Abstract
Introduction:
Intralesional steroid administration is a popular adjunct to scar management with numerous reports in the literature appraising this modality in hypertrophic and keloid scars. The percutaneous delivery of steroids using adhesive tape is an alternative modality, which was first described in the dermatological literature in the 1960s. It is infrequently used in most countries apart from the Orient, where it represents one of the mainstays of specialist scar management protocols.
Methods:
An English and Japanese literature review was performed and reports were stratified using the Joanna Briggs Institute Levels of Evidence. Data were extracted relating to the maximum dose of steroid that can be delivered safely, the reported therapeutic efficacy, as well as the side effects associated with the percutaneous delivery of steroids.
Discussion:
Steroid tape has the potential to be a safe and patient-friendly adjunct to scar management for carefully selected cases of keloid and hypertrophic scars. The main limitation for its widespread adoption is the lack of data to enable the determination of safe exposure thresholds in adult and paediatric patients.
Conclusion:
Despite the existing encouraging reports regarding the potential to be a useful adjunct in scar management, steroid tape is not widely used apart from a limited number of scar services worldwide. Further research is warranted to delineate the role of this modality in specialist scar management protocols.
Keywords: Steroid, tape, scar, keloid, hypertrophic, occlusion
Lay Summary
Steroid medications are frequently used to relieve symptoms as well as improve the appearance of bulky and unsightly scars. In most cases, this treatment involves injections; nevertheless, there is a more patient-friendly way to deliver this type of medicine to scars, which involves a sticky tape. Steroid tape is very popular in the East but is not commonly used in the rest of the world. We undertook this study to find out what has been written about this method of treatment in the skin disease literature and we focused our search on the treatment of scars in particular. We concluded that at present there some studies to support the safe use of steroid tape to treat carefully selected troublesome scars. Nevertheless, further research is needed to determine the maximum size of scar as well as duration of treatment that the steroid tape can be used for.
Introduction
Steroid medication is a commonly used adjunct in the management of hypertrophic and keloid scars. A recent systematic review of the literature regarding the outcome of intralesional steroid injections in scar management has revealed a paucity of large cohort studies for this commonly performed procedure. The outcome measures used in the literature are frequently poorly defined and non-standardised. Furthermore, the short follow-up period in the protocols employed may account for the reported variable success rate of the intervention.1
Aside from the injectable form of steroid administration, transdermal routes have been described including cream formulations in a small number of reports with mixed results. An observational study investigated the effectiveness of an emulsion consisting of a biosynthetic film component - cynthaskin in combination with triamcinolone acetonide in a prospective study in 32 hypertrophic and nine keloid patients. The formulation was applied twice a day on the scars and an adjacent part of the lesion on the same patient was left untreated in order to act as the comparison area. Assessment was performed using a four-point scale for five factors (colour, elevation, texture, itching and pain) and a beneficial result was considered if the scars showed improvement in at least three out of the five. In the hypertrophic scar group, 84.4% of patients showed improvement whereas this was 44.4% in the keloid group (P = 0.03). However, all patients achieved symptomatic relief of pain and itch.2
Another case series study investigated the response to 0.2% fluocinolone acetonide cream applied three times daily in 192 patients with a variety of skin conditions including chronic discoid lupus erythematosus, lichen planus and granuloma annulare as well as 12 cases of keloids. Two keloid patients showed clearance, seven showed significant response and three showed poor or no response. The authors did not speculate about the reasons for the variable response observed.3
The delivery of steroid to keloid/hypertrophic scars using a tape formulation was first reported in 1967 in a mixed cohort of patients with various dermatological disorders.4 Most interestingly, the most up-to-date Japanese literature suggests that steroid tape represents a mainstay in specialist centre scar management protocols.5 The aim of this work is to present an evidence-based search regarding the use of steroid tape in the dermatology and scar management literature, address issues pertinent to the safe administration of this modality and define directions for further research in the field.
Methodology
A detailed English literature search was performed using the PubMed database for the years 1966 to 2016 with the following keywords: steroid; tape; flurandrenolone; and flurandrenolide. Reference lists of relevant articles were scanned for additional pertinent literature. All articles were assessed for relevance to the study objective and a number of additional manuscripts were retrieved from the papers deemed appropriate for inclusion in the study.
Moreover, a separate Japanese literature database (‘Japan Medical Abstracts Society’) was searched using similar keywords for articles pertinent to the studies on the effectiveness of steroid tape in hypertrophic and keloid scars.
The papers were analysed and stratified according to levels of evidence using the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party.6 Data were extracted relating to the dose of steroid delivered to the scars [total body surface area (TBSA) / duration of use], any side effects reported as well as the therapeutic efficacy of the modality.
