digits Modalities Test) (22), and one test of general orientation and global cognition (Mini-Mental State Examination) (23). We combined the four tests into one composite standardized global cognitive measure by averaging the four tests after centering and scaling each to their baseline mean and standard deviation, thereby reducing the impact of large standard deviation in any one of the scales (24). The composite measures showed high correlation with each cognitive test (0.82–0.94) and predicted future clinical development of AD dementia. A participant whose composite performance matches the average participant at baseline had a composite cognitive score of 0. Participants with worse cognitive function had scores below 0 and those with better function had scores above 0.
Clinical Evaluation
Individuals sampled for clinical assessment underwent a uniform clinical evaluation that included a structured medical history, neurological examination, and a battery of 19 cognitive function tests. On the basis of this evaluation, a board-certified neurologist, who was unaware of previously collected data, diagnosed dementia and AD according to the NINCDS-ADRDA criteria (25). These require a history of cognitive decline and evidence of impairment in two or more cognitive domains, one of which must be memory for a diagnosis of AD dementia. Persons who did not meet dementia criteria but had impairment in one or more cognitive domains were classified as having MCI (26). Persons meeting these criteria have been shown to have intermediate levels of AD pathology (27), rates of cognitive decline (26), and risk of mortality (28) compared to no-cognitive-impairment and dementia subgroups.
Covariates
Our analyses adjusted for three demographic variables—age (centered at 75 years), sex (coded as males vs females), and educational attainment (measured in number of years of schooling completed centered at 12 years). Participants reported race at the time of their initial interview and were coded as AAs or EAs.
Statistical Analysis
Our estimation process had to take into account several characteristics of our study—race-specific change points for cognitive trajectories, inclusion of participants with no cognitive impairment, and differences prior to change point in cognitive test scores several years before cognitive turn. Our analysis started with descriptive statistics (means and standard deviations for continuous measures and percentages for categorical measures) at baseline assessment stratified by race (AAs and EAs) and cognitive status during the entire follow-up (no cognitive impairment, developing clinical AD dementia, and developing MCI). All descriptive comparisons were performed using two-sample t-tests for continuous measures and chi-squared test statistic for categorical measures. Following descriptive analysis, we performed a graphical analysis by plotting mean cognitive function at each population interview for the three subgroups of cognitive status in AAs and EAs. This provides some empirical evidence for differential change in cognitive function and the ability to detect cognitive turn prior to AD dementia and MCI.
We used a mixed effects model to identify subject-specific fixed change points prior to diagnosis of AD in AAs and EAs. A supremum test based on log-likelihood values was used to estimate change point in AAs and EAs (29). The change points were estimated using a grid search algorithm, where the algorithm evaluated the log-likelihood at vertices of a rectangular grid and chose the vertex with the highest value. During this search process, three vertices were evaluated—minimum, maximum, and midpoint—and vertex with highest log-likelihood met the global maxima criteria. The plot of the residual log-likelihood for estimating the fixed but unknown change point before developing AD for AAs and EAs is shown in Supplementary Figure 1a, and for MCI among AAs and EAs in Supplementary Figure 1b. The model was parameterized using four components: one linear component for participants with no cognitive impairment and three components for participants with an AD dementia or MCI diagnosis—a linear component for before the change point, a linear component for after the change point, and finally, a linear component for participants after the clinical diagnosis. As a consequence, three cognitive decline slopes are estimated in participants with AD/MCI—(1) cognitive decline before AD/MCI change point (2), cognitive decline after AD/MCI change point but before the clinical diagnosis of AD/MCI, and (3) cognitive decline after the clinical diagnosis of AD/MCI. Our time measure was set at zero for baseline assessment for participants with no cognitive impairment, and set at zero at each of the change point. The statistical models allowed participants with initial cognitive assessments after the turn to be treated as left censored, and time after change point to AD dementia recalibrated to account for censoring time. Our MCI regression models used a similar parameterization with all times focused on MCI diagnosis rather than AD dementia. Participants who developed MCI and then transitioned to AD were included in the AD group and not the MCI group. For both AD dementia and MCI change point models, we included a separate term to capture differences before change points in AD dementia and MCI among those who developed AD dementia and MCI at the earliest time available prior to their change point. Since CHAP data were collected 3 years apart, we performed additional sensitivity analysis by imputing yearly cognitive function data between two data collection periods using a mean imputation with random error around zero and a standard deviation of observed data during each time period. Racial differences were examined using appropriate interaction terms with segmented time. All demographic measures were treated as fixed and time treated as random. A bootstrap technique was used to estimate the variability of change points based on a 1,000 samples in AAs and EAs. All programming was done using SAS software (30). Additional sensitivity analysis using a joint modeling framework with fixed change points for longitudinal cognitive decline process and time-to-mortality was used to evaluate the impact of mortality on our cognitive decline estimates (31) and implemented using R program (32).
