Abstract

Increased burn wound healing time has been shown to influence abnormal scarring. This study hypothesised that scar severity increases commensurate to the increase in time to healing (TTH) of the wound.
Wound healing and scar data from burn patients treated by the Burn Service of Western Australia at Royal Perth Hospital were examined. The relationship between TTH and scar severity, as assessed by the modified Vancouver Scar Scale (mVSS), was modelled using regression analysis. Interaction terms evaluated the effect of surgery and total body surface area – burn (TBSA) on the main relationship. Maximum likelihood estimation was used to account for potential bias from missing independent variable data.
The sample had a median age of 34 years, TTH of 24 days, TBSA of 3% and length of stay of five days, 70% were men and 71% had burn surgery. For each additional day of TTH, the mVSS score increased by 0.11 points (P ≤ 0.001) per day in the first 21 days and 0.02 points per day thereafter (P = 0.004). The relationship remained stable in spite of TBSA or surgical intervention. Investigation of the effect of missing data revealed the primary model underestimated the strength of the association.
An increase in TTH within 21 days of injury is associated with an increase in mVSS or reduced scar quality. The results confirm that efforts should be directed toward healing burn wounds as early as possible.
Keywords: Burns, scar, time to healing, Vancouver Scar Scale, outcome, surgery
Lay Summary
Burns that take a long time to heal have a greater chance of a leaving a bad scar. We wanted to know whether daily increases in healing time in the early stages result in worse scars as assessed by experienced clinicians using a standard measure of scar quality. Our research found that in the first 21 days after the injury, each additional day that a burn wound takes to heal is associated with worsening scar quality.
Introduction
Burn scarring evokes physical, psychological, aesthetic and social consequences.1,2 Studies estimate 32–77% of cutaneous burn injuries result in pathological scarring.3 Physical issues related to the area of the scarring include the interference with sensory function, the inability to sweat and thermoregulate, chronic pain and itching. In addition, there are systemic pathophysiological impacts of the burn injury further complicating the psychological and social issues.4 The association between scarring and poor body image including post-traumatic stress and social avoidance has been extensively documented.1,3,5,6
Burn care is focused on survival and the quality of the survival with an emphasis on scar minimisation to limit physical, aesthetic and psychological sequelae post injury.7 Conservative and surgical interventions aim to facilitate expedient wound closure. This is a factor that can significantly impact scarring and quality of outcome.8–10 The time to healing (TTH) is influenced by patient, injury and treatment factors.11,12 Past research has demonstrated that increased TTH, beyond 21 days post burn, results in an increased risk of hypertrophic scarring.9,10,13 However, these studies were limited by lack of standardised scar outcome measures and involved animal, major burn or paediatric samples. In addition more recent research has noted that some small burns can have good results even when healing time is extended beyond 21 days.14,15 The gaps in the evidence leads this study to investigate the relationship between TTH and scar outcome across a spectrum of burn injury severity in adults.
An understanding of the natural history of the burn wound is essential in planning clinical intervention. This serves to optimise tissue salvage and minimise wound depth conversion. Clinicians seek to identify the points in the burn wound healing process where intervention can provide the best benefit with minimal risk of harm. Early intervention is widely discussed but requires clarification.16 Based on the wound assessment, ideally within 72 h, a wound predicted to take longer than 14 days to heal may be considered for surgical intervention. Where surgery is indicated, the goal is to undertake complete debridement and repair within one week from the time of the injury.17 Integral to the clinical care plan is the regular reappraisal of the progression of the healing. The inflammatory response is essential for wound healing but in burn injury excessive or prolonged inflammation may be implicated in the long-term scar outcome. Animal studies have demonstrated a greater cellular and cytokine response associated with a burn injury when compared with the equivalent excised wound.18 Further, the excision of the burn wound was associated with a more intense response when the excision was at day 6 compared with day 2 post injury.18 A decision of when and how to intervene in burn wound healing may be hampered by a lack of objective wound assessment tools and is often based on clinical judgment alone.11,19
This study aims to quantify the influence of TTH on modified Vancouver Scar Scale (mVSS) assessed scar quality after burn injury. This may assist the clinical decision-making process, particularly with partial thickness burns where there is ambiguity surrounding the diagnosis and comparison of the depth of injury between study cohorts. We hypothesised that, after adjusting for severity, increased burn wound healing time results in a worse mVSS score.
