Abstract
Keloid and hypertrophic scars are unique human dermal fibroproliferative disorders of the injured skin and are associated with pain, itch and can cause functional limitations. A number of genetic, systemic and local factors have been identified in the formation of keloids and hypertrophic scars. Studies have shown that adipose-derived stem cells have angiogenic and antiapoptotic properties which has effects on wound healing, soft-tissue restoration and scar remodelling, and thus may have a role in managing keloid scaring. However, this role is not well described in the literature. A systemic review of available literature was thus undertaken, regarding the use of fat grafting in treatment of keloids and hypertrophic scarring. In total, 858 articles were identified, with ten studies ultimately fulfilling inclusion criteria. There were no studies specifically isolating the keloids and hypertrophic group of patients, and thus quantitative data were completely lacking from the literature. There were, however, individual cases described, and qualitatively encouraging clinical results were reported for the use of fat grafting on keloids and hypertrophic scars. Combined with the current theoretical and immunohistochemical understanding through other laboratory and animal studies, fat grafting may play a role in the treatment of keloids and hypertrophic scaring; however, specific evidence is currently lacking. The role for further research is clear.
Keywords: Keloid, hypertrophic, scar, management, injection, scarring
Lay Summary:
Keloid and hypertrophic scars are difficult to treat conditions. Some studies have suggested that fat cells may have a role in managing these scars although this role is not well described in the literature. We perform a systematic review of available literature, examining the evidence for this.
Background
Keloids and hypertrophic scars are unique human dermal fibroproliferative disorders of the injured skin.1 They are associated with pain, itching and sensation of contraction, and can cause functional limitations.2–4 A number of genetic, systemic and local factors have been identified to affect the formation of keloids and hypertrophic scars, such as single nucleotide polymorphisms, circulating sex hormones and local mechanical forces.5–9 For the treatment of keloids and hypertrophic scars, the role of conservative or minimally invasive management such as splinting, silicon sheeting and steroid injections have been well described in the literature.10–16
Autologous fat grafting has been introduced as the treatment of atrophic scars and contour deformity. It not only serves to improve contour and to fill areas of deficiencies, but increasingly there has been a focus on its ability to regenerate and remodel surrounding tissues.17 Studies have shown that adipose-derived stem cells have angiogenic and antiapoptotic properties which has effects on wound healing, soft-tissue restoration and scar remodelling.18–20
Fat grafting was first described by Neuber in 1893 for filling a retracted scar at the infraorbital rim.21 Since then it has developed slowly over the next century and, in 1992, Coleman described his technique which improved overall adipose cell survival.22 The Coleman technique forms the basis of how fat graft is currently performed. Fat grafting has since been used increasingly in a wide spectrum of clinical applications. Currently, it is widely used in breast reconstruction and augmentation, as an adjunct procedure to improve contouring and to fine-tune the reconstructed breast or as a stand-alone technique for breast reconstruction.23–28 It has also frequently been used as an adjunct procedure in the treatment of facial aging and facial reconstruction.29
The use of fat grafting as a treatment option for scars has recently been popularised and there is an abundance of evidence in the literature which supports its application for the treatment of scars in general.18,30 However, its role specifically in keloids and hypertrophic scars is not well described. Our goal is to systemically review the available literature regarding the use of fat grafting in treatment of keloids and hypertrophic scarring.
Patients and methods
Literature search
A comprehensive search was conducted in September 2016 using the following terms in combination in PubMed, MEDLINE and EMBASE: ‘fat grafting’, ‘lipofilling’, ‘adipose cells’, ‘fat injection’, ‘autologous fat graft’, ‘keloid scar’, ‘hypertrophic scar’, ‘scar management’, ‘wound management’ and ‘burn scar’. Articles were screened and selected by the primary author (GL) with any concerns regarding article eligibility consulted and discussed with the two other senior authors.
Inclusion criteria
All study types including randomised controlled trials, prospective trials, retrospective cohort studies, case-control studies, case series and case reports were included. The articles needed to describe the use of autologous fat grafting and its effect for the treatment of keloids or hypertrophic scars. The terms ‘keloid’ and/or ‘hypertrophic scar’ needed to be specified in the population or outcome of the studies. There were no restrictions with regards to the number of patients, follow-up periods or languages of articles.