Results
During an average of 10.3 years of follow-up, 442 (21%) participants developed clinical AD dementia and 661 (31%) developed MCI. AAs were more likely to develop clinical AD dementia (24% vs 17%; p < .001) and MCI (46% vs 32%; p < .001) than EAs.
Baseline Characteristics
Baseline characteristics of AAs and EAs by their future cognitive status—no cognitive impairment, developing clinical AD dementia, and developing MCI—are shown in Table 1. In both racial groups, those developing clinical AD dementia and MCI were older, had less education, and had lower baseline cognitive function test scores than participants with no cognitive impairment. The baseline cognitive function test scores of AAs developing clinical AD dementia and MCI were significantly lower than EAs who developed AD dementia and MCI. However, the difference in cognitive function test scores between AAs developing AD dementia and no cognitive impairment were similar to the difference in EAs. Similarly, the difference in cognitive function test scores between those developing MCI compared to those with no cognitive impairment for AAs and EAs was not significant (p = .18).
Table 1.
Baseline Characteristics of 2,125 African Americans and European Americans With No cognitive Impairment, and Developing AD Dementia and MCI
| African Americans | European Americans | |||||
|---|---|---|---|---|---|---|
| No Cognitive Impairment | Developing AD Dementia | Developing MCI | No Cognitive Impairment | Developing AD Dementia | Developing MCI | |
| N = 474 | N = 275 | N = 409 | N = 548 | N = 167 | N = 252 | |
| Age, y | 69.9 (4.2) | 74.2 (5.2) | 72.2 (5.2) | 72.9 (5.5) | 77.9 (6.5) | 76.7 (6.2) |
| Education, y | 12.3 (3.0) | 11.3 (3.2) | 11.8 (3.2) | 14.5 (3.0) | 13.7 (3.4) | 14.1 (3.2) |
| Cognitive function | 0.572 (0.426) | 0.039 (0.596) | 0.290 (0.511) | 0.808 (0.366) | 0.332 (0.486) | 0.584 (0.389) |
| Females (%) | 294 (62%) | 168 (61%) | 269 (66%) | 337 (62%) | 106 (63%) | 151 (60%) |
| Income (%) | ||||||
| $0–$14,999 | 136 (29%) | 134 (49%) | 187 (46%) | 78 (14%) | 25 (16%) | 53 (21%) |
| $15,000–$29,999 | 206 (44%) | 102 (37%) | 152 (37%) | 147 (27%) | 65 (41%) | 91 (37%) |
| >$30,000 | 131 (28%) | 38 (14%) | 68 (17%) | 320 (59%) | 70 (44%) | 104 (42%) |
Note: Mean (SD) is presented for continuous measures and frequency (percentage) for categorical measures. AD = Alzheimer’s disease; MCI = mild cognitive impairment.
Characteristics at Clinical Evaluation of AD Dementia and MCI
Participants developing AD dementia were, on an average, about 4 years older than those with no cognitive impairment (Table 2). Participants were about 2 years older for those developing MCI compared to those with no cognitive impairment. AAs developing clinical AD dementia were about 3 years younger, and those developing MCI were about 4 years younger than EAs. AAs had slightly longer follow-up time to clinical diagnosis of AD dementia (0.3 years) and MCI (1.1 years) than EAs. The difference in cognitive function test scores among those with no cognitive impairment and those who developed MCI were significantly different between AAs and EAs. However, cognitive function test scores for those developing clinical AD dementia were not different between AAs and EAs (p = .50).
Table 2.