Advantages of steroid tape formulations
Weiner first reported steroid tape formulations in the dermatology (non-scar) literature in 1966. The original study compared flurandrenolone tape to cream in combination with plastic film occlusion as a paired comparison in ten patients with psoriasis. In all patients, clinical improvement began within a few days of therapy and the tape was found to be equal or superior to film in eight patients. Interestingly, all ten patients preferred tape over film occlusion based on its ease and greater convenience of application.7
Similar results proving the therapeutic advantage of tape versus cream formulation were obtained in a study of 18 patients with lichen simplex chronicus (70% of tape versus 25% of ointment patients showing complete involution of lesions with no recurrence at an average 12-month follow-up).8 Additionally, a small study using flurandrenolide tape (applied 16 h a day) versus diflorasone ointment in adult psoriatic patients reported greater success in terms of disease clearance in the tape group.9
The superior acceptability of steroid tape by patients has been reported in a number of publications due to the following reasons:4,8,10
Ease of application;
Better adherence/localisation to affected skin;
Inconspicuous aesthetic appearance;
Controlled medication dose release/better absorption relating to the occlusion effect;
Protection from mechanical irritation (e.g. clothing, scratching activity).
Steroid tape formulations and indications for clinical use
The steroid tape is available in the following three countries in slightly different preparations:
In the UK, the commercially available formulation comprises a fludroxycortide impregnated tape (4 μg/cm2), which represents a moderately potent steroid. The manufacturer’s indications are the ‘adjunctive therapy for chronic, localised recalcitrant dermatoses that may respond to topical corticosteroids’. Caution is advised against long-term continuous treatment periods to avoid hypothalamic–pituitary–adrenal axis suppression and in children a maximum duration of five days is recommended.11
In the USA, a preparation containing 4 μg/cm2 flurandrenolide (medium-strength steroid) is available with indications of use being the ‘relief of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses’. Similar precautions concerning long-term use, especially in special groups including pregnant, breastfeeding as well as paediatric patients, are given by the manufacturers.12
In Japan, two steroid tape formulations are available; a 4 μg/cm2 fludroxycortide type (medium-strength steroid) and a 20 μg/cm2 deprodone proprionate type (higher-potency steroid). The list of indications includes: eczema; psoriasis; lupus erythematosus; lichen; and other inflammatory skin conditions encompassing keloid and other scars. Caution is advised regarding the use of these tapes in pregnancy, children, elderly patients and large total body surface areas especially for prolonged periods.13,14
Steroid tape has several clinical indications in scar management including:5,15
First-line treatment for appropriately small-sized keloid and hypertrophic scars in paediatric and older patients (It is known that patients at the extremes of age have thinner skin, which facilitates steroid absorption);
Secondary adjunct following bulk reduction of the scar with steroid injections, radiotherapy or surgery;
Prophylaxis for patients at risk of scar hypertrophy, which can commence approximately one month after epithelialisation.
The steroid tape is applied by cutting the adhesive material to the size and shape of the scar with minimal overlap onto normal skin. Patients can shower as normal and pat dry over the tape, which should be changed every 24–48 h.5
Figures 1 and 2 show clinical results from the application of steroid tape in hypertrophic and keloid scars.
Figure 1.
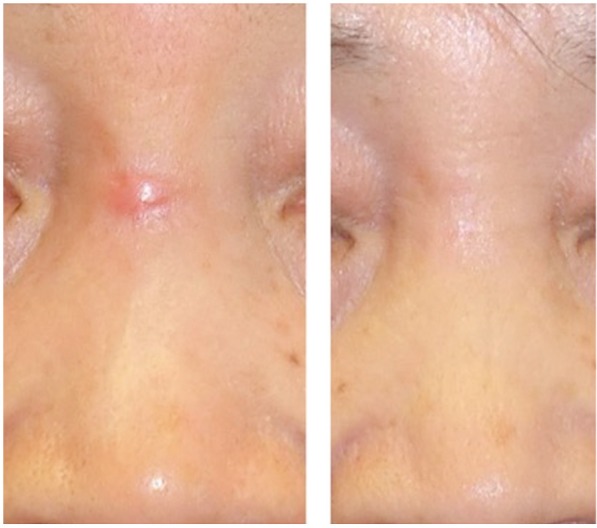
Hypertrophic scar over the nasal dorsum of a 73-year-old woman treated with deprodone propionate tape. Photograph before and two months after initiation of treatment.
Figure 2.