Exclusion criteria
Exclusion criteria were articles on fat grafting for acute wounds, contour deformity, non-keloid and non-hypertrophic scars. Animal studies and in vitro studies were also excluded.
Statistical analysis
A formal statistical analysis was not performed because of the methodologic heterogeneity demonstrated among the articles.
Results
In total, 858 articles were identified through database searching (Figure 1). A PRISMA (P referred R eporting I tems for S ystematic R eviews and Meta-Analyses) flowchart for literature attrition is included (Figure 1). Duplicates were removed and 562 distinctive articles were found. After screening through all remaining titles, 70 relevant abstracts were assessed after our inclusion criteria, of which 44 were excluded. The remaining 26 full text articles were reviewed in their entirety and ultimately ten studies were included (Table 1).
Figure 1.
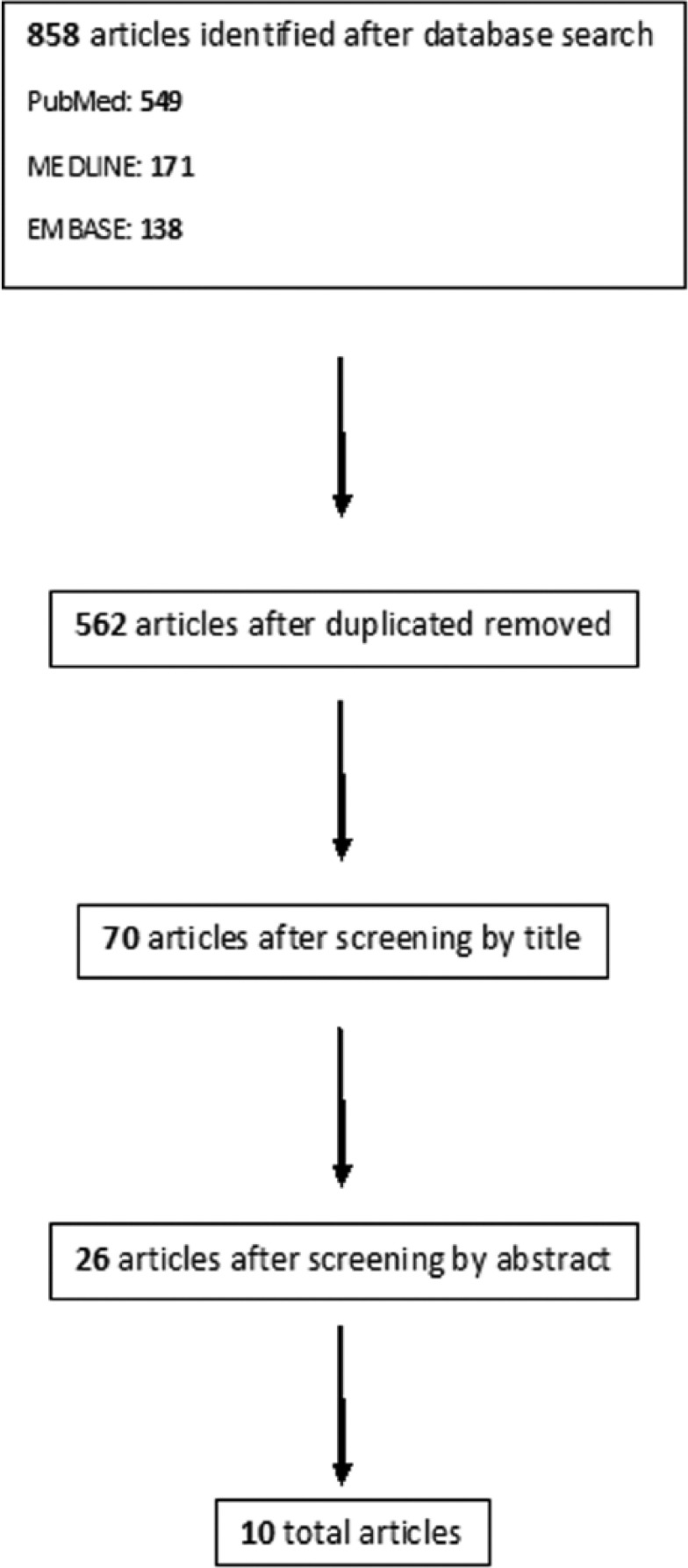
A PRISMA flowchart for literature attrition.
