Abstract

Objectives:
Skin graft failure is a recognised complication in the treatment of major burns. Little research to date has analysed the impact of the complex physiological management of burns patients on the success of skin grafting. We analysed surgical and anaesthetic variables to identify factors contributing to graft failure.
Methods:
Inclusion criteria were admission to our Burns Intensive Care Unit (BICU) between January 2009 and October 2013 with a major burn. After exclusion for death before hospital discharge or prior skin graft at a different hospital, 35 patients remained and were divided into those with successful autografts (n=16) and those with a failed autograft (n=19). For the purposes of this study, we defined poor autograft viability as requiring at least one additional skin graft to the same site. Logistic regression of variables was performed using SPSS (Version 22.0 IBMTM).
Results:
Age, Sex, %Total Burn Surface Area or Belgian Outcome Burns Injury score did not significantly differ between groups. No differences were found in any surgical factor at logistic regression (graft site, harvest site, infection etc.). When all operations were analysed, the use of colloids was found to be significantly associated with graft failure (p=0.035, CI 95%) and this remained significant when only split thickness skin grafts (STSGs) and debridement operations were included (p=0.034, CI 95%). No differences were found in crystalloid use, intraoperative temperature, pre-operative haemoglobin and blood products or vasopressor use.
Conclusions:
This analysis highlights an independent association between colloids and graft failure which has not been previously documented.
Keywords: Graft failure, burns, skin graft, colloids, anaesthetic management, burns intensive care unit
Lay Summary


After large burns injuries, patients require fluid to be given via an IV drip. This is often very large volumes of fluid, much higher than would normally be required in other medical conditions. When the area of burn is very large multiple operations are often required and during these operations, IV fluids are again used to keep the blood pressure normal and keep the patient hydrated. We looked at the hospital records of 35 patients to see if there was any link between the drugs and fluids given during these operations and the success of the skin grafts performed in these operations. We found that using a particular type of fluid called a colloid fluid, which allows smaller volumes to be given for the same effect on blood pressure and hydration, was more common in patients whose skin grafts subsequently required further surgery due to poor healing. A larger study would now be required to see if this type of fluid is causing poor healing or whether it is a coincidental finding only.
Introduction
The treatment of burns is complex due to the combination of hypermetabolic physiological response and the need for good functional and cosmetic recovery. Critical care input is often required and a recent systematic review of European burns epidemiology data revealed that up to 22% of patients presenting with burns require specialist treatment on a Burns Intensive Care Unit (BICU).1 Although the global incidence of burns is decreasing and survival has increased predominantly due to the advent of early excision and grafting,2,3 mortality from late complications persist. The major cause of death from burns is now pneumonia secondary to inhalational injury.2,3 Reducing the time spent on ventilators and in BICU where patients are susceptible to increasing numbers of multi-resistant bacteria can only help to further reduce mortality.
In addition to maintaining end organ perfusion, fluid resuscitation also helps prevent progression of burns. Three zones within a burn wound are described: necrotic; static; and hyperaemic. With physiological optimisation of the patient, the areas of stasis can be prevented from becoming necrotic4 and may therefore remain viable. Early debridement of the necrotic areas, usually within 48 h, reduces the hypermetabolic response to burns injury5 and early skin grafting is beneficial to prevent contractures.4 Split-thickness skin grafts (STSGs) are commonly used to cover large areas of burn and allow re-harvesting from the donor site.4 STSG failure is usually caused by infection, haematoma or seroma formation and necrosis; failed STSGs often require re-grafting.4
Although a multi-specialty approach to burns patients has been developed in the modern BICU, there is little research at present on wider factors which may influence graft failure such as the effect of interventions made by intensivists. Although infection and bacterial colonisation are widely known to cause graft failure,6 there are often instances of graft failure in non-infected grafts. Considering the hypermetabolic status of the patient at the time of grafting and the sometimes complex co-morbidities in this group of patients, it is likely that there are other factors which contribute and it is this which was the focus of our study.
Our BICU is a tertiary referral centre for London and patients are jointly managed by intensive care specialists, specialist burns surgeons and burns anaesthetists. The aim of our study was to assess whether interventions made intraoperatively contributed to autograft failure.
