Abstract
Introduction:

Current guidelines suggest a multimodal approach to treating scars but there is no gold standard for treatment; however, there is exciting therapeutic potential for the use of autologous fat grafting (AFG). Functional and aesthetic improvements have been reported, including pain relief and scar quality improvement.
Aims:
To explore the current evidence regarding the use of AFG in hypertrophic and painful scars.
Methods:
A systematic review of the literature was conducted using 11 MeSH terms in PubMed, Medline and EMBASE. English studies that used AFG to treat scars in human participants were included.
Results:

A total of 746 studies were found and 23 studies (from 2008 to 2016) were included: five studies were evidence level V; nine studies were evidence level IV; eight were evidence level III; and one study was evidence level II. A total of 1158 patients were assessed for improvement in scar characteristics including colour, thickness, volume, pain and restoration of function at affected sites, following treatment. Positive outcomes were noted for all parameters and a significant improvement in AFG’s analgesic effect was recorded in 567 out of 966 patients, P < 0.05.
Discussion:

AFG is a minimally invasive and safe approach to treating scars, a promising alternative to surgical excision. The technique of blunt cannula insertion optimises the release of scar retraction, which contributes to the analgesic effect of this treatment method. The evidence supports current theories of mesenchymal stem cell’s regenerative and anti-inflammatory properties responsible for scar healing. There are limited high quality studies to support its use and future randomised controlled trials should be conducted.
Keywords: Autologous fat grafting, fat transfer, healing, lipofilling, pain, remodelling, scars
Lay Summary
Currently there is no gold standard treatment for scars, however there is exciting potential for the use of autologous fat grafting (AFG). This technique uses liposuction to extract fat from areas of the body where it is readily available. It is then processed to remove all debris and injected under a scar. We carried out a study to review its use in clinical practice.
Results of the literature suggest fat contains specialised stem cells that possess properties which improve the quality of scars. Fat injection has been shown to decrease tension which softens scar tissue and provides pain relief. Analgesic effects are caused by nerve repair and scar entrapment release. As well as functional benefits, the evidence also shows fat grafting improves the cosmetic appearance of a scar. In conclusion, AFG is a safe treatment method with few complications. This method poses promising benefits in future treatment of scar-related conditions.
Introduction
There is currently no gold standard treatment for scars; however, there is promising therapeutic potential for autologous fat grafting (AFG), a treatment pioneered by numerous institutions.1,2 The key aims of scar revision are to camouflage the tissue to the surrounding skin and restore functional capacity at the site.3 Neuropathic pain may also be experienced in scars, caused by the entrapment of nerve fibres in scar tissue. AFG harnesses the regenerative properties of adipose derived stem cells (ADSC) to restore function, improve appearance and provide analgesia.4
Current guidelines for scar treatment support a multi-modal approach, with the best results following combination therapy of laser treatment, 5-fluoracil and corticosteroids for hypertrophic and keloid scars. Newer therapies include mitomycin c and onion extract, which are currently growing in interest alongside AFG.1,2
AFG has had an expanding role in plastic surgery due to its filling effects and regenerative properties.5 Historically it was used to treat wounds with tissue loss and congenital deformities.6 However, the introduction of liposuction increased its availability and its clinical applications to treat facial lipoatrophy, breast surgery and buttock augmentation.7–9 Coleman (1995) further evolved its application when he described a reproducible technique of injecting lipoaspirates.10 This increased the survival of harvested fat, which is now an established method of administering autologous grafts.
Rigotti et al. (2007) first described the regenerative capacity of ADSC to treat chronic oncological lesions after radiation therapy.7 They reported an improvement of lesions that had previously been irreversible, with hydration and neo-angiogenesis. Following this, Klinger et al. (2008) documented the first use of AFG in treating scars.11 This case series included three patients with burns of the face, hands, trunk and limbs, who were treated with two sessions of AFG and followed up after six months. A positive outcome was reported with increased softness and, improved texture and thickness of scar tissue. Histological staining also demonstrated tissue architectural remodelling, supporting Riggoti et al.’s theory of ADSC’s regenerative potential.7,11
Aims
The aim of this study was to carry out a detailed search of the current literature assessing the efficacy of AFG in treating scars and analgesia. In doing so, we aim to test the hypothesis of the regenerative capabilities of adipose tissue and develop further evidence of the role of ADSC in scar healing and analgesia. The evidence can be used to guide future treatments and construct further research in this area.