Materials and methods
Study sample
Those included in this study comprised a subset of adult burns patients who received a scar assessment at Royal Perth Hospital (RPH) from January 2006 to March 2013. As part of routine clinical care, patients were scheduled for scar assessment using the mVSS at four to six weeks and again at three, six, 12 and 24 months post burn to guide management. The study sample was a subset of those in the scar outcome data base whose TTH was either recorded or retrieved from the medical records. The most recent scar, visible at 3 m, with the highest mVSS score, obtained within six months of injury, was used to determine if scar outcome was associated with TTH. This time point was chosen to maximise the availability of the data for the most mature scar as there is significant loss to follow-up beyond this point.20
Data collection
Data were collected routinely using the mVSS as part of the RPH scar assessment protocol.21 This involved two trained, experienced occupational therapists who viewed the scar with all pressure garments and bandages removed at least 15 min prior to the formal scar review. A 3 × 3 cm area of the worst scar on each body segment (limbs, chest, back, head) was identified by the assessor and quantified by the mVSS criteria (Table 1).22 The highest mVSS score for each patient on the database was the value used for analysis.
Table 1.
mVSS categories.
| Pigmentation | Vascularity | Pliability | Height |
|---|---|---|---|
| 0 = normal | 0 = normal | 0 = normal | 0 = normal/flat |
| 1 = hypo-pigmentation | 1 = pink | 1 = supple | 1 = > 0–1 mm |
| 2 = mixed pigmentation | 2 = red | 2 = yielding | 2 = > 1–2 mm |
| 3 = hyperpigmentation | 3 = purple | 3 = firm | 3 = > 2–4 mm |
| 4 = banding | 4 = > 4 mm | ||
| 5 = contracture |
TTH data were accessed from the patient’s medical record. As per standard practice, TTH was established after assessment by a senior clinician and documented in the medical record. Wound healing was assessed through visual evaluation which is a common and reliable method.23,24 The criteria used to establish final TTH was if 95% of the original total body surface area – burn (TBSA) burn had epithelialised and surgical intervention was not warranted or if active dressings were discontinued. The choice of definition was sourced from other studies using burn final wound healing as the outcome.25,26 In the RPH setting, wounds were assessed and dressed every two to five days depending on time from injury, thus it is possible for TTH to be overestimated as epithelialisation may have occurred in the days prior to review.
Other variables routinely recorded for each patient include TTH, age, gender, TBSA, length of stay (LOS) and surgical intervention (incidence of split skin graft). When available, data of interest missing from the scar database were retrospectively sourced from either the medical record or the burns patient information databases.
Outcome measure
The mVSS, is one of the most widely used tools for assessing and quantifying severity of an abnormal scar.27 The original VSS was developed in 1990.28 This became well established both clinically and in the literature when a modified version was issued.22 Its routine use was instigated at RPH in 2006 and has resulted in a large quantity of mVSS data being made available for analysis.29 Subsequently, a patient component was introduced with the Patient and Observer Scar Scale (POSAS) and has since been added to the scar assessment battery.30
The mVSS provides a numerical score of the worst portion of a scar, rating characteristics of pigmentation, vascularity, pliability and height (Table 1).31 Though the pigmentation component is not ordinal, researchers and clinicians have adopted an aggregate score to describe scar quality with increased scores generally indicative of a worse scar.32–34 The mVSS total score has the advantages of allowing a wide variety of scars to be measured and has demonstrated good inter-observer reliability (intraclass correlation coefficient [ICC] = 0.81), particularly when referring to the worse area of the scar, as was the case in this study.21,35 Reliablity of the mVSS individual components has been found to be reduced due to the limited range of values in each category which magnify small differences between raters.36
While validity of the scale has not been conclusively demonstrated, several studies have found the total score to provide some indication of scar severity. Nedelec et al. found that mVSS subscales aggregated (except pigmentation) consistently rated the most severe scar higher than the least severe scar. This aggregated score has been used as the cutoff for a receiver operating characteristic (ROC) analysis of other scar assessment tools.37 A second paper from the sample reported concurrent validity between each mVSS subscale and objective electronic scar assessement and concluded all were assessing the same traits.36 Wei found mVSS vascularity and pigmentation were correlated with dermoscopy while Draaijers demonstrated convergent validity between mVSS and POSAS (r = 0.89, P < 0.001).30,38 A study by Stewart in 2005 used linear least squares regression to show that mVSS total was associated with scar perfusion as measured by laser Doppler (r = 0.94) and laser speckle imaging (r = 0.89).39 Kaartinen employed Bayesian networks to demonstrate that mVSS and POSAS were highly dependent on each other and mVSS was dependent on pliability score.40 Further, in post-surgical scars the total score has been associated with time from surgery (P < 0.001) and scar adherence (r = 0.59, P < 0.001).41,42 Recently, a study demonstrated that of all the subscales, height dichotomised as 0, ≥ 1 had high sensitivity and specificity, concluding that it was the strongest indicator of hypertrophic scarring.43
Data analysis
Analysis of non-identifiable data was conducted using statistical software (STATA version 12, StataCorp, LP, TX, US). Significance was set at a level of P <0.05.
Mann–Whitney and Chi-square tests were used to compare characteristics of patients with and without TTH data and investigate potential bias introduced by missing data.