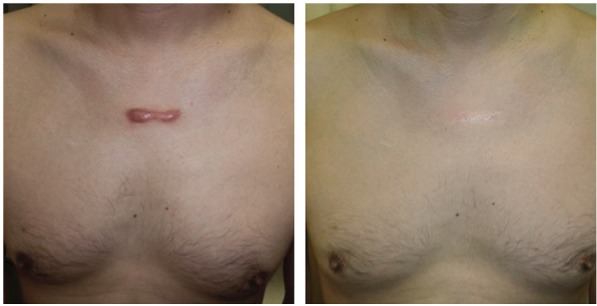
Pre-sternal keloid scar in a 45-year-old man treated with deprodone propionate tape. Photograph before and 36 months after initiation of treatment.
Mechanisms of action of steroid tape
The mechanisms of action of percutaneous steroid delivery can be divided into:
- Pharmacological effects of the steroid, which are thought to relate to:16,17
- decrease in collagen/glycosaminoglycan synthesis;
- antimitotic effect via a decrease in fibroblast proliferation;
- reduction in inflammatory response via inhibition of leucocyte and monocyte activity;
- reduction of plasma protease inhibitors, which promotes collagenase activity;
- effect on growth factor levels (decrease in transforming growth factor beta 1 and increase in beta fibroblast growth factor);
- hypoxia through a vasoconstriction effect.
- Non-pharmacological effects of the carrier tape:
- Occlusion: it is widely accepted that occlusion enhances the percutaneous absorption of corticosteroids18 but it appears that occlusion has a number of other effects on the skin. A study was conducted to delineate the effect of occlusion and percutaneous (tape) corticosteroid application to the skin. Flurandrenolide tape at 4 and 20 μg/cm2 produced a statistically significant reduction in the epidermal mitotic count (P <0.01) but no significant difference was observed between the two different concentrations of steroid. The conclusion from this study was that potent antimitotic effects occur with semi- and non-occlusive tapes containing steroids and total occlusion is not necessary for the physiological effect of the application to materialise. Additionally, a non-occlusive vehicle would assist in preventing certain adverse effects like irritation; this probably offsets the theoretical advantage of maximising the percutaneous absorption of the steroid via complete occlusion.19
- An additional hypothetical occlusion-related mechanism stems from the experience with silicone products for scar management. These have been shown to produce a favourable environment of hydration secondary to occlusion (decrease in water vapour transmission), which is thought to be responsible for a decrease in capillary activity and a reduced collagen deposition.22
- Vasoconstriction: it has been shown that steroid applied topically to the skin produces a vasoconstrictive effect and this phenomenon is enhanced by occlusion.23 The underlying mechanisms for vasoconstriction are not fully established but it is recognised that its degree correlates with corticosteroid clinical efficacy in inflammatory skin disease.24 Underlying theories include the effect of local norepinephrine release causing smooth muscle contraction,25 the inhibition of cutaneous mast cell function26 and the effect of histamine and arachidonic acid metabolites on altering phospholipase A activity.24 There appears to be a consensus that these effects are mediated via the action of steroids on glucocorticoid receptors.27
- Scar support: it is widely recognised that tension across a scar is an initiating factor for stretched/hypertrophied scars and hence mechanical support is a valid strategy to reduce unfavourable morphology.28 To this effect, the use of other tape formulations like microporous tape is used to control scar tension and prevent hypertrophic scarring as applied at two weeks to fresh surgical incisions.29 It is possible that scar support is one of the ways steroid tape may contribute to the involution of keloid and hypertrophic scars.
Safety data/risk of systemic toxicity
One of the pertinent questions in terms of intralesional as well as percutaneous steroid application relates to the maximum total body surface area and duration of use before systemic side effects develop.
A systematic review in 2013 identified 18 adult and paediatric cases of Cushing’s syndrome in patients receiving intralesional triamcinolone for keloid and hypertrophic scars. The study revealed that very limited objective evidence exists to allow the establishment of definitive treatment protocol recommendations. Central to the uncertainty regarding exact intralesional steroid dosology lies the lack of data on absorption of injectable glucocorticoids as well as variations in individuals’ sensitivity to steroid administration.30
The status of the steroid tape literature is very similar and given that the majority of steroid tape publications relate to the non-scar dermatological literature, we decided to appraise these studies with reference to safety data using the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party.6
Table 1 summarises the findings from the dermatological literature relating to levels of maximum exposure to steroid tape preparations in both adult and paediatric patients.
Table 1.