Table 1.
Studies included in this review.
| Reference | Study design | Keloids and/or hypertrophic scars (n) | OCEBM |
|---|---|---|---|
| Klinger et al., 201331 | Case-control | Not specified | III |
| Bruno et al., 201332 | Case-control | Not specified | III |
| Klinger et al., 200833 | Case series | 3 | IV |
| Brongo et al., 201234 | Case series | 18 | IV |
| Piccolo et al., 201535 | Case series | Not specified | IV |
| Mazzola et al., 201336 | Case series | 10 | IV |
| Byrne et al., 201637 | Case series | > 1 | IV |
| Fredman et al., 201638 | Case series | > 1 | IV |
| Huang et al., 201539 | Case series | > 2 | IV |
| Admani et al., 201540 | Case report | 1 | V |
In the ten studies, the majority of the scars identified are as results of burns or thermal injuries. A total of 1092 patients were included in these ten studies but unfortunately most did not specify the number of keloids or hypertrophic scars. In two of the studies, keloid scars were excluded.
Keloids and hypertrophic scars may in fact represent a small portion of this group of patients. Despite this, all ten studies reported promising results of fat grafting on the appearance, thickness, elasticity of the scars and its associated functionality.
Case-control studies
Klinger et al. enrolled 694 patients and performed fat grafting on 20 of them.31 Hypertrophic scars were described but the exact number was not specified. Keloids were excluded. Each scar was divided into two parts, one part fat grafted and the other infiltrated with normal saline. Assessment was made with durometer measurements and the Patient and Observer Scar Assessment Scale (POSAS) and results were statistically analysed. Statistically significant improvement was noted in skin hardness and all POSAS parameters except itching.
Similarly, Bruno et al. divided 93 burn scars into two homogenous portions with fat grafting performed in one portion and the other portion as control.32 Significant histochemical and antibody expression changes such as VEGF, β-catenin, TGF-β, Ki-67, P53 and P63 were noted. Clinical outcome was evaluated with modified Vancouver scar scale and patient satisfaction questionnaire which demonstrated functional and aesthetical improvement in hypertrophic scars.
Case series
In a different study, Klinger et al. reported improvement in skin elasticity, texture and thickness in three patients with hemifacial hypertrophic scars and keloids secondary to severe burns.33 Histological examinations by punch biopsies before and after fat grafting demonstrated new collagen deposition, local hypervascularity and dermal hyperplasia. Magnetic resonance scans were also performed showing similar and relatively symmetrical soft tissue signal enhancement on both the affected and unaffected sides.
Brongo et al. performed fat grafting on 18 patients with post-burn hypertrophic scars and keloids, noting better colour, texture, thickness, elasticity and a reduction of scar retraction.34 New collagen deposition, dermal hyperplasia and neoangiogenesis was also demonstrated on histological examination.
Piccolo et al. included 240 patients with acute, subacute burn wounds and burn scars.35 Subjective aesthetic improvement was reported with clinical photographs demonstrating visible and, in one case, complete remission of hypertrophic scar volume. However, the exact number of keloids or hypertrophic scars was not specified.
Mazzola et al. reported significant improvement in scar thickness and colour in a series of ten patients with retracted and/or hypertrophic scar secondary to tracheostomy.36
Byrne et al. described functional improvement in a series of 13 patients with burn scars in the hand. Evaluation was made with Total Active Movement (TAM), the Disabilities of Arm, Shoulder and Hand (DASH) Questionnaire and Michigan Hand Outcome Questionnaire (MHQ). However, only one of the patients was specified as having hypertrophic scar.37
Fredman et al. focused on the improvement of neuropathic pain assessed with Patient-Reported Outcomes Measurement Information System (PROMIS), also reporting improvement of colour, texture, contour, pliability and pruritus.38 One of seven patients had hypertrophic scar secondary to chemical burn; others were not specified.