Linear regressions were performed to investigate associations between TTH (dependent variable) and covariates: age, gender, TBSA, surgery and LOS. A log transformation was applied to TTH to normalise the data for this analysis. Beta coefficients which are defined as the change in standard deviations of the outcome for a one standard deviation change in the covariate are reported along with unstandardised coefficients. Coefficients were exponentiated to produce estimates of proportional changes in TTH in order to be interpreted in terms of untransformed TTH. Model diagnostics were performed including a test for heteroskedasticity and normality of residuals. Due to departure of the residuals from normality, the final model was bootstrapped to produce robust standard error estimates.
The relationship between TTH and mVSS (dependent variable) was examined using scatter plots with linear and LOWESS fits and confirmatory spline regression models. A similar process was followed with TBSA. The mean mVSS scores in each of ten quantiles of TTH were plotted to investigate potential points at which the relationship (slope) changed. The choice of break point was verified against alternatives using Akaike’s Information criteria.
A multivariable linear piecewise regression was performed including all covariates (age, gender, TBSA, surgery, LOS). Non-significant variables were removed sequentially. Interaction terms were added to the regression analyses to evaluate whether the relationship between TTH, as a continuous variable, and mVSS changed according to surgery versus conservative management, age, gender and TBSA. This is the recommended statistical approach for evaluating differences in the relationship of interest between subgroups.44 Model diagnostics were performed.
A linear regression of mVSS was conducted with TTH ranked in ascending order to investigate the possible inaccuracies caused by potential over-estimation of TTH in some cases.
A second piecewise regression was performed using maximum likelihood estimation (MLE) to investigate potential bias due to missing TTH data in the sample. MLE computes a likelihood function for cases with complete data on all variables and a second for those with complete data on some variables and then maximises the two likelihoods. Rather than estimating a value for each missing data point, MLE estimates the mean that is most likely, from the observed data and is known to produce unbiased estimates.45
Many advocate the use of the individual components rather than the overall mVSS. In this instance, analysis of the mVSS components was hampered by failure to satisfy critical assumptions of the appropriate regression techniques such as the proportional odds assumption of ordinal logistic regression. This was likely due, in part, to the small number of patients with higher scores in each of the mVSS categories. Collapsing the categories within the component scores is one possible solution. However, this loses information and is counter-productive to the goal of grading the severity of the scar. Thus, scatter plots fitted with LOWESS curves were generated to illustrate the relationship between TTH and the subscales.
Ethical approval
This study was approved by the Western Australian Health Department Human Research Ethics Committee (reference 13-163). The project uses non-identifiable routinely collected data, under a waiver of consent for use in research purposes. Confidentiality and anonymity of participant data was maintained throughout. Patient privacy and confidentiality was fully protected in digital and hard copy formats.
Results
Sample information
There were 567 patients in the scar dataset, 295 of these were included in the analyses. Thirty-three (n = 33) were excluded as they received only ambulatory care as were an additional 39 with no assessment within six months of burn, leaving 494. A further 199 were excluded due to missing TTH data.
The demographic, injury and treatment characteristics of the sample are described in Table 2. Minor burns (≤ 15% TBSA) had a median time to healing of 23 days, compared with major burns (34 days).
Table 2.
Sample demographic, injury and treatment information.
| Variable | Summary information |
|---|---|
| Age (years) | 34 (15–85, 25) |
| TTH (days) | 24 (6–122, 16) |
| TBSA | 3 (0.05–45, 5) |
| LOS | 5 (1–71,10) |
| Surgery | 209 (71%) |
| Male gender | 206 (70%) |
| mVSS total | 5 (1–12, 3) |
| Pliability | 1 (0–5, 1) |
| Height | 1 (0–4, 1) |
| Vascularity | 2 (0–3, 2) |
| Pigmentation | 2 (0–3, 0) |
| TBSA 0–15% | 276 (94%) |
| TBSA > 15% | 19 (6%) |
For categorical variables: number and percentage presented.
For continuous variables: median, range and interquartile range presented.
LOS, length of stay; mVSS, modified Vancouver Scar Scale; TBSA, total body surface area – burn; TTH, time to healing.
The excluded group had a significantly higher proportion of surgical cases (P = 0.004), higher TBSA (P = 0.02), LOS (P = 0.01) and mVSS scores (P = 0.001).
Associations between covariates and TTH
A bootstrapped multivariable linear regression found that log TTH was significantly associated with surgery, TBSA and age as shown in Table 3. After transformation back to the original scale, TTH was found to be 31% higher in surgical than non-surgical patients and 15% higher in women compared to men. A one unit (1%) increase in TBSA was found to be associated with a 1.6% increase in time to healing while each additional year of age was associated with a 0.5% increase in TTH.
Table 3.