Methods
A systematic literature search was carried out by two independent authors using the PubMed, EMBASE and Medline databases. 11 MeSH terms were used to conduct the search, based on keywords used in literature on AFG treatment of scars (Table 1).12,13 Variations of the terms were included to ensure inclusion of all relevant articles, e.g. ‘fat graft’ could be substituted with ‘adipose graft’ or ‘lipofilling’. All possible combinations of terms were entered in the databases. Filters were applied, in accordance with pre-determined inclusion criteria (Table 2). Studies using human participants, written in the English language and fully accessible via PubMed, EMBASE and Medline, were included.
Table 1.
MeSH terms used in literature search.
| MeSH terms used for search |
|---|
| Autologous fat grafting |
| Fat transplant/transfer/grafting |
| Scar healing/remodelling/correction |
| Wound healing |
| Analgesia/pain |
| Lipofilling |
Table 2.
Inclusion criteria to filter articles.
| Inclusion criteria |
|---|
| Studies written in English language |
| Human studies |
| Interventional studies using autologous fat grafting for scars including case series, case-control, cohort studies and randomised controlled trials |
| Full article accessible |
| All scar types were included, e.g. retractile, keloid and hypertrophic |
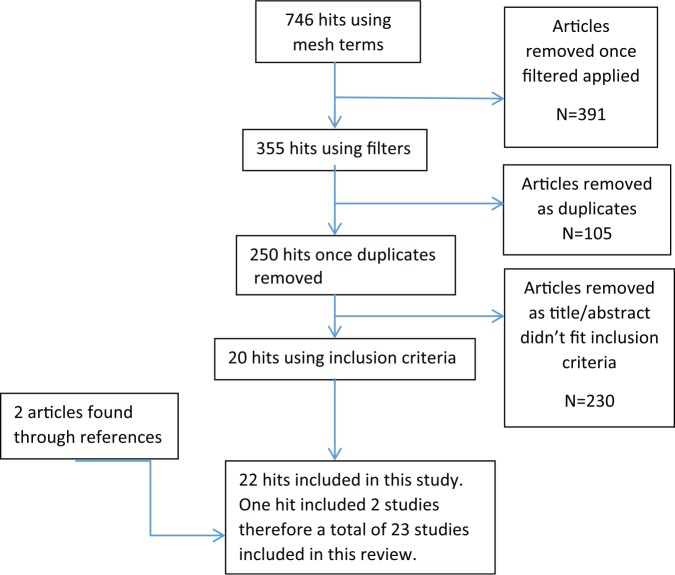
Articles returned from the search were screened using titles and abstracts. Eligibility was assessed independently by two authors. Studies that reported the outcomes of AFG as an intervention for scar healing and analgesia were included. The search was not restricted to any one scar type and included studies that used AFG with or without supplementary treatments. References of articles included in the search were reviewed to assess if further studies could be included (Figure 1). Full texts of the articles were read to further determine compliance with inclusion criteria and aims of this review. All articles returned from the search were categorised into levels of evidence based on the Oxford Centre of Evidence-Based Medicine (OCEBM) levels of evidence (Table 3).14 No formal statistical analysis was carried out due to the heterogeneity of outcome measures and quantitative data in the studies included.
Figure 1.
Diagram showing method used to determine articles included in this review.
Table 3.
Levels of evidence per OCEBM.