A summary of the different studies investigating the use of steroid tape in non-scar, dermatological patients categorised based on the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party levels 1–5.6
| Authors | Type of study/Level of evidence | Disease | Patient no. | Age range (years) | Steroid | Total body surface area | Daily duration of application | Duration of treatment | Symptomatic response |
|---|---|---|---|---|---|---|---|---|---|
| Labow TA et al., 196931 | Double-blinded controlled trial- 1 | Psoriasis | 21 (32 sites treated with steroid tape and 38 with placebo) | Unspecified range; median: 51 years | Flurandrenolone tape (4 μg/cm2) | Four sites treated per patient (two with active tape and two with placebo) | 24 h | 2 weeks (2 dropouts) | 26/32 steroid tape vs. 6/38 placebo sites showed overall improvement (P <0.001) |
| Kikuchi I and Jono M, 197632 | Case series – 4 | Granuloma gluteale infantum | 2 | 3- and 6-month-old | 0.025% fluocinolone acetonide (case 1) and flurandrenolide tape (case 2) | Unspecified | 1–2 months of daily application (case 1), unspecified (case 2) | 1–2 months (case 1); unspecified (case 2) | Good response (noticeable flattening of lesions) |
| Berger JE and Helm F, 197010 | Case series– 4 | Range of subacute and chronic dermatoses | 155 | Unspecified | Flurandrenolone tape (4μg/cm2) | Areas of 1–24 inches in maximum dimension | 3–24 h daily | 1 day to 6 weeks | 61/155 showed good results and 38/155 moderate results |
| Gomez F and Schorr WF, 196833 | Case series– 4 | Dermatoses including psoriasis, lichen, contact dermatitis | 42 | Unspecified | Flurandrenolone tape (4μg/cm2) | Unspecified | 24 h | 3 weeks (one dropout at day 6) | 36/42 showed excellent to good results |
| Riley K, 196918 | Case series– 4 | Dermatoses including psoriasis, lichen disorders | 39 | 8–77 years | Flurandrenolone tape (4μg/cm2) | Maximum dimension 4 inches | Night-time or 24 h on–24 h off regimen | Unspecified | 28/39 showed good to excellent results, 7 partial improvement |
| Burrows D, 196934 | Case series– 4 | Dermatoses including lupus erythematosus, psoriasis, lichen disorders | 41 | Unspecified | Flurandrenolone tape (4μg/cm2) | Less than 10% of total body surface area | 12–24 h | Unspecified | 19/41 obtained clearance of lesions and 12/41 showed improvement |
| Sellers FM, 197035 | Case series – 4 | Psoriasis and lichen simplex/ planus | 15 | Unspecified | Flurandrenolone tape (4 μg/cm2) | Unspecified | 12 h | Unspecified | All patients reported improvement |
| Ronchese F, 196936 | Case series – 4 | Dermatoses including lichen chronicus, psoriasis | 126 | Unspecified | Flurandrenolone tape (4μg/cm2) | Unspecified | Bedtime | Unspecified | Poor reporting of outcomes but positive descriptive data provided |
| Nurse DS, 197437 | Case series – 4 | Dermatoses including, psoriasis and eczema | 172 | Unspecified | Flurandrenolone tape (4μg/cm2) | Unspecified | Unspecified | 2–63 days | Excellent results in 63 patients and good results in 60 patients |
| Fields RJ and Magpantay R, 196838 | Case series – 4 | Dermatoses including psoriasis, eczema, contact dermatitis | 56 | Unspecified | Flurandrenolone tape (4μg/cm2) | Unspecified | Overnight-24 h | Unspecified | Excellent response in 15/56 and good in 29/56 patients |
| Boatwright H, 196939 | Case series– 4 | Dermatoses including lichen, atopic/contact dermatitis, lupus erythematosus | 915 | Unspecified | Flurandrenolone tape (4μg/cm2) | Unspecified | Unspecified | Unspecified | Excellent response in 42.19%, good response in 30.27% and partial improvement in 14.86% |
| Kestel JL, 197140 | Case report – 4 | Neurodermatitis | 1 | 14 | Flurandrenolone tape (4μg/cm2) | Unspecified | Overnight | Unspecified | Good response |
| Azulay DR, 198541 | Case report– 4 | Isotretinoin associated granulation tissue | 1 | Unspecified | Flurandrenolide tape | Unspecified | Unspecified | Unspecified | Good response |
| Ronchese F, 197442 | Case report– 4 | Recurrent periungual myxoid cysts | 5 | Unspecified | Flurandrenolone tape (4μg/cm2) | Small area corresponding to periungual myxoid cysts | Cycle of 24 h tape application followed by 24 h of steroid cream | 2–3 months | Excellent response with no recurrence for up to 3 years |
In conclusion, there is a single level 1 study in psoriasis patients, which indicates that a two-week treatment of 24 h long application of flurandrenolone tape (4 μg/cm2) is effective without any systemic side effects.31
The remaining studies have several significant limitations; these include incomplete datasets (especially in terms of treatment duration and TBSA) as well as the use of subjective outcome measures. This makes any conclusions about safe maximum exposure to steroid tape in dermatological cohorts very challenging.