Regression model showing relationship between log TTH and covariates (n = 295, R2 = 0.1).
| Coefficient | P | 95% CI | Beta coefficient | |
|---|---|---|---|---|
| Surgery | 0.28 | < 0.001 | 0.15, 0.42 | 0.23 |
| TBSA | 0.02 | < 0.001 | 0.01, 0.03 | 0.19 |
| Gender (female) | 0.14 | 0.065 | −0.01, 0.28 | 0.11 |
| Age | 0.005 | 0.03 | 0.001, 0.01 | 0.13 |
| Constant | 2.70 | < 0.001 | 2.5, 2.9 |
TBSA, total body surface area – burn; TTH, time to healing.
Relationship between TTH and mVSS-total
Univariate analysis of covariates found that TTH (linear coefficient 0.04, 95% CI 0.03, 0.05, P < 0.001), TBSA (coefficient 0.12, 95% CI 0.08, 0.15, P < 0.001), LOS (coefficient 0.12, 95% CI 0.09, 0.14, P < 0.001), surgery (coefficient 1.53, 95% CI 1.02, 2.04, P < 0.001) and age (coefficient 0.02, 95% CI 0.01, 0.04, P = 0.009) were significantly associated with mVSS-total while gender (coefficient 0.32, 95% CI −0.22, 0.85, P = 0.24) and time since burn were not associated (3 months vs. 1 month: coefficient 0.04, 95% CI −0.61, 0.69, P = 0.9; 6 months vs. 1 month: coefficient −0.28, 95% CI −0.87, 0.31, P = 0.35). Co-linearity between TBSA and LOS was also identified (IRR = 1.11, P < 0.001) resulting in TBSA being selected for inclusion in the final multivariable model.
A scatter plot fitted with a LOWESS curve demonstrated the relationship between TTH and mVSS to be non-linear with a flattening of the slope noted around the three-week mark. A plot of the mean mVSS scores in each of ten quantiles of TTH confirmed 21 days as the point of change in the curve.
The piecewise regression found that TTH, TBSA and surgery were identified to be significantly associated with mVSS (Table 4). Age became non-significant when included with the other variables. After adjusting for TBSA and surgery each additional day of healing during the first 21 days was associated with an increase in mVSS. After 21 days, the rate of increase in mVSS was slower (Figure 1).
Table 4.
Piecewise regression model of TTH and mVSS total score (n = 295, R2 = 0.3).
| Coefficient | P | 95% CI | Beta coefficient | |
|---|---|---|---|---|
| TTH days <= 21 | 0.11 | < 0.001 | 0.05, 0.17 | 0.19 |
| TTH days > 21 | 0.02 | 0.004 | 0.01, 0.03 | 0.16 |
| TBSA | 0.10 | < 0.001 | 0.07, 0.13 | 0.30 |
| Surgery | 1.13 | < 0.001 | 0.66, 1.70 | 1.60 |
| Constant | 1.77 | 0.002 | 0.67, 2.87 |
mVSS, modified Vancouver Scar Scale; TBSA, total body surface area – burn; TTH, time to healing.
Figure 1.
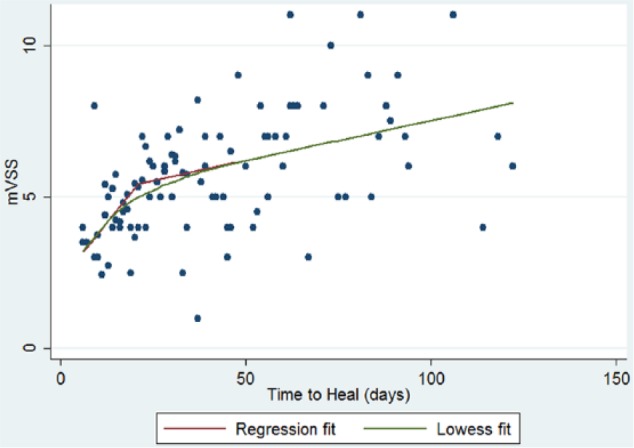
Scatter plot with LOWESS curve of piecewise regression model of relationship between TTH and mVSS total score.
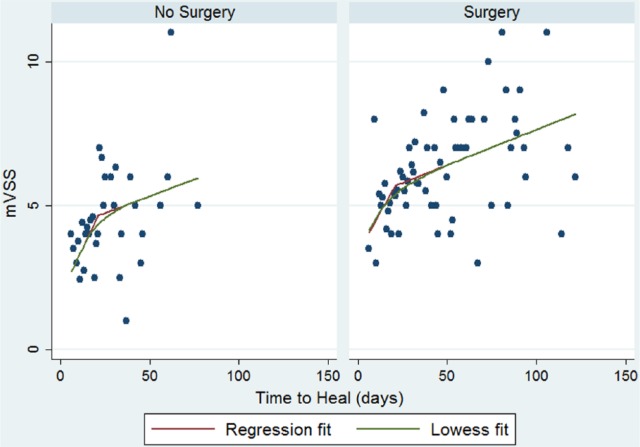
Use of interaction terms to investigate potential effect modification of the relationship between TTH and mVSS did not identify any significant variation in the relationship between TTH and mVSS due to surgical or conservative management, age, gender or size of burn. There may be unfamiliarity with interaction terms and concerns regarding potential differences in the pathophysiological process of healing of surgical compared to conservatively managed burn wounds. Therefore, these two groups were separated and individual regression models produced to further illustrate that the main relationship detected applies to both groups. Figure 2 shows that differences between the two separate models are small. The model for surgical patients most closely resembled the model for the overall sample given the greater proportion of this type of patient (71%).