Characteristics of 2,125 African Americans and European Americans With No cognitive Impairment, and Developing AD Dementia and MCI at the Time of Clinical Evaluation for Diagnosis of AD Dementia and MCI
| African Americans | European Americans | |||||
|---|---|---|---|---|---|---|
| No Cognitive Impairment | Developing AD Dementia | Developing MCI | No Cognitive Impairment | Developing AD Dementia | Developing MCI | |
| Age at diagnosis, y | 78.3 (5.7) | 82.6 (5.7) | 80.4 (5.9) | 81.8 (6.1) | 85.2 (6.1) | 84.0 (6.3) |
| Time to diagnosis, y | 8.8 (4.0)a | 8.4 (3.9) | 8.4 (4.0) | 8.2 (3.9)a | 7.3 (3.6) | 7.3 (3.8) |
| Cognitive function | 0.356 (0.411) | −0.597 (0.718) | 0.007 (0.510) | 0.593 (0.390) | −0.549 (0.739) | 0.247 (0.520) |
Note: Mean (SD) is presented for age at diagnosis, time to diagnosis, and cognitive function. AD = Alzheimer’s disease; MCI = mild cognitive impairment.
aTime to diagnosis of participants with no cognitive impairment is their average follow-up time.
Graphical Analysis
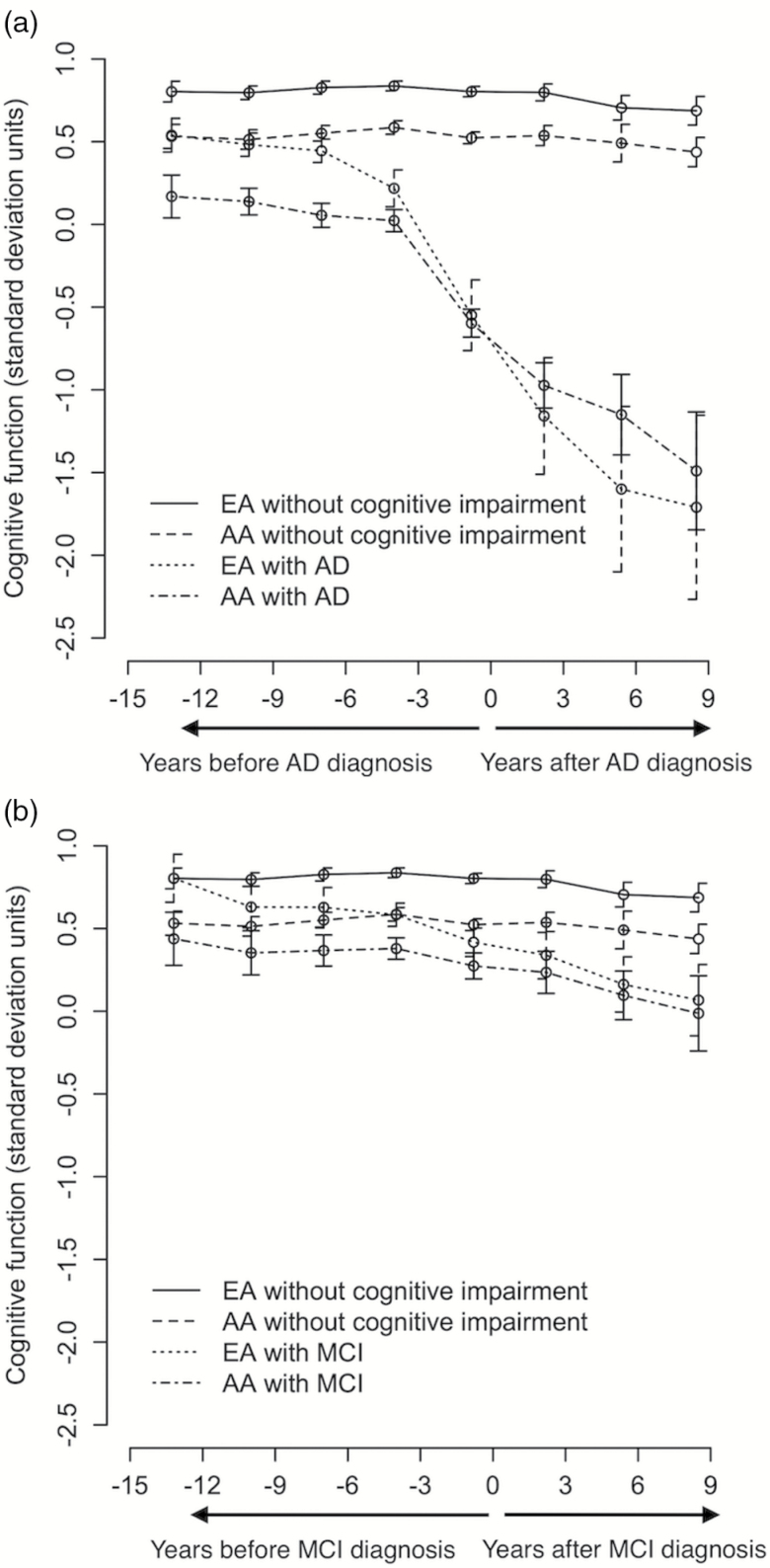
As shown in Figure 1a, AAs developing AD dementia had significantly lower cognitive function scores almost 16 years prior to AD assessment with a rapid decline in cognitive function several years prior to developing AD dementia compared to participants with no cognitive impairment. EAs developing AD dementia had cognitive function test scores similar to AAs with no cognitive impairment but showed rapid cognitive decline reaching levels of cognitive function similar to AAs about the time of clinical diagnosis of AD dementia. A similar pattern in Figure 1b, but less rapid cognitive decline was observed in EAs and AAs developing MCI than participants with no cognitive impairment. EAs developing MCI seemed to exhibit levels of cognition higher than AAs developing MCI.
Figure 1.
Time-specific mean and 95% CIs for change in cognitive function among AA and EA with no cognitive impairment, and developing AD dementia (a) and MCI (b). AA = African Americans; AD = Alzheimer’s disease; EA = European Americans; MCI = mild cognitive impairment.