Methods
Clinical governance
This study followed the UK Good Code of Practice (GCP) in research, Patient’s Protection Act 1998 and had obtained NHS approval, Registration Reference Number 506.
Study sample
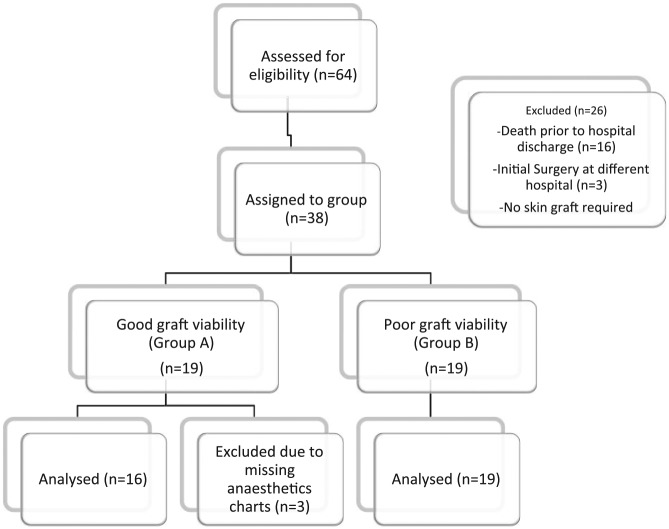
The cases-notes of any patient admitted to BICU with a major burn (as defined by the American Burns Association, Table 1) at Chelsea and Westminster Hospital (London, UK) between January 2009 and October 2013 were retrospectively reviewed. Data were collected from surgical and anaesthetic records on all surgical procedures for each patient. After exclusion for death prior to hospital discharge, initial skin grafting before admission to Chelsea and Westminster and missing anaesthetics records, 35 patients’ records remained for analysis. Of these records, there were a total 191 surgical procedures performed. The patients who were included were divided into two groups based on whether they had any degree of autograft failure at any point. In the group with entirely successful autografts (Group A) there were 16 patients with 53 surgical procedures. In the group of patients who had any degree of autograft failure (Group B, n = 19) there were 138 surgical procedures (Figure 1). Graft failure was defined as any graft which required return to theatre for re-grafting as identified by the surgical team.4 A total of 191 surgical procedures were included in the analysis.
Table 1.
| American Burn Association Major Burn Criteria |
|---|
| >25% TBSA in any age group |
| >20% TBSA aged <10 years or >40 years |
| Full thickness burns >10% TBSA |
| Burns to the perineum, genitalia, face or neck or burns likely to cause function impairment (e.g. burns to joints) |
| Concomitant inhalational injury |
| Electrical burns |
| Burns associated with other major trauma (e.g. head injury, fractures) |
| Patients with multiple co-morbidities |
Figure 1.
CONSORT 2010 diagram showing patient selection.
Study variables
Data were collected by the researchers on any variable likely to affect skin graft viability. These were divided into patient factors, surgical factors and anaesthetic factors. Surgical and patient factors were collected for each patient and the anaesthetic factors were collected for each surgical procedure for each patient. All variables analysed are listed in Table 2. Patient factors included age, % total burn surface area (TBSA), Belgian Outcome of Burns Injury (BOBI) score, pre-existing co-morbidities and systemic complications of injury. Surgical factors were sites of autograft, sites of skin harvest, burn aetiology, length of stay and total number of surgeries for each patient. Wound colonisation by type of bacteria isolated on skin swab was also analysed along with number of different antimicrobials given per patient. Anaesthetics data were collected on intraoperative management and analysed type and total volume of fluid (crystalloid and colloid) and blood products, preoperative haemoglobin (Hb), inotrope use, intraoperative temperature and choice of inhalational agent.
Table 2.
Table to show variables analysed by category.
| Patient variables | Surgical variables | Anaesthetic variables |
|---|---|---|
|
|
|
Data analysis
Given the large number of categorical variables, a descriptive analysis of all data was performed initially. Fisher’s exact test (two-tailed) and T-tests were employed to identify variables where significant differences existed between the groups. Variables which showed significant differences (P <0.05) were entered into univariate logistic regression. Those which maintained significance were taken forward to the multivariate logistic regression and combined with the anaesthetic data.