Figure 2.
Scatter plot with LOWESS curve showing separate piecewise regression models of relationship between TTH and mVSS total score for conservatively and surgically managed patients.
A significant linear association was detected between TTH, presented as categorical data ranked in ascending order, and mVSS (coefficient: 0.01, 95% CI 0.004, 0.009, P < 0.001). This confirmed the positive association demonstrated previously by the piecewise regression model using continuous TTH data. The linear relationship equated to a beta coefficient of 0.28 which was slightly higher than the comparable coefficient for actual TTH (assuming a linear association) of 0.25. This suggests that the use of actual TTH was likely to be a more conservative estimate of the association.
Investigation of missing data
MLE estimates of the piecewise coefficients for TTH and mVSS total indicated a daily change of 0.14 (95% CI 0.07, 0.22, P < 0.001) for the first 21 days and 0.02 (95% CI 0.008, 0.04, P = 0.003) for greater than 21 days. This suggests that the missing data have contributed to an underestimation of the effects of TTH on mVSS_total, particularly for the first 21 days.
Relationship between TTH and mVSS component scores
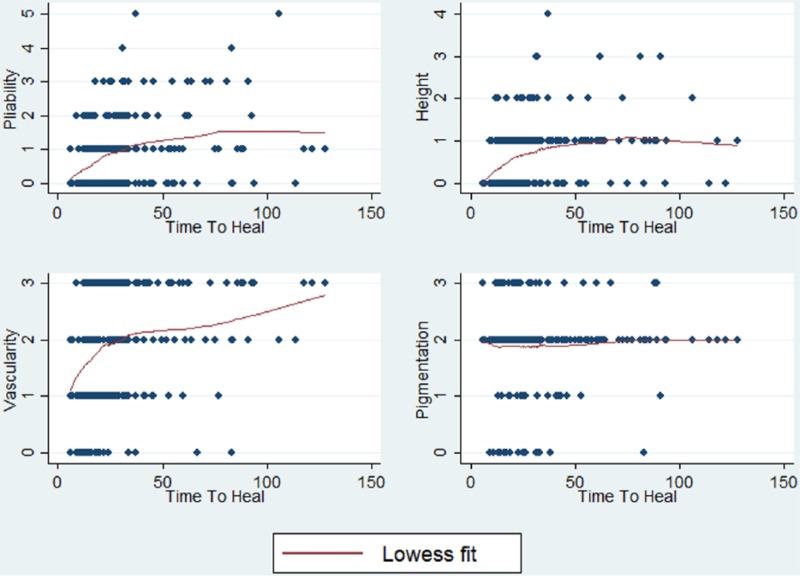
The association between TTH and the individual category scores was examined using scatter plots fitted with LOWESS curves which showed that pattern of the relationship of TTH with pliability and height was most similar to the overall mVSS score (Figure 3). In approximately 90% of the sample, pigmentation was rated as mixed and was essentially constant, having little influence on the overall total score.
Figure 3.
Scatter plots of TTH and mVSS component scores.
Discussion
This study indicates that scar quality, as measured by the mVSS, deteriorates with an increase in wound healing time in a sample of adults with burn scars. The results suggest that the majority of the risk of poorer scarring occurs in the first 21 days of healing. Scar quality worsened with every day post burn but with a relatively slower reduction with increased TTH beyond 21 days. This information reinforces the importance of achieving early wound healing and adds to the understanding of the relationship between TTH and mVSS rated scar outcome.
This study showed that an increase in standardised scar assessment scores, which probably indicates worsening scar severity, is associated with longer healing times. This occurred at a higher rate in the first 21 days post burn. Previous studies describing the effect of burn TTH on scar outcome have investigated the risk of developing hypertrophic or pathological scar.14,46 Findings based on samples of adults and children have showed that TTH greater than 21 days is a major determinant of whether or not a scar is hypertrophic.10,13
This study investigated the worst scars of a cohort of patients. It excluded those that did not have an observable scar, did not present for scar assessment or did not have TTH recorded. In addition, the sample comprised mostly small TBSA burns (< 15%) with relatively low severity scars as the median mVSS total score of five for the sample had been defined previously as a ‘good’ or low severity scar.29,33,47 It was not possible to retrospectively determine the scar severity of the patients lost to follow-up. Therefore, those who were more likely to scar could not be fully characterised. For those with missing TTH, MLE produced larger estimates of the effect of TTH when excluded sample data were incorporated in the analysis; however, the overall nature of the relationship between TTH and mVSS score was not affected. This was not unexpected given that burn injury characteristics (surgical intervention, TBSA, LOS, mVSS score) for those without TTH recorded were significantly worse than those with information available. Further, this may indicate the difficulty in establishing final wound closure for those with more severe burns.