Linear Estimates of Cognitive Decline
The earliest observable preclinical differences in cognitive function test scores, termed pre-change point cognitive difference, was substantially lower in participants developing clinical AD dementia and MCI than participants with no cognitive impairment (Table 3). However, pre-change point differences in AD dementia and MCI cognitive test scores were not significantly different between AAs and EAs (p = .17).
Table 3.
Linear Model-Based and Change Point Model-Based Estimates of Cognitive Decline (CD measured in SD units per year) Among African Americans and European American Participants With No Cognitive Impairment, and Developing AD Dementia and MCI
| African Americans | European Americans | |
|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | |
| Pre-change point AD dementia level | 0.316 (0.228, 0.404) | 0.274 (0.193, 0.354) |
| Pre-change point MCI level | 0.139 (0.068, 0.209) | 0.095 (0.033, 0.159) |
| Linear model-based estimates | ||
| CD – no cognitive impairment | 0.016 (0.012, 0.020) | 0.021 (0.017, 0.025) |
| CD – developing ad dementia | 0.090 (0.080, 0.100) | 0.157 (0.139, 0.174) |
| CD – developing MCI | 0.034 (0.028, 0.040) | 0.051 (0.043, 0.059) |
| Change point model-based estimates | ||
| Time of change prior to ad dementia | 4.3 (3.9, 4.7) y | 4.7 (4.3, 5.1) y |
| CD – before AD dementia change point | 0.013 (0.009, 0.017) | 0.020 (0.016, 0.024) |
| CD – after AD dementia change point | 0.171 (0.153, 0.189) | 0.233 (0.209, 0.256) |
| CD – after developing AD dementia | 0.150 (0.119, 0.181) | 0.224 (0.177, 0.271) |
| Time of change prior to MCI | 3.6 (3.3, 3.9) y | 3.0 (2.7, 3.3) y |
| CD – before MCI change point | 0.011 (0.007, 0.015) | 0.021 (0.017, 0.025) |
| CD – after MCI change point | 0.084 (0.068, 0.099) | 0.102 (0.084, 0.119) |
| CD – after developing MCI | 0.091 (0.068, 0.115) | 0.107 (0.076, 0.138) |
Note: AD = Alzheimer’s disease; CD = cognitive decline; MCI = mild cognitive impairment.