Evidence stemming from reports with scar patient cohorts
English literature
The first report investigating the use of steroid tape in scar patients was a case series of 285 patients with a variety of dermatological disorders including psoriasis (n = 98), lichen planus (n = 13), eczematous dermatitis (n = 28) and keloids (n = 2) using two different concentrations of flurandrenolone tape (1 and 4 μg/cm2). The age range of the cohort was 2 months to 77 years and ‘with few exceptions the tape was not used for over more than 5–10% of body surface area.’ No data on steroid absorption or pituitary depression were collected either; nevertheless, no systemic toxicity was reported. The authors provide limited result data on the cohort; 61 patients had an excellent response and 14 had a good response, while six had only partial improvement. In 12 patients, there was no effect of the tape and in one case it aggravated the underlying disease process (psoriasis).4
Alden et al.43 studied the use of flurandrenolone tape (4 μg/cm2) in a mixed cohort of 100 patients, which included eight patients with small hypertrophic and keloid scars. The tape was used for up to eight weeks, on a 24 h or 12h (nigth-time) daily basis. It was concluded that the tape treatment was superior or better compared to traditional therapies in 63% of cases, especially in those with nummular psoriasis and discoid lupus erythematosus as opposed to vesicular eruptions and large psoriatic plaques. In terms of scar patients, the authors reported that ‘all patients obtained some relief and comfort from the tape’.43
A study of 74 patients with a variety of dermatological diseases including atopic/contact dermatitis, lichen planus as well as hypertrophic/keloid scarring investigated the effect of flurandrenolone tape (4 μg/cm2).
The group of hypertrophic scarring comprised 19 patients and were defined with scars having been present for less than 12 months. Overall, 28 scars were treated, some of which had multiple treatment episodes during the study. The age range was between 10–75 years old with an average of 37 years. The treatment duration range was between 2–120 days with an average of 51 days. Excellent response, as defined by complete disappearance of excessive scarring manifestations, was observed in eight patients, whereas a good response was seen in ten, and partial in four.
The keloid group (defined as scar duration of >12 months) comprised 12 patients with an age range of 3–70 (average age, 32 years) and treatment duration between 7–288 days (average, 77 days). Two patients had excellent and four good results, while partial improvement was seen in another four patients.
The application duration for each individual tape episode varied between 12 h and seven days for the whole cohort and it was observed that the tape worked best in cases of 72–96 h long applications with intervals not exceeding 1 h before the next piece of tape was applied.
The author of this work states: ‘Individuals with partial and good improvement in the hypertrophic scar group could be possibly be converted to the excellent category by greater persistence in the regularity of tape application and by increasing the period of treatment’. Additionally, in well-established keloid cases, longer treatment periods were needed. Interestingly, upon interruption of therapy, once the keloid height had decreased to skin level, recurrences seemed to respond to reapplication of tape for shorter periods.44
Another clinical trial involved 40 patients at Glasgow Western Infirmary, UK with a variety of dermatoses including psoriasis, lichen simplex, discoid lupus erythematosus and 11 keloid scars. The age range for the keloid group was 2–76 years and these patients applied the tape for 12–24 h/day over the 18 month period of the trial. The area of application was in most cases less than 0.5% TBSA and only two children with keloids had up to 5% treated with tape. The author stated that ‘the most gratifying response to the application of the tape was in the cases of keloids, notably in children’. The majority of the scars became flatter and paler than the surrounding skin and traces of redness and irritation disappeared in the most severely affected patients.45
Japanese literature
An open trial investigated the effectiveness of deprodone propionate plaster on a large cohort of dermatological diseases including 24 hypertrophic and 30 keloids. The subcohort included 22 male and 32 female patients with an age range of 10–70 years. The aetiology of scarring and TBSA of application were not specified; nevertheless the tape was applied for 15 h/day over six weeks. The outcomes were judged based on subjective reporting on behalf of the physician with the tape found to be very effective in three patients, effective in 21 patients, while a minor response was seen in 18 and no response in 12 patients.46