Evaluation of data in this study was based on seven years of scar assessments conducted using the mVSS. At the time, this was the most widely used clinical tool. During this period, published data demonstrated the psychometric properties of the POSAS and this tool was added to the battery of scar outcome measures; however, not enough data were available to present as part of this research. The mVSS total score, along with the individual components, was explored due to issues of interpretability associated with incorporating categorical items. The mVSS total score is made up of a combination of scores from three ordinal items and one categorical item (pigmentation). Appropriate modelling of the individual components was not possible without combining categories resulting in a loss of information. Therefore, the mVSS total was retained as the main outcome. There are multiple other validity studies involving the mVSS that have not employed regression analyses of the components.36,38–41 Further, the mVSS total score was also responsive to change in TTH confirming previous responsiveness related to improved scores before and after AlloDerm® graft treatment of dyspigmented scars.48
As is to be expected in a sample that presented for scar assessment, the majority experienced skin graft surgery. The Burn Service of WA reviews wounds within seven days and surgical intervention planned if the burn is predicted to take longer than 14 days to heal with conservative treatment. Therefore, initiation of surgery is often indicative of a deeper, more severe injury than those to be managed conservatively. Patients who underwent skin graft surgery produced higher scar scores than conservatively managed patients. In this study, surgical intervention did not negatively influence the effect of TTH on mVSS score more than conservatively managed burns. Thus, scarring may be related more to the depth of the burn itself.10,49
The analysis identified some outliers where some wounds with longer TTH produced good scar scores. In the study sample, ten patients had mVSS scores ≤ 5 and a TTH ≥ 50, a similar finding observed by Hassan et al.14 Crude comparisons suggest that LOS was slightly longer, TBSA was smaller and age slightly higher for these patients. This group was consistent with the main sample for surgery and gender. However, this sample is too small for any formal analysis. Studies are planned to elucidate other factors influencing scar outcome, including use of the POSAS.
The findings of this study were limited by the retrospective nature of the data. While the addition of known covariates aimed to reduce variability in the relationship, information on other aspects purporting to influence both main predictor variable and outcome were not available. Psychological factors and co-morbidities such as diabetes are also known to affect TTH.50 Lack of data describing ethnicity and skin type of the sample along with other unknown factors that could impact on mVSS score may also affect generalisability of the results. In addition, although TTH may be overstated by up to five days, analysis of rank ordered TTH confirmed the influence of TTH on mVSS score. Finally, both variations in type and adherence to scar management may have had an impact on scar outcome despite the application of standard care. These issues will be addressed in future studies.
In adults who have a visible scar, increased burn wound TTH is related to worsening scar quality as rated by the mVSS. This effect is greater within the first 21 days post burn injury. Further investigation to evaluate the impact of patient factors and various interventions on time to healing along with other potential predictors of scar outcome may provide a more comprehensive picture of this relationship.
Acknowledgments
The authors thank the Burn Service of Western Australia Occupational Therapists involved in assessing scars using the modified Vancouver Scar Scale resulting in the substantial database that enabled this study. We are grateful to the Katie Piper Foundation for supporting the publication of this article.
Footnotes
Disclaimer: The views expressed in this article are the authors’ own and not an official position of the Fiona Stanley Hospital.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The Fiona Wood Foundation supported this work.