Linear estimates of cognitive decline suggest faster cognitive decline among EAs than AAs for participants developing clinical AD dementia and MCI, but not among those without any cognitive impairment (Table 3). In a linear mixed model adjusted for age, sex, and educational attainment, participants with no cognitive impairment had a cognitive decline of 0.016—units per year among AAs that was not different from a cognitive decline of 0.021—units per year among EAs (p = .15). EAs developing clinical AD dementia (p < .001) and developing MCI (p = .006) exhibited faster cognitive decline than AAs. Specifically, cognition declined about 75% faster in EAs who developed clinical AD dementia compared to AAs who developed clinical AD dementia.
Change Point Model-Based Estimate of Cognitive Decline
Our graphical analyses suggests that cognitive decline among participants developing clinical AD dementia and MCI might not be uniform during the preclinical phase. Hence, to provide a better understanding of the race-specific cognitive trajectories, we used change point-based models to capture cognitive decline before and after change points among AAs and EAs.
A single cognitive change point among AAs was observed approximately 4.3 (95% CI = 3.9, 4.7) years before developing clinical AD dementia, while this was approximately 4.7 (95% CI = 4.3, 5.1) years among EAs. Cognitive decline before AD dementia change point was similar to linear cognitive decline among those with no cognitive impairment among AAs and among EAs. Cognitive decline after AD dementia change point increased by about 13-fold change per year among AAs, and by about 12-fold change per year among EAs compared to before change point. Cognitive decline after AD dementia change point was significantly higher among EAs than AAs (0.233 vs 0.171; p = .002). The change in cognitive decline after developing AD dementia was not significantly different from the change in cognitive decline after AD dementia change point among AAs (p = .74) and among EAs (p = .87).
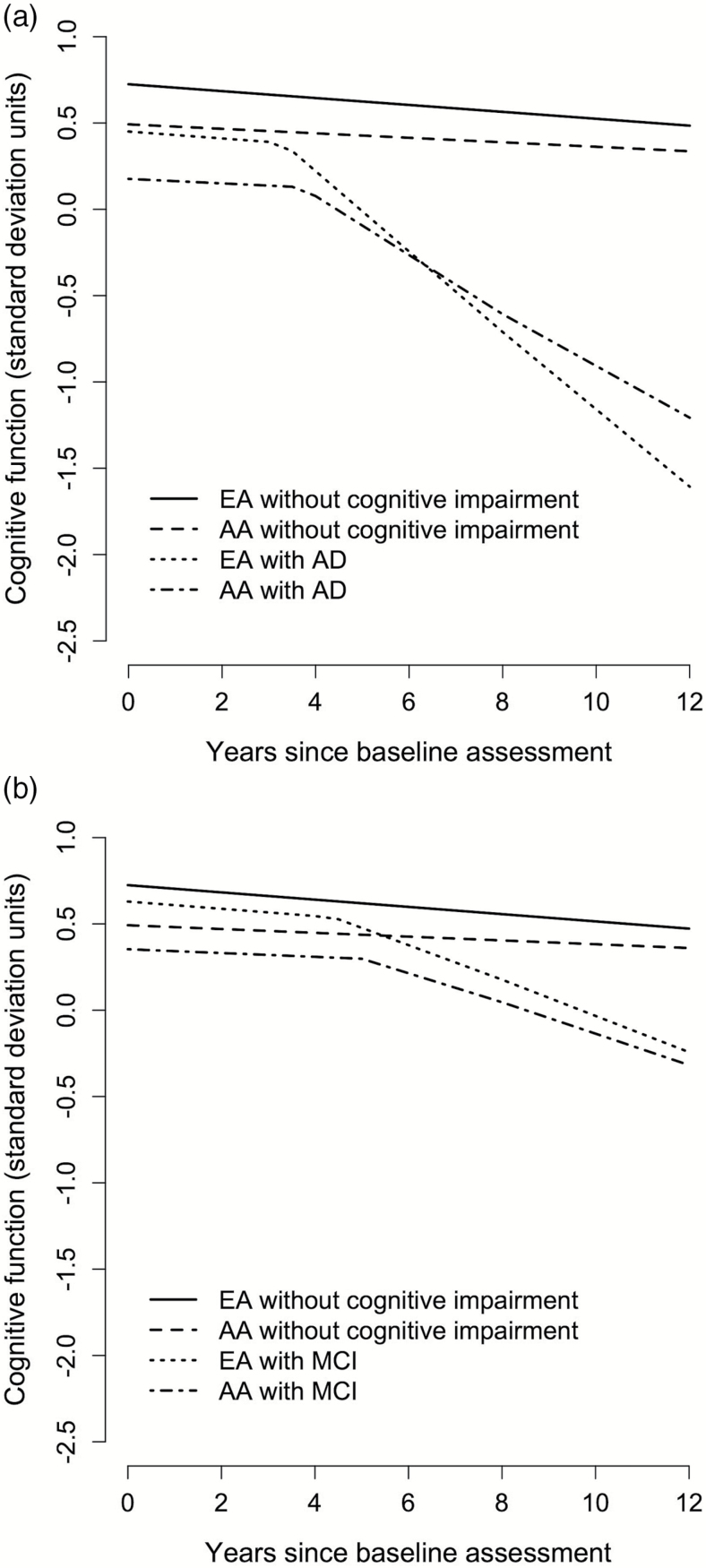
Figure 2a shows the predicted change in cognitive function before and after AD dementia change point and after developing AD dementia over 12 years of follow-up for a hypothetical participant with clinical diagnosis for AD dementia at year 8. AD dementia change point occurred earlier among EAs, and their cognitive decline after AD dementia change point was notably faster than AAs.