Anaesthetics data were collected for each of the 191 surgical procedures performed. Categorical variables were again initially analysed by descriptive statistics. Univariable logistic regression was carried out for continuous data. Important variables which were likely to be confounders were also carried forwards to the multivariate analysis. Temperature was analysed by quartile to account for a number of missing data. The minimum and maximum temperatures from each operation were recorded where available. Surgical procedures fell into three categories: debridement only (n = 32); STSGs (n = 81); and change of dressings (CODs) (n = 78). Three different multivariate regression analyses were performed according to surgery type. STSG only, STSG + Debridement and All Surgical Procedure. As there was a significant difference in the number of surgical procedures between the two groups, repeated measures were accounted for by performing averages for each variable for each patient so that each patient had equal weighting within the analysis.
The multivariate logistic regression was performed with backwards exclusion of variables. Important covariates were included throughout. Data analysis was performed using SPSSTM Version 22.0 (IBM). Hosmer and Lemeshow test was used to confirm goodness of fit of the model.
Results
Of the 35 patients included in the final analysis, a total of 16 had successful autografts with 53 surgical procedures between them (group A) and 19 had autografts which required at least one further surgery with a total 138 surgical procedures between them (group B). The length of total hospital stay was significantly longer in patients who had autograft failure compared to those who did not (83.84 days vs. 36.19 days, P = 0.05) and the length of stay on BICU was longer although not significantly (22.84 days vs. 12.56 days, P = 0.129). No significant differences were found in age, BOBI score or TBSA between the groups (Table 3). A higher proportion of patients in the graft failure group were women but this was not significant (47.3% vs. 25%, P = 0.29).
Table 3.
Breakdown of patient characteristics by group. Length of hospital stay, number of surgeries and length of follow-up were significantly longer in those with graft failure.
| Variable | Median | Range | P value* | |
|---|---|---|---|---|
| BOBI score | Group A (n = 16) | 2 | 0–5 | 0.448 |
| Group B (n = 19) | 2 | 0–7 | ||
| TBSA% | Group A (n = 16) | 19 | 1–90 | 0.167 |
| Group B (n = 19) | 31 | 3–90 | ||
| Age | Group A (n = 16) | 38.38 | 17.72–67.09 | 0.397 |
| Group B (n = 19) | 42.78 | 16.07–88.72 | ||
| Surgeries (n) | Group A (n = 16) | 2 | 1–11 | 0.003 |
| Group B (n = 19) | 7 | 2–18 | ||
| BICU duration (days) | Group A (n = 16) | 4 | 1–66 | 0.129 |
| Group B (n = 19) | 14 | 1–60 | ||
| Hospital stay (days) | Group A (n = 16) | 21 | 5–164 | 0.005 |
| Group B (n = 19) | 70 | 21–194 | ||
| Inhalational injury | Group A (n = 16) | n = 6 | N/A | 1.000 |
| Group B (n = 19) | n = 7 | |||
| Follow-up days | Group A (16) | 139 | 0–945 | 0.033 |
| Group B (19) | 391 | 0–1458 |
Using Fisher’s exact (two-tailed) and T-test.
Descriptive statistics
No differences were found in descriptive analysis of the site grafted, wound colonisation and antibiotics usage or burn aetiology. Significantly more patients in the failure group had autograft taken from the buttock (P = 0.049). No other differences in the site of autograft harvest were found between the groups. Equally, Matriderm biological scaffold was used in significantly more patients who had graft failure than those who did not (P = 0.049). Interestingly there were more diabetic patients in the successful graft group as opposed to the graft failure group (4 vs. 0, P = 0.035). There was no difference in the rates of any other pre-existing co-morbidities or systemic complications. No differences were found in rates of graft colonisation with Gram-positive, Gram-negative or mixed bacteria. No differences were found in rates of MRSA colonisation between patients or use of multiple courses of antibiotics. A full table of descriptive statistics is found in appendix i.