References
- 1. Williams N, Stiller K, Greenwood J, et al. Physical and quality of life outcomes of patients with isolated hand burns- a prospective audit. J Burn Care Res 2012; 33(2): 188–198. [DOI] [PubMed] [Google Scholar]
- 2. Wang Z, Zhang J, Lu S. Objective evaluation of burn and post-surgical scars and the accuracy of subjective scar type judgement. Chin Med J (Eng) 2008; 20(121): 2517–2520. [PubMed] [Google Scholar]
- 3. Lawrence J, Mason S, Schomer K, et al. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. Journal of Burn Care & Research 2012; 33(1): 136–146. [DOI] [PubMed] [Google Scholar]
- 4. van Loey N, van Son M. Psychopathology and psychological problems in patients with burn scars- Epidemiology and management. Am J Clin Dermatol 2003; 4(4): 245–272. [DOI] [PubMed] [Google Scholar]
- 5. Falder S, Browne A, Edgar D, et al. Core outcomes for adult burns survivors: a clinical overview. Burns 2009; 35(5): 618–631. [DOI] [PubMed] [Google Scholar]
- 6. Martin L. Social challenges of visible scarring after severe burn: A qualitative analysis. Burns 2017; 43(1): 76–83. [DOI] [PubMed] [Google Scholar]
- 7. Finnerty C, Jeschke M, Branski L, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016; 388(10052): 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gangemi E, Carnino R, Stella M. Videocapillaroscopy in postburn scars: in vivo analysis of the microcirculation. Burns 2010; 36(6): 799–805. [DOI] [PubMed] [Google Scholar]
- 9. Chan Q, Harvey J, Graf NS, et al. The correlation between time to skin grafting and hypertrophic scarring following an acute contact burn in a porcine model. J Burn Care Res 2012; 33(2): e43–48. [DOI] [PubMed] [Google Scholar]
- 10. Deitch EA, Wheelaham TM, Rose MP, et al. Hypertrophic burn scars: analysis of variables. J Trauma 1983; 23: 895–898. [PubMed] [Google Scholar]
- 11. Monstrey S, Hoeksema H, Verbelen J, et al. Assessment of burn depth and burn wound healing potential. Burns 2008; 34(6): 761–769. [DOI] [PubMed] [Google Scholar]
- 12. Hoeksema H, Van de, Sijpe K, Tondu T, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns 2009; 35(7): 36–45. [DOI] [PubMed] [Google Scholar]
- 13. Cubison TCS, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald. Burns 2006; 32(8): 992–999. [DOI] [PubMed] [Google Scholar]
- 14. Hassan S, Reynolds G, Clarkson J, et al. Challenging the dogma: relationship between time to healing and formation of hypertrophic scars after burn injuries. J Burn Care Res 2014; 35(2): 118–124. [DOI] [PubMed] [Google Scholar]
- 15. Makboul M, Makboul R, Abdelhafez AH, et al. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J Cosmet Dermatol 2014; 13(3): 169–179. [DOI] [PubMed] [Google Scholar]
- 16. Janzekovic Z. The burn wound from a surgical point of view. J Trauma 1975; 15(1): 42–62. [PubMed] [Google Scholar]
- 17. Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma 1970; 10(12): 1103–1108. [PubMed] [Google Scholar]
- 18. Fear V, Poh W, Valvis S, et al. Timing of excision after a non-severe burn has a significant impact on the subsequent immune response in a murine model. Burns 2016; 42(4): 815–824. [DOI] [PubMed] [Google Scholar]
- 19. Jeng J, Bridgeman A, Shivnan L, et al. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgement: a prospective blinded trial. Burns 2003; 29(7): 665–670. [DOI] [PubMed] [Google Scholar]
- 20. Finlay V, Burke K, van de, Ruit C, et al. Assessing the impact of missing data in evaluating recovery from minor burn injury. Burns 2009; 35(18): 1086–1091. [DOI] [PubMed] [Google Scholar]
- 21. Gankande TU, Wood FW, Edgar DW, Duke JM, DeJong H, et al. A modified Vancouver Scar Scale linked with TBSA (mVSS-TBSA): Inter-rater reliability of an innovative burn scar assessment method. Burns 2013; 39(6): 1142-9. [DOI] [PubMed] [Google Scholar]
- 22. Baryza M, Baryza G. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil 1995; 16(5): 535–538. [DOI] [PubMed] [Google Scholar]
- 23. Bloemen M, van Zuijlen P, Middlekoop E. Reliability of subjective wound assessment. Burns 2011; 37(4): 566–571. [DOI] [PubMed] [Google Scholar]
- 24. Gravante G, Montone A. A rerospective analysis of ambulatory burn patients: focus on wound dressings and healing times. Ann R Coll Surg Engl 2010; 92(2): 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verbelen J, Hoeksema H, Heyneman A, et al. Aquacel(®) Ag dressing versus Acticoat™ dressing in partial thickness burns: a prospective, randomized, controlled study in 100 patients. Part 1: burn wound healing. Burns 2014; 40(3): 416–427. [DOI] [PubMed] [Google Scholar]
- 26. Sharpe J, Booth S, Jubin K, et al. Progression of wound pH during the course of healing in burns. J Burn Care Res 2013; 34(3): e201–208. [DOI] [PubMed] [Google Scholar]
- 27. Tyack Z, Simons M, Spinks A, et al. A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 2012; 38(1): 6–18. [DOI] [PubMed] [Google Scholar]