Figure 2.
A 12-y course of change in cognitive function of a hypothetical participant with clinical diagnosis for AD dementia (a) and MCI (b) at year 8 for AA and EA. AA = African Americans; AD = Alzheimer’s disease; EA = European Americans; MCI = mild cognitive impairment.
AAs developing MCI had cognitive change point approximately 3.6 years before diagnosis, whereas EAs had cognitive change point approximately 3.0 years before diagnosis. Cognitive decline before MCI change point was similar to participants with no cognitive impairment and participants developing AD dementia among AAs and EAs. Cognitive decline after MCI change point was about 8-fold higher among AAs and about 5-fold higher among EAs. However, the rate of cognitive decline after MCI change point was higher among EAs compared to AAs (0.102 vs 0.084).
Figure 2b shows the predicted change in cognitive function before and after MCI change point and after developing MCI over 12 years of follow-up for hypothetical participants for clinical diagnosis of MCI at year 8. Cognitive change point before MCI change point occurred earlier among AAs, but cognitive decline after MCI change point was somewhat faster among EAs than AAs. A similar trend was observed among AAs and EAs after developing MCI.
Sensitivity Analysis
A sensitivity analyses was performed to examine how time between cognitive assessments impacted our change points. Using an interpolation, annual imputed cognitive assessments were randomly generated from a normal distribution with mean based on the interpolation mean and error based on the variance of the interpolated means. The change point for developing AD dementia remained within a 0.2-year margin, thereby, suggesting that our 3-year interval had very little influence on our change point estimates (data not shown). An additional sensitivity analysis for truncation due to mortality with change points fixed suggested that our cognitive decline estimates were conservative among AAs and EAs, but differences in cognitive decline still persisted between the two groups (Supplementary Table 1).
Discussion
Our study builds on previous studies of change points in cognitive decline (17,18) before dementia in several ways: The data are from 18-year observation of an older population sample, the pre-change point and post-change point courses of people who developed incident AD dementia, MCI, and those remaining without substantial cognitive impairment during the study are clearly distinguished, and pre-change point differences between people who develop AD dementia and MCI and people without substantial cognitive impairment identified. Of substantial relevance, levels of and changes in cognition among AAs and EAs can be compared including pre-change point and post-change point findings: Pre-change point, EAs had higher levels of cognitive performance than AAs, the slopes of decline were similar, and for those developing AD dementia, the change point was slightly earlier for EAs, but the 95% CI for AD dementia change point for EAs overlapped with AAs. Post-change point AA/EA differences were more evident: For both those developing AD dementia and those developing MCI, slopes of cognitive decline were substantially greater for EAs. At baseline, EAs that developed AD dementia had higher level of cognitive function compared to AAs. However, at the time of AD dementia diagnosis, no difference in the level of cognitive function was seen between EAs and AAs with AD dementia. This progression continued to the end of study, where EAs with AD dementia had poorer performance than AAs with AD dementia. This reversing of the level of cognitive function between EAs and AAs was mostly due to higher slope of cognitive decline among AAs following the AD dementia change point.