An observational study investigated the use of fludroxycortide tape in 60 hypertrophic and keloid scars in 30 adult patients (age range, 23–67 years; mean age, 37 years) and 30 paediatric patients (age range, 2–15 years; mean age, 7.2 years) involving various anatomical sites including the trunk and extremities. The scars were over twelve months old and the tape was used for at least one year for 24 h/day. There are no specific data on the TBSA of application. The study employed the Japan Scar Workshop (JSW) scar scale, which is based on risk factors as well as symptoms in scars to assign a score of 0–25 (0–5, mature scar; 6–15, hypertrophic character, 16–25, keloid-type scar). Results suggest that 20% of adult patients and 80% of paediatric patients improved with fludroxycortide tape from 18 to less than 2 points (almost mature scar). The ‘non-responsive’ adult subcohort (n = 24) was started on deprodone propionate tape after twelve months of fludroxycortide application. Following instigation of the higher potency tape, 17 out of 24 cases (70.8%) improved to less than 2 points. The authors concluded that adults needed a stronger (deprodone propionate) tape to reach similar responses in terms of scar maturation, while the strength of the fludroxycortide tape appeared to be sufficient for paediatric patients.47
Side effects
It is accepted that the higher the concentration of steroid tape, the more pronounced the associated side effects are likely to be.4 A variety of side effects have been reported in the literature arising from the use of steroid tape and these include in descending order of incidence:
Burning/stinging sensation: mean incidence 12.21% (range, 1.67–30%);4,8,35,37,39,44
Contact dermatitis: mean incidence 11% (range, 5–20%).31,44 The senior author has a long experience of treating over 1500 scars per year using steroid tape. Before 2013, fludroxycortide was used for the majority of patients and the incidence of contact dermatitis was approximately 20%. Interestingly, this fell to less than 5% when the majority of patients were treated with deprodone propionate tape (unpublished data);
Skin irritation: mean incidence 10.6% (range, 2.4–30%);8,18,34,37,43
Folliculitis: mean incidence 8.79% (range, 0.56–13.35%);33–35,37,39
Erosion formation: incidence 4.9%;34
Erythema: incidence 3.6%;38
Eczematous reaction: incidence 2.9%;37
Aggravation of pre-existing dermatitis: mean incidence 0.84% (range, 0.56–1.12%);4
Hypopigmentation (transient) has also been reported in a single literature report; this appeared within 48 h of night-time application and gradually resolved within one week following cessation of application.40
Concluding remarks
This work appraised the literature concerning the use of steroid tape in dermatological disorders with a focus on potential applications in scar management. The percutaneous mode of steroid administration has several advantages including its ease of application and non-invasive nature, making it attractive for children as well as adults who do not tolerate injectable steroid formulations.
Additionally, it is plausible that the tape formulation has the potential to maintain a continuous level of steroid concentration to the scar as opposed to the peaks and troughs produced by injectable formulations. The resulting more uniform level of steroid concentration might be a beneficial strategy to control the inflammatory state of the scar milieu, which appears to be central to the pathophysiology of the hypertrophic and keloid scars.48,49
Having reviewed the number of literature reports, it becomes evident that following a peak of research activity in the 1960s and 1970s, there has been some resurgence of interest in this modality for scar management which represents standard of care in Japan despite the existing low levels of evidence.5,47 One of the most significant limiting factors for the widespread adoption of percutaneous steroid administration is the lack of absorption data for adult and paediatric patients. It is very challenging to draw valid conclusions regarding the maximum size of scar (TBSA) and duration of treatment from studies incorporating scar subcohorts. Their limitations relate to non-heterogenous designs, incomplete result data and the use of non-standardised outcome measures. Nevertheless, it appears that daily application of a medium strength tape (e.g 4 μg/cm2 flurandrenolone) to small hypertrophic and keloid scars of up to 5% TBSA for at least six to eight weeks is associated with no reported systemic side effects. The most recent publication from Japan reported the use of the medium strength steroid-fludroxycortide for at least one year in adults and children with no reports of systemic complications.47
In conclusion, steroid tape administration appears to be a promising adjunct for the management of carefully selected keloid and hypertrophic scars. Further research should focus on attaining absorption data to guide steroid tape treatment regimens; additionally, studies comparing steroid tape to other adjuncts are warranted in order to determine a role for this modality in international scar management protocols.