In terms of the descriptive analysis of anaesthetic data, no differences in choice of inhalational agent were found (Table 4). All operations used an inhalational maintenance agent. There was no use of total intravenous anaesthesia (TIVA). Platelets were only given in 4/191 operations (2%) and fresh frozen plasma in 15/191 operations (7.8%). Neither were found to be significant by two-tailed T-test and so were not carried forward to the logistic regression analysis. No significant differences were found in minimum and maximum intraoperative temperatures when analysed using Fisher’s exact test (Table 5). Noradrenaline infusion was used in 10/53 (18.87%) surgeries in Group A and 36/138 (26.09%) surgeries in Group B. This was not found to be significant (P = 0.348, Fisher’s exact two-sided). Metaraminol and Ephedrine were given as boluses and were therefore analysed by univariate logistic regression.
Table 4.
Maintenance inhalational agent by group.
| Variable | Group | n | P value* |
|---|---|---|---|
| Sevofluorane | Group A (n = 16) | 59 | 0.280 |
| Group B (n = 19) | 17 | ||
| Isoflourane | Group A (n = 16) | 53 | 0.257 |
| Group B (n = 19) | 32 | ||
| Desfluorane | Group A (n = 16) | 8 | 0.726 |
| Group B (n = 19) | 2 |
Using Fisher’s exact (two-tailed).
Table 5.
Table showing difference between groups in terms of minimum and maximum intraoperative temperature. Temperatures were divided by interquartile range. No differences were found in proportion of missing data between the groups.
| Temperature by quartile | Group | n | P value* |
|---|---|---|---|
| MinQ1 | Group A (n = 16) | 8 | 0.319 |
| Group B (n = 19) | 31 | ||
| MinQ2 | Group A (n = 16) | 13 | 0.210 |
| Group B (n = 19) | 22 | ||
| MinQ3 | Group A (n = 16) | 11 | 0.847 |
| Group B (n = 19) | 32 | ||
| MinQ4 | Group A (n = 16) | 14 | 0.231 |
| Group B (n = 19) | 25 | ||
| MinMissing | Group A (n = 16) | 7 | 0.301 |
| Group B (n = 19) | 29 | ||
| MaxQ1 | Group A (n = 16) | 11 | 0.842 |
| Group B (n = 19) | 27 | ||
| MaxQ2 | Group A (n = 16) | 11 | 1.000 |
| Group B (n = 19) | 30 | ||
| MaxQ3 | Group A (n = 16) | 13 | 0.436 |
| Group B (n = 19) | 27 | ||
| MaxQ4 | Group A (n = 16) | 11 | 0.677 |
| Group B (n = 19) | 24 | ||
| MaxMissing | Group A (n = 16) | 7 | 0.223 |
| Group B (n = 19) | 30 |
Using Fisher’s exact (two-tailed).
Univariate logistic regression
None of the differences found between the groups in terms of buttock harvest site, matriderm scaffold or pre-existing diabetes retained significance when entered in a univariate logistic regression. These were therefore not carried forwards to multivariate regression.
In univariate analysis, colloid use was significantly associated with graft failure in the analysis of STSG only surgical procedures (P = 0.040) and retained near significance in the STSG + Debridement surgical procedures analysis (P = 0.080) and All Surgical Procedure analysis (0.063) (Table 6).
Table 6.
Univariate analysis of anaesthetics data.
| STSG only | STSG + Debridement | All Surgical Procedure | ||||
|---|---|---|---|---|---|---|
| OR | P value | OR | P value | OR | P value | |
| Colloid | 1.002 | 0.040 | 1.002 | 0.080 | 1.003 | 0.063 |
| Crystalloid | 1.000 | 0.309 | 1.000 | 0.593 | 1.000 | 0.341 |
| Preop Hb | 0.952 | 0.752 | 0.976 | 0.896 | 0.862 | 0.460 |
| Red blood cells | 1.288 | 0.273 | 1.481 | 0.172 | 1.357 | 0.352 |
| Metaraminol | 0.861 | 0.361 | 0.779 | 0.227 | 0.695 | 0.152 |
| Ephedrine | 1.060 | 0.445 | 1.041 | 0.572 | 1.055 | 0.569 |
Multivariate logistic regression
There was no difference in crystalloid, preoperative Hb or inotrope use between the groups. In the STSG only and All Surgical Procedure analysis, colloid use remained positively associated with graft failure in multivariate logistic regression (Tables 7 and 8). In the STSG + Debridement models, colloids were not significant although the P value was 0.056 (Table 9).