A clear separation in the cognitive trajectories of participants with no cognitive impairment and participants developing AD dementia and MCI could be seen over the entire duration of our 18-year study. Our sensitivity analysis suggested that our change point estimates were robust to the 3-year interval between cognitive assessments. Given that mortality rates were higher among those developing AD dementia, cognitive decline following the diagnosis of AD dementia was conservative.
The EA findings from our study largely agree with previous cognitive change point studies. A cognitive change point among EAs was found to be around 5 years before dementia diagnosis (17). However, this study did not report pre-change point differences between participants developing AD dementia and MCI and participants with no cognitive impairment, and understanding of such pre-change point differences is an important aspect of our cognitive change point study. Cognitive decline in participants with no cognitive impairment was similar among EAs and AAs, consistent with previous reports (4–8). Earlier reports of race-specific differences may be explained by informative change point-based models: As those EAs with higher cognitive decline (9–13) likely were enriched with subjects who went on to develop AD dementia and MCI, hence, faster cognitive decline could be observed in the combined group. However, a higher fraction of AAs from post-cognitive change point can explain findings of faster cognitive decline compared to EAs (14–16). Our population-level study that did not account for heterogeneity in cognitive trajectories, most importantly, among those who developed AD dementia, reported no significant racial difference in cognitive trajectories (J. Weuve, L. L. Barnes, C. F. Mendes de Leon, et al., unpublished data).
The pattern of steeper cognitive decline among EAs (9–13), who had initial higher level of cognitive function (11,12), is usually observed in individuals with higher cognitive reserve (10,12), since these individuals are usually more resilient to cognitive change but decline faster after a threshold has been reached. The change point curves from Figures 1 and 2 suggest the cognitive reserve phenomenon. EAs have higher education and cognitive function test scores than AAs, which some have suggested reflects higher cognitive reserve (33). Also, years of schooling may be important for baseline cognitive performance when patients have not yet developed a dementia syndrome, confirming the effects of cognitive reserve, but this protection is lost when the burden of Alzheimer’s pathology starts to increase, leading to faster cognitive decline. Cognitive trajectories and change points may also be associated with APOE genotype, cardiovascular disease risk factors, and other factors not examined here.
There are several strengths of our study. Cognitive assessments were performed over 18 years in 2,125 participants with a clinical diagnosis of AD and MCI. Our study sample consists of a large proportion, about 55%, of AAs. The cognitive change point preceding AD in AAs has not been reported in the literature. Our approach partitions cognitive trajectories and provides a change point among those who develop AD and among those who develop MCI. Our data had very little nonparticipation, and we found no evidence to suggest that this was related to cognitive outcomes.
Limitations of this study need to be noted. Data collection cycles were 3 years apart, which may have obscured more short-term changes in cognitive function. Nonetheless, in sensitivity analysis, our estimates of change point times and cognitive trajectories remained steady, suggesting little bias in our estimates in length of follow-up, likely due to continual follow-up of subjects over the entire duration of follow-up cycles. Even though we had a small fraction of participants with non-AD dementia diagnosis, we did not have sufficient sample size to study cognitive preceding non-AD dementia participants. Our analyses are based on a composite measure of cognitive function test scores, which may vary for specific cognitive domains among AAs and EAs using a single change point. One study has found two cognitive change points before clinical diagnosis of AD dementia (34). However, our study does not have enough power to detect a second change point prior to the clinical diagnosis of AD dementia. In our study, we found a change point prior to the diagnosis of AD dementia and MCI using neuropsychological tests. However, it is possible that change points using other markers of preclinical change in cognition preceding AD dementia and MCI may provide an earlier change point than the change points here. Also, the average time to diagnosis for AD dementia was less than 10 years, which may limit our ability to detect a change point earlier than our measurement window.
Early differences in cognitive function exist over about 2 decades prior to developing AD dementia and MCI among both AAs and EAs (1). Consistent with previous results (17,18), a cognitive change point followed by substantially steeper cognitive decline was seen several years prior to diagnosis among those developing AD dementia or MCI among both AAs and EAs. Cognitive decline after the AD dementia change point was steeper among EAs than AAs, even though EAs had higher cognitive scores than AAs. The benefits of cognitive reserve among EAs are lost after the AD dementia change point. The mechanisms underlying this marked acceleration of cognitive decline during the development of dementia need to be examined further. Further research studies are needed to identify factors associated with this cognitive turning point could provide novel targets for interventions designed to delay cognitive disability in old age.