| Author and year | Study design | OCEBM level | n |
|---|---|---|---|
| Byrne et al. (2016)19 | Case series | IV | 13 |
| Admani et al. (2015)15 | Case report | V | 1 |
| Guerrissi et al. (2015)25 | Case series | IV | 4 |
| Huang et al. (2015)3 | Case series | IV | 13 |
| Klinger et al. (2015)27 | Case report | V | 1 |
| Lisa et al. (2015)28 | Case report | V | 1 |
| Bollero et al. (2014)17 | Case series | IV | 19 |
| Zellner et al. (2015)33 | Case-control | III | 35 |
| Dini et al. (2014)23 | Case report | V | 1 |
| Gentile et al. (2014)24 | Case-control | III | 30 |
| Pallua et al. (2014)31 | Case series | IV | 26 |
| Maione et al. (2014)29 | Case-control | III | 36 |
| Azzam et al. (2013)16 | Prospective cohort study | III | 20 |
| Bruno et al. (2013)18 | Case-control | III | 93 |
| Klinger et al. (2013)5 | Case series | IV | 694 |
| Klinger et al. (2013)5 | Case control | III | 20 |
| Mazzola et al. (2013)30 | Case series | IV | 10 |
| Guisantes et al. (2012)26 | Case series | IV | 8 |
| Ulrich et al. (2012)32 | Prospective cohort study | III | 20 |
| Caviggioli et al. (2011)21 | Retrospective cohort study | II | 113 |
| Cervelli et al. (2011)22 | Prospective cohort study | III | 60 |
| Caviggioli et al. (2008)20 | Case report | V | 1 |
| Klinger et al. (2008)11 | Case series | IV | 3 |
Results
All 23 studies adopted liposuction to harvest autologous fat grafts. 15 studies processed AFG according to Coleman’s technique involving 1062 patients.3,5,17–21,24–29,31 Additional methods included simple filtration and Telfa rolling.23,33 Most commonly the aspirate was centrifuged at 3000 rpm for 3–5 min. Aspirate was injected under scar tissue at the dermal-hypodermal junction and a technique of retrograde fat deposition was used, allowing fat distribution in multiple planes, dispersed in a web-like matrix. Eight studies reported that fat distribution was matched in importance by the scar release induced through needle insertion at the site.3,5,11,19,21,26,27,30 This technique achieved reduced skin contracture and increased tissue viability.
Donor sites included the abdomen, medial thigh, hips and trochanteric regions. The abdomen was used as a donor site in 16 studies and was the only site in 11 studies, in 340 patients.3,5,11,15,17–22,26–28,30,31 This donor site is preferable due to the abundance of fat and ease of access with the patient supine.
The number of sessions varied among studies, with 13 studies using one session of AFG treatment.3,5,18,20–24,28,29,31,33 Ten articles report using more than one session in some or all participants.10,15–17,19,25–27,30 Mazzola et al. (2013) states that volume filling is the first step and further scar quality is achieved through a second session.30 Studies were guided by further treatment based on contour and volume filling or by outcome improvement, e.g. pain, itch and restoration of function. Average follow-up was 11.4 months (range 3–60 months). Long-term follow-up mostly occurred up until 12 months and loss to follow-up was only reported by Caviggioli et al. (2011).21
All 23 articles assessed cosmetic changes post treatment, e.g. colour, scar appearance in relation to surrounding skin and thickness. 12 articles studied pain relief; six articles used the Patient and Observer Scar Assessment Scale (POSAS), which included assessment of relief of pain and itch alongside pliability, vascularisation and scar surface area;3,5,19,21,24,27–32 two articles used histological outcomes to assess microscopic changes at the cellular level; and six articles assessed functionality at the site of the scar post treatment.10,17–20,23,25,27 Outcome measures were heterogeneous, involving both quantitative and qualitative data.
Volume injected
Volume of fat injected was in the range of 0.5–80 mL, reported in 15 articles.3,5,15,20–23,25–28,30,32,33 Guerrissi et al. (2015) reported the largest volume injection of 80 mL in a 21-year-old man with circumferential burns of the right leg.25 The volume injected was proportional to the extent of scarring and surface area affected. Klinger et al. (2013) suggests that 1 mL AFG for each 3.5cm2 should be used and Piccolo et al. (2015) suggested 1.6–2.0 mL per 10 cm2.5,34 No other studies reported a volume : scar size method for calculating injected AFG volume. The degree of scar retraction more commonly guided volume of fat injected.