Table 7.
Multivariate logistic regression STSG only (Hosmer and Lemeshow; Chi squared 6.217, df 7, Sig. 0.515).
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Pre-op Hb | 1.037 | 0.709 | 1.518 | 0.850 |
| Crystalloid | 1.000 | 0.999 | 1.000 | 0.384 |
| Colloid | 1.003 | 1.000 | 1.005 | 0.036 |
| Metaraminol | 0.782 | 0.528 | 1.157 | 0.218 |
| Ephedrine | 1.100 | 0.952 | 1.272 | 0.194 |
Table 8.
Multivariate logistic regression for All Surgical Procedures (Hosmer and Lemeshow; Chi-squared 11.711, df 7, sig 0.110).
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Preop Hb | 0.981 | 0.590 | 1.634 | 0.943 |
| Crystalloid | 1.000 | 0.999 | 1.001 | 0.551 |
| Colloid | 1.004 | 1.000 | 1.007 | 0.036 |
| Metaraminol | 0.567 | 0.272 | 1.181 | 0.129 |
| Ephedrine | 1.086 | 0.874 | 1.348 | 0.943 |
Table 9.
Multivariate logistic regression for STSG + Debridement (Hosmer and Lemeshow; Chi-squared 11.173, dF 7, Sig. 0.131).
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Preop Hb | 1.073 | 0.713 | 1.615 | 0.735 |
| Crystalloid | 1.000 | 0.999 | 1.000 | 0.325 |
| Colloid | 1.002 | 1.000 | 1.004 | 0.056 |
| Metaraminol | 0.596 | 0.940 | 1.280 | 0.238 |
| Ephedrine | 1.097 | 0.470 | 1.234 | 0.269 |
Discussion
Principal findings
An indisputable finding of our study is that patients who had graft failure were seriously disadvantaged in terms of overall length of hospital stay, length of stay on BICU, length of follow-up and total numbers of operations. Since there was very little difference in terms of age and severity of initial injury, we can suggest that this discrepancy was due to the graft failure itself. Therefore, graft failure is a serious complication and indirectly increases the risk of hospital-acquired infections associated with BICU stay and subsequently requires longer periods of follow-up for rehabilitation and reconstructive surgeries when finally discharged from hospital. From the perspective of the health service this also results in significantly increased costs.
Ultimately no significant differences were found in terms of surgical factors meaning that autograft failure was not found to be influenced by site of the burn, site of skin harvest, biological scaffold use and graft colonisation or infection.
Our finding that there was an association between total volume of colloid use intraoperatively and skin graft failure is interesting and is further discussed below. Only fluids given intraoperatively were analysed in this study. Although the monitoring of specific fluid balance was beyond the scope of this paper, what was measured was the total colloid and total crystalloid use in each surgery. Larger volumes of colloids were given intraoperatively in the graft failure group but the overall fluid volumes given were the same between groups meaning that the group with graft failure were given higher proportions of colloids rather than larger volumes of fluids overall.
Comparison with existing literature
To our knowledge this is the first study to look at the how anaesthetic factors may specifically affect the likelihood of graft failure. It is widely accepted that bacterial colonisation and infection of grafts may result in failure.4,6 There is still debate about the benefits and drawbacks of the use of colloids. On the one hand, fluid overload is known to be associated with poorer outcomes in ITU patients and colloids allow smaller volumes to be given for the same effect on preload. Theoretically a ratio of 1:4 has been quoted although a recent meta-analysis found that on average only 1.5 times more crystalloid than colloid was required to produce the same effect on haemodynamics including for sepsis.7 On the other hand, the large colloid particles stay in the body for longer and in patients with septic shock it is hypothesised that these particles leak out into the peripheral tissues due to increased vascular permeability. This is likely due to disruption of the glycocalyx layer on the endothelium which is thought to be the first barrier to physiological intravascular colloid loss.8 Once in the tissues, the colloid particles cause increased oncotic pressure resulting in oedema. The shock seen in burns injury is similar to that seen in sepsis and it could be this effect that may negatively impact graft healing.