Similarly, Huang et al. reported an improvement of neuropathic pain in a series of 13 patients evaluated by Visual Analog Scale (VAS) and Neuropathic Pain Symptom Inventory (NPSI).39 One patient had keloid on his left knee from friction burn and another had hypertrophic scar on her knee from an avulsion injury; others were not known.
Case reports
Admani et al. reported a multimodal approach with the combination of fat grafting, ablative fractional lacer resurfacing, laser-assisted corticosteroids delivery and pulsed-dye laser treatments for a mixed hypertrophic/atrophic facial scar from a dog bite in a three-year-old girl.40 Improvement in facial asymmetry, texture and colour was described and demonstrated by clinical photographs.
Discussion
To our knowledge, there is currently no study in the literature that focuses on the use of fat grafting in treatment of keloids or hypertrophic scars. However, there is substantial evidence that fat grafting has a positive effect on wound healing and scars in general. Two systematic reviews were recently published in this topic. Negenborn et al. reviewed fat grafting for treatment of all scar-related conditions which included 26 studies with 906 patients in total.30 The main outcome measures were scar appearance, skin characteristics, restoration of volume and contour, itch and pain. Other therapies, such as platelet-rich plasma and laser therapy in combination with fat grafting, were also described. Conde-Green et al. instead focused on burn wound healing and burn scars.18 Six murine and 12 human studies were included in the review with 818 human cases. Despite the lack of randomised controlled trials and comprehensive statistical analysis, overall it was suggested that fat grafting has beneficial effects on scars with few side effects.
With regard to the underlying pathophysiology and mechanism of fat grafting, several in vitro and murine studies suggested multiple immunohistochemical pathways where fat grafting may have positive effects on keloids and hypertrophic scars. Sultan et al. demonstrated early revascularisation in burn scars that has been fat grafted in a murine model.41 As a result, this reduces TGF-β1 upregulation, which is an important regulator for collagen production and fibrosis. Spikeman et al. demonstrated that adipose tissue-derived stromal cells inhibit TGF-β1-induced proliferation of human dermal fibroblasts in a paracrine fashion.42 Similarly, TGF-β1-induced myofibroblast contraction and collagen III gene expression is also suppressed in keloid scar-derived fibroblasts by adipose tissue-derived stromal cell-conditioned medium. Li et al. demonstrated that adipose tissue-derived stem cell conditioned media reduce collagen deposition, scar formation and suppress hypertrophic scar fibrosis by inhibiting the p38/MAPK signalling pathway.43 The author proposed that adipose tissue-derived stem cell conditioned medium is a potential therapeutic strategy for treatment of hypertrophic scaring.
Furthermore, in a human study, Bruno et al. demonstrated changes in histochemistry and antibody expressions in burn scars after fat grafting.32 Expression of Ki-67 suggested cellular proliferation and restoration to a normal environment post treatment.
It is important to note that the level of evidence of studies included in this review were relatively low with lack of proper statistical analysis. Most of the studies had relatively small number of cases and lacked controls. Most importantly, the exact number of keloids and hypertrophic scars were not specified in the majority of the articles. There is also an inherent bias for publication of positive results.