Acknowledgments
Mr Ioannis Goutos wishes to acknowledge the Dowager Countess Eleanor Peel Trust and the HCA International Foundation for their sponsorship during his scar management fellowship scheme in Tokyo, Japan.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Perdanasari T, Torresetti M, Grasetti L, et al. Intralesional injection treatment of hypertrophic scars and keloids: A systematic review regarding outcomes. Burns & Trauma 2015; 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yii NW, Frame JD. Evaluation of cynthaskin and topical steroid in the treatment of hypertrophic scars and keloids. Eur J Plast Surg 1996; 19: 162–165. [Google Scholar]
- 3. Marsden CW. Fluocinolone acetonide 0.2% cream-a co-operative clinical trial. Br J Derm 1968; 80: 614–617. [DOI] [PubMed] [Google Scholar]
- 4. Goldman L, Igelman JM, Kitzmiller KW. Clinical investigative studies with flurandrenolone tape. Cutis 1967; 3: 367–371. [Google Scholar]
- 5. Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and hypertrophic scars can now be cured completely: Recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch 2016; 83: 46–53. [DOI] [PubMed] [Google Scholar]
- 6. The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party*. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. Adelaide: The Joanna Briggs Institute, 2014. Available at: joannabriggs.org. [Google Scholar]
- 7. Weiner MA. Flurandrenolone tape. A new preparation for occlusive therapy. J Invest Dermatol 1966; 47: 63–66. [PubMed] [Google Scholar]
- 8. Bard JW. Flurandrenolone tape in the treatment of lichen simplex chronicus. J Ky Med Assoc 1969; 67: 668–670. [PubMed] [Google Scholar]
- 9. Krueger GG, O’Reilly MA, Weidner M, et al. Comparative efficacy of once-daily flurandrenolide tape versus twice-daily diflorasone diacetate ointment in the treatment of psoriasis. J Am Acad Dermatol 1998; 38: 186–190. [DOI] [PubMed] [Google Scholar]
- 10. Berger JE, Helm F. Flurandrenolone tape. New means of achieving occlusive therapy. NY J Med 1970; 70: 406–408. [PubMed] [Google Scholar]
- 11.Available at: www.drugs.com/uk/haelan-tape.html (accessed 10 December 2016).
- 12. Cordran tape. Available at: www.drugs.com/cdi/cordran-tape.html (accessed 10 December 2016).
- 13. Dorenison tape. Available at: http://www.teikoku.co.jp/bin/img_pdf/attached/ED76_att.pdf (accessed 10 December 2016).
- 14. Eclar plaster. Available at: http://www.hisamitsu.co.jp/medical/data/eclarp_t.pdf (accessed 10 December 2016).
- 15. Rauscher GE, Kolmer WL. Treatment of recurrent earlobe keloids. Cutis 1986; 37: 67–68. [PubMed] [Google Scholar]
- 16. Niessen FB, Spauwen P, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999; 104: 1435–1438. [DOI] [PubMed] [Google Scholar]
- 17. Roques C, Teot L. The use of corticosteroids to treat keloids: a review. Int J Lower Extr Wounds 2008; 7: 137–145. [DOI] [PubMed] [Google Scholar]
- 18. Riley K. Flurandrenolone (Cordran) tape as occlusive therapy. JSC Med Assoc 1969; 65: 171–172. [PubMed] [Google Scholar]
- 19. Fisher LB, Maibach HI, Trancik RJ. Effects of occlusive tape systems on the mitotic activity of epidermis. With and without corticosteroids. Arch Dermatol 1978; 114: 384–386. [PubMed] [Google Scholar]
- 20. Tillman WJ, Higuchi T. Quantitative evaluation of the interaction of callus strips with some hydroxylic solvents. J Invest Derm 1961; 27: 87. [DOI] [PubMed] [Google Scholar]
- 21. Frank L, Rapp Y. Occlusive topical corticosteroids and heat in psoriasis. Arch Derm 1963; 87: 32. [DOI] [PubMed] [Google Scholar]
- 22. Niessen F, Spauwen P, Robinson P, et al. The use of silicone occlusive sheeting (Sil-K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plast Reconstr Surg 1998; 102: 1962. [DOI] [PubMed] [Google Scholar]
- 23. McKenzie A. Perutaneous absorption of steroids. Arch Dermatol 1962; 86: 91–94. [Google Scholar]
- 24. Marks R, Sawyer M. Glucocorticoid-induced vasoconstriction in human skin. Arch Dermatol 1986; 122: 881–883. [PubMed] [Google Scholar]