Synthetic colloids have been in use for more than 40 years and classically concerns have focused on the use of hydroxyethyl starch based solutions which have been found in randomised controlled trials to be associated with increased kidney injury and, in patients with sepsis, death. In the UK their use has fallen out of favour due to these safety concerns.9 A recent study published in the BMJ looked at the use of 6% HES and 5% albumin solutions in perioperative management of elective surgery and found that patients receiving these fluids had a higher incidence of acute kidney injury and other complications compared with patients who had received neither.10 This shows clearly that certain types of colloids present safety concerns even in non-critically ill patients. It is not only hydroxyethyl starch based solutions which have been associated with increased rates of renal failure however. Gelatin solutions have also been found to results in higher numbers of patients requiring renal replacement therapy compared to those given only crystalloids.11 All of the patients in our study received gelatin-based colloids as this was the colloid in use at our hospital during the dates of the study. An animal model which evaluated the use of colloids on wound healing in rats found that gelatin-based solutions were detrimental to wound healing when compared with HES solutions and crystalloids.12 This was hypothesised to be due to the effect of clot formation. To our knowledge, no studies have previously identified a link between colloid use and graft failure.
Strengths and limitations
The key strength of our data lies in the number of variables considered. We looked at both the surgical and anaesthetic factors which may lead to autograft failure and found no difference in the key variables which are often quoted as the cause of skin graft failure such as temperature and infection. Equally our groups were well matched in terms of age and TBSA%. We limited our study to patients in intensive care so that they were reasonable well matched in terms of physiological stability. There were no significant differences in the instability of patients between the two groups when factors such as preoperative Hb, intraoperative temperature and vasopressor use are taken as proxy measures of this.
We had small numbers of patient in both groups, which may limit the size of the effects seen. We also excluded patients who had died as it was impossible to tell whether had they survived, they would have had completely successful skin grafts. It is difficult in a single-centre study to produce larger numbers of patients given the relative rarity of severe burn injuries. Additionally, graft failure is ill-defined in the pre-existing literature. For our study we defined skin graft failure as any patient who required a return trip to theatre for re-grafting. If only part of the skin graft fails to take, then there is likely to be variability between individual surgeons over which patients may require re-grafting and which may not.
Conclusions and further works
Although we have small numbers of patients included in the study, the finding that colloids could be associated with graft failure is interesting and has a theoretical basis to support it. This study did not find differences in the numbers of colonised skin grafts meaning that this commonly accepted cause of graft failure has not compounded our findings. A further prospective study with larger numbers is required to determine whether the association of colloid use with autograft failure is indeed a true finding. It would also be important to look at the types of fluids used during initial resuscitation and maintenance on BICU.
Acknowledgments
We would like to acknowledge the help of our statistician Sudhiya Mandalia for her advice on the correct statistical methods
Appendix
Appendix i.
Surgical data descriptive statistics.