There is a lack of evidence in the literature on the use of fat grafting specifically on hypertrophic and keloid scarring. And with the limited papers on this, few describe scars from non-burn-related injuries. It will be interesting to study the effect of fat grafting on hypertrophic scars and keloid scars from different aetiologies. Despite the limitations and the small number of articles in the literature, encouraging clinical results have been reported in the use of fat grafting on keloids and hypertrophic scars. Together with the current theoretical and immunohistochemical understanding through other laboratory and animal studies, the literature does support that fat grafting may have a role to play in the treatment of keloids and hypertrophic scaring, but there is still much more yet to be explored and investigated in this therapy. The need for further research is thus apparent.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Tredget EE, Nedelec B, Scot PG, et al. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am 1997; 77: 701–703. [DOI] [PubMed] [Google Scholar]
- 2. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003; 326: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell L, McAdams T, Morgan R, et al. Pruritis in burns: A descriptive study. J Burn Care Rehabil 1988; 9: 305–308. [PubMed] [Google Scholar]
- 4. McOwan CG, MacDermid JC, Wilton J. Outcome measures for evaluation of scar: A literature review. J Hand Ther 2001; 14: 77–85. [DOI] [PubMed] [Google Scholar]
- 5. Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch 2016; 83(2): 46–53. [DOI] [PubMed] [Google Scholar]
- 6. Nakashima M, Chung S, Takahashi A, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010; 42: 768–771. [DOI] [PubMed] [Google Scholar]
- 7. Moustafa MF, Abdel Fattah MA, Abdel Fattah DC. Presumptive evidence of the effect of pregnancy estrogens on keloid growth. Case report. Plast Reconstr Surg 1975; 56: 450–453. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen 2012; 20: 149–157. [DOI] [PubMed] [Google Scholar]
- 9. Murray JC. Scars and keloids. Dermatol Clin 1993; 11: 697. [PubMed] [Google Scholar]
- 10. Niessen FB, Spauwen PM, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: A review. Plast Reconstr Surg 1999; 104(5): 1435–1458. [DOI] [PubMed] [Google Scholar]
- 11. Lawrence WT. In search of the optimal treatment of keloids: Report of a series and a review of the literature. Ann Plast Surg 1991; 27: 164. [DOI] [PubMed] [Google Scholar]
- 12. Perkins K, Davey RB, Wallis KA. Silicone gel: A new treatment for burn scars and contractures. Burns Incl Therm Inj 1983; 9: 201. [DOI] [PubMed] [Google Scholar]
- 13. Gold MH. Topic silicone gel sheeting in the treatment of hypertrophic scars and keloids. A dermatologic experience. J Dermatol Surg Oncal 1993; 19: 912. [DOI] [PubMed] [Google Scholar]
- 14. Dockery GL, Nilson RZ. Treatment of hypertrophic and keloid scars with Silastic gel sheeting. J Foot Ankle Surg 1994; 33: 110. [PubMed] [Google Scholar]
- 15. Akaishi S, Akimoto M, Kyakusoku H, et al. The tensile reduction effects of silicone gel sheeting. Plast Reconstr Surg 2010; 126: 109e–111e. [DOI] [PubMed] [Google Scholar]
- 16. Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg 2010; 125: 557–568. [DOI] [PubMed] [Google Scholar]
- 17. Conde-Green A, Baptista LA, de Amorin NF, et al. Effects of centrifugation on cell composition and viability of aspirated adipose tissue processed for transplantation. Aesthet Surg J 2010; 30: 249–255. [DOI] [PubMed] [Google Scholar]
- 18. Conde-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose derived regenerative cells in burn wound healing and scarring: A systematic review of the literature. Plast Reconstr Surg 2016; 137(1): 302–312. [DOI] [PubMed] [Google Scholar]
- 19. Francois S, Mouiseddine M, Mathieu N, et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol 2007; 86: 1–8. [DOI] [PubMed] [Google Scholar]
- 20. Agay D, Scherthan H, Forcheron F, et al. Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: Development of a new minipig model. Esp Hematol 2010; 38: 945–956. [DOI] [PubMed] [Google Scholar]
- 21. Neuber G. Fat transplantation. Verh Dtsch Ges Chir 1893; 22: 66. [Google Scholar]