Supplementary Material
Please visit the article online at http://biomedgerontology.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institutes of Health (NIH) grant R01-AG051635. The Chicago Health and Aging Project were funded by National Institutes of Health (NIH) grants R01-AG11101 and R01-AG09683.
Conflict of Interest
The authors report no conflicts of interest.
Supplementary Material
References
- 1. Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology. 2015;85:898–904. doi:10.1212/WNL.0000000000001774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dilworth-Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S; Social, Behavioral and Diversity Research Workgroup of the Alzheimer’s Association Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008;4:305–309. doi:10.1016/j.jalz.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 3. Early DR, Widaman KF, Harvey D, et al. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28:633–645. doi:10.1037/a0031645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atkinson HH, Cesari M, Kritchevsky SB, et al. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi:10.1111/j.1532-5415.2005.53362.x [DOI] [PubMed] [Google Scholar]
- 5. Masel MC, Peek MK. Ethnic differences in cognitive function over time. Ann Epidemiol. 2009;19:778–783. doi:10.1016/j.annepidem.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castora-Binkley M, Peronto CL, Edwards JD, Small BJ. A longitudinal analysis of the influence of race on cognitive performance. J Gerontol B Psychol Sci Soc Sci. 2015;70:512–518. doi:10.1093/geronb/gbt112 [DOI] [PubMed] [Google Scholar]
- 7. Marsiske M, Dzierzewski JM, Thomas KR, et al. Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. J Aging Health. 2013;25(suppl 8):103S–127S. doi:10.1177/0898264313497794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gross AL, Mungas DM, Crane PK, et al. Effects of education and race on cognitive decline: an integrative study of generalizability versus study-specific results. Psychol Aging. 2015;30:863–880. doi:10.1037/pag0000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry. 1999;156:58–65. doi:10.1176/ajp.156.1.58 [DOI] [PubMed] [Google Scholar]
- 10. Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry. 2005;13:968–975. doi:10.1176/appi.ajgp.13.11.968 [DOI] [PubMed] [Google Scholar]
- 11. Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community-dwelling older adults. Gerontology. 2009;55:32–40. doi:10.1159/000137666 [DOI] [PubMed] [Google Scholar]
- 12. Wolinsky FD, Bentler SE, Hockenberry J, et al. A prospective cohort study of long-term cognitive changes in older Medicare beneficiaries. BMC Public Health. 2011;11:710. doi:10.1186/1471-2458-11-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older Black and White persons. Psychol Aging. 2015;30:279–285. doi:10.1037/pag0000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. J Gerontol B Psychol Sci Soc Sci. 2005;60:P242–P250. [DOI] [PubMed] [Google Scholar]
- 15. Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: results from the AHEAD sample. Res Aging. 2007;29:73–94. doi:10.1177/0164027506294245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170:331–342. doi:10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19:1555–1566. [DOI] [PubMed] [Google Scholar]
- 18. Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi:10.1001/archneurol.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5:349–355. [DOI] [PubMed] [Google Scholar]
- 20. Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- 21. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 22. Smith A. Symbol Digits Modalities Test. Los Angeles, California: Western Psychological Services; 1982. [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24. Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54:P155–P160. [DOI] [PubMed] [Google Scholar]
- 25. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 26. Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LE, Evans DA. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951–955. doi:10.1212/WNL.0b013e3181d64786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi:10.1212/01.WNL.0000152982.47274.9E [DOI] [PubMed] [Google Scholar]
- 28. Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66:767–772. doi:10.1001/archneurol.2009.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng G, Chen Z. Comparison of maximum statistics for hypothesis testing when a nuisance parameter is present only under the alternative. Biometrics. 2005;61:254–258. doi:10.1111/j.0006-341X.2005.030531.x [DOI] [PubMed] [Google Scholar]
- 30. SAS Institute Inc. SAS 9.4 Help and Documentation. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 31. Rizopoulos D. Joint Models for Longitudinal and Time-to-Event Data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. Version 2.15.2. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 33. Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 34. Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging. 2012;27:1008–1017. doi:10.1037/a0029857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.