| Variable | Group | n | P value* | Variable | Group | n | P value* | Variable | Group | n | P value* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graft site | Neck | A (n = 16) | 9 | 0.3179 | Biological scaffold | Alloderm | A (n = 16) | 1 | 1.0000 | Co-morbidity | Neurological | A (n = 16) | 3 | 0.0856 |
| B (n = 19) | 7 | B (n = 19) | 1 | B (n = 19) | 0 | |||||||||
| Head | A (n = 16) | 12 | 0.4928 | Matriderm | A (n = 16) | 0 | 0.0493 | Endocrine | A (n = 16) | 1 | 0.4571 | |||
| B (n = 19) | 12 | B (n = 19) | 5 | B (n = 19) | 0 | |||||||||
| Anterior Trunk | A (n = 16) | 6 | 0.1811 | Biobrane | A (n = 16) | 2 | 1.000 | Renal | A (n = 16) | 1 | 1.000 | |||
| B (n = 19) | 12 | B (n = 19) | 3 | B (n = 19) | 1 | |||||||||
| Posterior trunk | A (n = 16) | 6 | 0.3145 | Recell | A (n = 16) | 0 | 0.1088 | |||||||
| B (n = 19) | 11 | B (n = 19) | 4 | |||||||||||
| Left arm | A (n = 16) | 12 | 1.000 | MySkin | A (n = 16) | 1 | 1.000 | Aetiology | Unknown | A (n = 16) | 1 | 0.4571 | ||
| B (n = 19) | 15 | B (n = 19) | 2 | B (n = 19) | 0 | |||||||||
| Right arm | A (n = 16) | 12 | 1.000 | Electrical | A (n = 16) | 1 | 1.000 | |||||||
| B (n = 19) | 2 | B (n = 19) | 14 | |||||||||||
| Buttock | A (n = 16) | 3 | 1.000 | Infection | MRSA + | A (n = 16) | 0 | 0.2336 | Chemical | A (n = 16) | 1 | 0.4571 | ||
| B (n = 19) | 3 | B (n = 19) | 0 | B (n = 19) | 3 | |||||||||
| Genitalia | A (n = 16) | 1 | 1.000 | >4 Antibiotics | A (n = 16) | 4 | 0.4759 | Scald | A (n = 16) | 0 | 1.000 | |||
| B (n = 19) | 2 | B (n = 19) | 8 | B (n = 19) | 1 | |||||||||
| R leg | A (n = 16) | 8 | 1.000 | No isolated bacteria | A (n = 16) | 7 | 0.3110 | Contact | A (n = 16) | 1 | 1.000 | |||
| B (n = 19) | 10 | B (n = 19) | 5 | B (n = 19) | 1 | |||||||||
| L leg | A (n = 16) | 5 | 0.1759 | Gram +ve only | A (n = 16) | 0 | 1.000 | Flash | A (n = 16) | 0 | 0.4891 | |||
| B (n = 19) | 11 | B (n = 19) | 0 | B (n = 19) | 2 | |||||||||
| Gram –ve only | A (n = 16) | 5 | 0.4906 | Flame | A (n = 16) | 12 | 0.7233 | |||||||
| B (n = 19) | 9 | B (n = 19) | 13 | |||||||||||
| Donor Site | Thigh | A (n = 16) | 10 | 0.2453 | Both Gram +ve and Gram –ve | A (n = 16) | 3 | 0.7003 | Median (range) | |||||
| B (n = 19) | 16 | B (n = 19) | 5 | |||||||||||
| Buttock | A (n = 16) | 0 | 0.0493 | Yeasts | A (n = 16) | 2 | 1.0000 | Surgery type | Total no. surgeries | A (n = 16) | 2 (1–11) | 0.03 | ||
| B (n = 19) | 5 | B (n = 19) | 3 | B (n = 19) | 7 (2–18) | |||||||||
| Abdomen | A (n = 16) | 2 | 0.0712 | Debridement | A (n = 16) | 0 (0–1) | 0.131 | |||||||
| B (n = 19) | 8 | B (n = 19) | 0 (0–3) | |||||||||||
| Back | A (n = 16) | 4 | 0.2928 | Co-morbidity | Smoker | A (n = 16) | 11 | 0.4906 | Autograft | A (n = 16) | 1 (0–2) | 0.072 | ||
| B (n = 19) | 9 | B (n = 19) | 10 | B (n = 19) | 3 (0–6) | |||||||||
| Leg/feet | A (n = 16) | 4 | 0.2928 | Diabetic | A (n = 16) | 4 | 0.0348 | Allograft | A (n = 16) | 1 (0–6) | 0.361 | |||
| B (n = 19) | 9 | B (n = 19) | 0 | B (n = 19) | 2 (0–5) | |||||||||
| Scalp | A (n = 16) | 1 | 1.0000 | Illicit drugs | A (n = 16) | 2 | 0.5820 | Auto + Allo | A (n = 16) | 0 (0–2) | 0.728 | |||
| B (n = 19) | 2 | B (n = 19) | 1 | B (n = 19) | 0 (0–2) | |||||||||
| Upper limb | A (n = 16) | 0 | 0.2336 | Alcohol | A (n = 16) | 5 | 0.2075 | SW | A (n = 16) | 0 (0–5) | 0 | |||
| B (n = 19) | 3 | B (n = 19) | 2 | B (n = 19) | 0 (0–3) | 0.561 | ||||||||
| Psychiatric | A (n = 16) | 4 | 0.4928 | COD | A (n = 16) | 0 (0–3) | 0.000 | |||||||