- 28. Sullivan T, Smith J, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil 1990; 11: 256–260. [DOI] [PubMed] [Google Scholar]
- 29. Gankande TU, Wood FM, Edgar DW, et al. A modified Vancouver Scar Scale linked with TBSA (mVSS-TBSA): Inter-rater reliability of an innovative burn scar assessment method. Burns 2013; 39(6): 1142–1149. [DOI] [PubMed] [Google Scholar]
- 30. Draaijers L, Tempelman F, Botman Y, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004; 113(7): 1966–1967. [DOI] [PubMed] [Google Scholar]
- 31. Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the Vancouver scar scale and scar volume. J Burn Care Rehabil 2000; 21: 205–212. [DOI] [PubMed] [Google Scholar]
- 32. Lau J, Li-Tsang C, Zheng Y. Application of tissue ultrasound palpation system (TUPS) in objective scar evaluation. Burns 2005; 31(4): 445–452. [DOI] [PubMed] [Google Scholar]
- 33. Thompson C, Hocking A, Honari S, et al. Genetic risk factors for hypertrophic scar development. J Burn Care Res 2013; 34(5): 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hultman C, Friedstat M, Edkins R, et al. Laser resurfacing and remodeling of hypetrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg 2014; 260(3): 519–529. [DOI] [PubMed] [Google Scholar]
- 35. van der Wal MBA, Verhaegen PDM, Middlekoop E, et al. A clinimetric overview of scar assessment scales. J Burn Care Res 2012; 33(2): 79–87. [DOI] [PubMed] [Google Scholar]
- 36. Nedelec B, Correa J, Rachelska G, et al. Quantitative measurement of hypertrophic scar: Interrater reliability and concurrent validity. J Burn Care Res 2008; 29(3): 501–511. [DOI] [PubMed] [Google Scholar]
- 37. Nedelec B, Correa J, Rachelska G, et al. Quantitative measurement of hypertrophic scar: intrarater reliability, sensitivity and specificity. J Burn Care Res 2008; 29(3): 489–500. [DOI] [PubMed] [Google Scholar]
- 38. Wei Y, Li-Tsang C, Luk D, et al. A validation study of scar vascularity and pigmentation assessment using dermoscopy. Burns 2015; 41(8): 1717–1723. [DOI] [PubMed] [Google Scholar]
- 39. Stewart C, Frank R, Forrester K, et al. A comparison of two laser-based methods for determination of burn scar perfusion: laser Doppler versus laser speckle imaging. Burns 2005; 31(6): 744–752. [DOI] [PubMed] [Google Scholar]
- 40. Kaartinen I, Valisuo P, Bochko V, et al. How to assess scar hypertrophy - a comparison of subjective scales and spectocutometry: a new objective method. Wound Rep Reg 2011; 19(3): 316–323. [DOI] [PubMed] [Google Scholar]
- 41. Truong P, Abnousi F, Yong C, et al. Standardized assessment of breast cancer surgical scars integrating the Vancouver Scar Scale, Short-Form McGill Questionnaire and patients’ perspectives. Plast Reconstr Surg 2005; 116(5): 1291–1299. [DOI] [PubMed] [Google Scholar]
- 42. Ferriero G, Vercelli S, Salgovic L, et al. Validation of a new device to measure post surgical scar adherence. Phys Ther 2010; 90(5): 776–783. [DOI] [PubMed] [Google Scholar]
- 43. Thompson C, Sood R, Honari S, et al. What score on the Vancouver Scar Scale constitutes a hypertrophic scar? Results from a survey of North American burn-care providers. Burns 2015; 41(7): 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altman D, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003; 326(7382): 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Enders C, Bandalos D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling 2001; 8(3): 430–457. [Google Scholar]
- 46. Gangemi E, Gregori D, Berchialla P, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 2008; 10(2): 93–102. [DOI] [PubMed] [Google Scholar]
- 47. Lim J, Lum C, Tan A, et al. Long term sensory function after minor partial thickness burn: a pilot study to determine if recovery is complete or incomplete. Burns 2014; 40(8): 1538–1543. [DOI] [PubMed] [Google Scholar]
- 48. Oh S, Kim Y. Combined AlloDerm® and thin skin grafting for the treatment of postburn dyspigmented scar contracture of the upper extremity. J Plast Reconstr Aesthet Surg 2011; 64(2): 229–233. [DOI] [PubMed] [Google Scholar]
- 49. Pape S, Baker R, Wilson D, et al. Burn wound healing time assessed by laser Doppler imaging (LDI). Part 1: Derivation of a dedicated colour code for image interpretation. Burns 2012; 38(2): 195–202. [DOI] [PubMed] [Google Scholar]
- 50. Wilson E, Wisely J, Weardon A, et al. Do illness perceptions and mood predict time to healing for burn wounds? A prospective, preliminary study. J Psychosom Res 2011; 71(5): 364–366. [DOI] [PubMed] [Google Scholar]
How to cite this article
- Finlay V, Burrows S, Burmaz M, et al. Increased burn healing time is associated with higher Vancouver Scar Scale score. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/2059513117695324. [DOI] [PMC free article] [PubMed] [Google Scholar]