- 25. Solomon LM, Wentzel HE, Greenberg MS. Studies in the mechanism of steroid vasoconstriction. J Invest Derm 1965; 44: 129–131. [DOI] [PubMed] [Google Scholar]
- 26. Goldsmith P, Bunker C, Leslie T, Foreman J, et al. The effect of topical steroid on the actions of vasoconstrictor and vasodilator peptides in human skin. Skin Pharmacol 1996; 9: 289–297. [DOI] [PubMed] [Google Scholar]
- 27. Marks R, Barlow JW, Funder JW. Steroid induced vasoconstriction: Glucocorticoid antagonist studies. J Clin Endocrinol Metab 1982; 54: 1075–1077. [DOI] [PubMed] [Google Scholar]
- 28. Widgerow AD, Chait LA, Stals R, et al. New innovations in scar management. Aesth Plast Surg 2000; 24: 227–234. [DOI] [PubMed] [Google Scholar]
- 29. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg 2002; 110: 560–571. [DOI] [PubMed] [Google Scholar]
- 30. Fredman R, Tenenhaus M. Cushing’s syndrome after intralesional triamcinolone acetonide: A systematic review of the literature and multinational survey. Burns 2013; 39: 549–557. [DOI] [PubMed] [Google Scholar]
- 31. Labow TA, Eisert J, Sanders SL. Flurandrenolide tape in the treatment of psoriasis. NY State J Med 1969; 69: 3138–3140. [PubMed] [Google Scholar]
- 32. Kikuchi I, Jono M. Flurandrenolide-impregnated tape for granuloma gluteale infantum. Arch Dermatol 1976; 112: 564. [PubMed] [Google Scholar]
- 33. Gomez F, Schorr WF. Flurandrenolone tape. A new therapeutic measure in dermatology. Wis Med J 1968; 67: 596–599. [PubMed] [Google Scholar]
- 34. Burrows D. Flurandrenolone therapy. Trans St Johns Hosp Dermatol Soc 1969; 55: 103–104. [PubMed] [Google Scholar]
- 35. Sellers FM. Investigative study of flurndrenolone tape in a series of ambulatory outpatients. J Ind St Med Assoc 1970; 63: 34–36. [PubMed] [Google Scholar]
- 36. Ronchese F. Flurandrenolone tape therapy. RI Med J 1969; 52: 389–390. [PubMed] [Google Scholar]
- 37. Nurse DS. Letter: Haelan tape. Australasian J Dermatol 1974; 15: 152. [DOI] [PubMed] [Google Scholar]
- 38. Fields RJ, Magpantay R. Studies with flurandrenolone tape. Med Ann Dist Columbia 1968; 37: 272. [PubMed] [Google Scholar]
- 39. Boatwright H. Use of flurandrenolone tape in lupus erythematosus and other diseases. J S C Med Ass 1969; 65: 173–175. [PubMed] [Google Scholar]
- 40. Kestel JL., Jr. Hypopigmentation following the use of Cordran tape. Arch Dermatol 1971; 103: 460. [PubMed] [Google Scholar]
- 41. Azulay DR, Azulay-Abulafia L. Isotretinoin associated granulation tissue treated with occlusive corticosteroid tape. J Am Acad Derm 1985; 13: 670–671. [DOI] [PubMed] [Google Scholar]
- 42. Ronchese F. Treatment of myxoid cysts with flurandrenolide tape. R I Med J 1974; 57: 154–155. [PubMed] [Google Scholar]
- 43. Alden HS, Cronce PC. Experiences with the use of flurandrenolone tape in dermatology. J Med Assoc Ga 1969; 58: 433–435. [PubMed] [Google Scholar]
- 44. Goldblatt S. The use of Cordran tape in dermatology. Ohio State Med J 1969; 65: 1118–1121. [PubMed] [Google Scholar]
- 45. Ratzer MA. A clinical trial of flurandrenolone tape. Br J Clin Pract 1970; 24: 185–189. [PubMed] [Google Scholar]
- 46. DP Study Group. Analysis of clinical outcomes of deprodone propionate plaster for various skin diseases. Rinsholyaku 1989; 5: 2177–2185 (in Japanese). [Google Scholar]
- 47. Ogawa R, Akashi S. Effectiveness of corticosteroid tape/plaster for keloids and hypertrophic scars – Comparative study of fludroxycortide and deprodone tape/plasters. Scar Management 2016; 10: 55–60 (in Japanese). [Google Scholar]
- 48. Huang C, Akaishi S, Hyakusoku H, et al. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int Wound J 2014; 11: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang C, Murphy GF, Akaishi S, et al. Keloids and hypertrophic scars: Update and future directions. Plast Reconstr Surg Glob Open 2013; 1: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
How to cite this article
- Goutos I, Ogawa R. Steroid tape: A promising adjunct to scar management. Scars, Burns & Healing, Volume 3, 2017; DOI: 10.1177/2059513117690937. [DOI] [PMC free article] [PubMed] [Google Scholar]