| B (n = 19) | 7 | B (n = 19) | 1 (0–8) | |||||||||||
| Cardiovascular | A (n = 16) | 5 | 0.4236 | Tracheostomy | A (n = 16) | n = 3 | 0.4605 | |||||||
| B (n = 19) | 3 | B (n = 19) | n = 6 | |||||||||||
| Respiratory | A (n = 16) | 3 | 0.6418 | Escharotomy | A (n = 16) | n = 2 | 0.6657 | |||||||
| B (n = 19) | 2 | B (n = 19) | n = 4 |
Footnotes
Declaration of conflicting interests: The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The anaesthetics data were originally presented by CE Isitt and KA McCloskey at ISICEM conference in Brussels, Belgium in March 2015 and the surgical data were presented at the Nordic Burn meeting in Linkoping, Sweden in May 2014 by A Caballo.
Ethical approval: The authors confirm that the necessary written, informed consent was obtained from patients for this article. This study followed the UK Good Code of Practice (GCP) in research, Patient’s Protection Act 1998 and obtained NHS approval, Registration Reference Number 506.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Chelsea and Westminster Health Charity Research Fund Number 556.
References
- 1. Brusselaers N, Monstrey S, Vogelaers D, et al. Severe burn injury in Europe: a systematic review of the incidence, aetiology, morbidity and mortality. Critical Care 2010; 14(5):R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harbin KR, Norris TE. Anaesthetic management of patients with major burn injury. AANA Journal 2012; 80(6): 430–439. [PubMed] [Google Scholar]
- 3. Weber DJ, van Duin D, DiBiase L, et al. Healthcare-associated infections among patients in a large burn intensive care unit: incidence and pathogens, 2008–2012. Infect Control Hosp Epidemiol 2014; 35(10): 1304–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orgill DP, Ogawa RO. Current methods of burn reconstruction. Plast Reconstr Surg 2013; 131(5): 827e–836e. [DOI] [PubMed] [Google Scholar]
- 5. Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004; 363: 9424. [DOI] [PubMed] [Google Scholar]
- 6. Ryseel H, Gazyakan E, Germann G, et al. Antiseptic therapy with a polyacticacid-acetic acid matrix in burns. Wound Repair Regen 2010; 18: 439–444. [DOI] [PubMed] [Google Scholar]
- 7. Orbegozo Cortes D, Gamarano Barros T, Nijmi H, et al. Crystalloids versus colloids: exploring differences in fluid requirements by systematic review and meta-regression. Anesth Analg 2015; 120(2): 389–402. [DOI] [PubMed] [Google Scholar]
- 8. Aditianingsih D, George YWH. Guiding principles of fluid and volume therapy. Best Pract Res Clin Anaesthesiol 2014; 28: 249–260. [DOI] [PubMed] [Google Scholar]
- 9. Hartog CS, Nantanson C, Sun J, et al. Concerns over use of hydroxyethyl starch solutions. BMJ 2014; 349: g5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Operer M, Poeran J, Rasul R, et al. Use of perioperative Hydroxyethyl starch 6% and albumin 5% in elective joint arthroplasty and association with adverse outcomes: a retrospective population based analysis. BMJ 2015; 350: h1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayer O, Schwarzkopf D, Doenst T, et al. Perioperative fluid therapy with tetrastarch and gelatin in cardiac surgery: a prospective sequential analysis. Crit Care Med 2013; 41(11): 2532–2542. [DOI] [PubMed] [Google Scholar]
- 12. Eroglu E, Eroglu F, Yavuz L, et al. The effect of colloidal fluid replacement on wound healing in an experimental sublethal hemorrhagic shock model. Eur J Emerg Med 2005; 12(6): 282–284. [DOI] [PubMed] [Google Scholar]