- 22. Coleman SR. Long-term survival of fat transplants: Controlled demonstrations. Aesthet Plast Surg 1995; 19: 421–425. [DOI] [PubMed] [Google Scholar]
- 23. Hsu VM, Stransky CA, Bucky LP, et al. Fat grafting’s past, present, and future: Why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J 2012; 32(7): 892–899. [DOI] [PubMed] [Google Scholar]
- 24. Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg 2005; 116(5): 1300–1305. [DOI] [PubMed] [Google Scholar]
- 25. Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 2009; 29(5): 360–376. [DOI] [PubMed] [Google Scholar]
- 26. Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: the management of acquired contour deformities. Plast Reconstr Surg 2009; 124(2): 409–418. [DOI] [PubMed] [Google Scholar]
- 27. Babovic S. Complete breast reconstruction with autologous fat graft – a case report. J Plast Reconstr Aesthetic Surg 2010; 63(7): e561–e563. [DOI] [PubMed] [Google Scholar]
- 28. Panettiere P, Accorsi D, Marchetti L, et al. Large-breast reconstruction using fat graft only after prosthetic reconstruction failure. Aesthetic Plast Surg 2011; 35(5): 703–708. [DOI] [PubMed] [Google Scholar]
- 29. Glasgold RA, Lam SM, Glasgold MJ. Facial fat grafting: the new paradigm. Arch Facial Plast Surg 2008; 10(6): 417–418. [DOI] [PubMed] [Google Scholar]
- 30. Negenborn VL, Groen JW, Smit JM, et al. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions: A systematic review. Plast Reconstr Surg 2016; 137(1): 31e–43e. [DOI] [PubMed] [Google Scholar]
- 31. Klinger M, Caviggioli F, Linger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg 2013; 24: 1610–1615. [DOI] [PubMed] [Google Scholar]
- 32. Bruno A, Delli SG, Fasciani L, et al. Burn scar lipofilling: Immunohistochemical and clinical outcomes. J Craniofac Surg 2013; 24: 1806–1814. [DOI] [PubMed] [Google Scholar]
- 33. Klinger M, Marazzi M, Vigo D, et al. Fat injection for cases of severe burn outcomes: A new perspective of scar remodelling and reduction. Aesthetic Plast Surg 2008; 32: 465–469. [DOI] [PubMed] [Google Scholar]
- 34. Brongo S, Nicoletti GF, La Padula S, et al. Use of lipofilling for the treatment of severe burn outcomes. Plast Reconstr Surg 2012; 130: 374e–376e. [DOI] [PubMed] [Google Scholar]
- 35. Piccolo NS, Piccolo MS, Piccolo MT. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin Plast Surg 2015; 42: 263–283. [DOI] [PubMed] [Google Scholar]
- 36. Mazzola IC, Cantarella G, Mazzola RF. Management of tracheostomy scar by autologous fat transplantation: A minimally invasive new approach. J Craniofac Surg 2013; 24: 1361–1364. [DOI] [PubMed] [Google Scholar]
- 37. Byrne M, O’Donnell M, Fitzgerald L, et al. Early experience with fat grafting as an adjunct for secondary burn reconstruction in the hand: Technique, hand function assessment and aesthetic outcomes. Burns 2016; 42: 356–365. [DOI] [PubMed] [Google Scholar]
- 38. Fredman R, Edkins RE, Hultman CS. Fat grafting for neuropathic pain after severe burns. Ann Plast Surg 2016; 76: 295–303. [DOI] [PubMed] [Google Scholar]
- 39. Huang SH, Wu SH, Chang KP, et al. Alleviation of neuropathic scar pain using autologous fat grafting. Ann Plast Surg 2016; 74: 99–104. [DOI] [PubMed] [Google Scholar]
- 40. Admani S, Gertner JW, Gosman A, et al. Multidisciplinary, multimodal approach for a child with a traumatic fascial scar. Semin Cutan Med Surg 2015; 34: 24–26. [DOI] [PubMed] [Google Scholar]
- 41. Sultan SM, Bar JS, Butala P, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J Plast Reconstr Aesthet Surg 2012; 65: 219–227. [DOI] [PubMed] [Google Scholar]
- 42. Spiekman M, Przybyt E, Plantinga JA, et al. Adipose tissue-derived stromal cells inhibit TGF-β1- induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg 2014; 134(4): 699–712. [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Zhang W, Gao JX, et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signalling pathway. Stem Cell Res Ther 2016; 7: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
How to cite this article
- Lee G, Hunter-Smith DJ, Rozen WM. Autologous fat grafting in keloids and hypertrophic scars: a review. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/2059513117700157. [DOI] [PMC free article] [PubMed] [Google Scholar]