Pain and itch
The analgesic effect of AFG was assessed in 966 patients.3,5,19,21,24,27–32 In total, 832 patients had an improvement in pain following treatment; of these, 567 showed a significant improvement. This was measured using POSAS, visual analogue scale (VAS), the neuropathic pain symptom inventory (NPSI), Manchester scar scale (MSS), present pain intensity index and the McGill pain questionnaire. Caviggioli et al. (2011) used AFG to treat post-mastectomy pain and 28 out of 34 patients withheld analgesia following lipofilling.21 Furthermore, pain reduction post AFG was significant, P < 0.05 in the treatment group compared with controls. AFG’s analgesic effects were reported for facial scars, perineal post-surgical scars, burns and vaginal lacerations. Pain relief was reported as early as 14 days’ follow-up.
POSAS outcomes were assessed for 126 patients in six studies; however, data were available for 95 patients only. An improvement in patient and observer outcomes for scar colour, regularity and thickness were reported. A total of 82 patients reported a significant improvement in pain and itch following treatment.5,19,24,28,29,31
Restored function
Five articles aimed to restore function at the site of the scar tissue in 713 patients, summarised in Table 4.5,19,20,25,27 Functional deficits included limited facial movements with facial burns and reduced mobility of joints due to scar retraction. AFG restored functional capacity in these patients. Guerrissi et al. (2015) demonstrated this with restoration of leg extension after treatment of retracted scars following burn injury.25
Table 4.
Studies assessing the effect of AFG on functionality.
| n | Functional deficit | Outcome | |
|---|---|---|---|
| Byrne et al. (2016)19 | 13 | Range of motion of hands | + |
| Guerrissi et al. (2015)25 | 4 | Leg extension + flexion Ring finger immobility Thumb opposition Cervical immobility |
+ + + + |
| Klinger et al. (2015)27 | 1 | Facial movements | + |
| Klinger et al. (2013)5 | 694 | Joints Eyelids Facial movements Nasal valve |
+ + + + |
| Caviggioli et al. (2008)20 | 1 | Eyelid function | + |
Scar appearance and tissue hardness
All 23 articles commented on the scar appearance and cosmetic restoration post AFG treatment. Positive outcomes were reported in all studies regarding appearance of scars, including erythema, contouring, elasticity, thickness, texture, softness and colour. Significant results were reported in four articles. Klinger et al. (2013) reports improvement in all POSAS parameters, both patient and observer after three months of follow-up.5 All these parameters were significant compared to controls except pain and itch (Tables 5 and 6).
Table 5.
Summary of effect of supplementary additions on scar tissue.
| OCEBM | n | Supplementary treatment | Outcome | |
|---|---|---|---|---|
| Admani et al. (2015)15 |
V | 1 | AFG + PDL + AFR | Improved skin contracture, erythema + contouring with combination treatment |
| Gentile et al. (2014)24 |
III | 30 | SFV vs. platelet-rich plasma (PRP) vs. AFG | Stromal vascular fraction (SVF) and PRP enhanced AFG showed a greater improvement than AFG alone, P < 0.05. |
| Azzam et al. (2013)16 |
III | 20 | Fractional CO2 laser vs. AFG | Greatest improvement in AFG group compared with fractional CO2 laser |
| Cervelli et al. (2011)22 |
III | 60 | Laser treatment vs. PRP vs. both | Laser treatment + PRP enhanced AFG treatment improved scars more than either treatment alone |
PDL: pulse dye laser; AFR: ablative fractional laser resurfacing.
Table 6.
Summary of scar features using POSAS.
| Observer assessment of
scar |
Patient assessment of
scar |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascularity | Pigmentation | Thickness | Relief | Pliability | Surface area | Pain | Itch | Colour | Stiffness | Thickness | regularity | |
| Byrne et al. (2016)19 |
NA | NA | NA | NA | NA | NA | * | * | † | † | † | † |
| Lisa et al. (2015)28 |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gentile et al. (2014)24 |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Maione et al. (2014)29 |
† | † | † | † | † | † | † | ‡ | † | † | † | † |
| Pallua et al. (2014)31 |
‡ | † | ‡ | ‡ | † | ‡ | † | † | ‡ | † | ‡ | † |
| Klinger et al. (2013)5 |
† | † | † | † | † | † | † | + | † | † | † | † |
No improvement post AFG treatment.
Significant improvement post AFG treatment (P < 0.05).
Improvement post AFG treatment.
NA, information not available.
Durometer measurements were used to report tissue hardness. Maione et al.29 and Klinger et al.5 both report a significant reduction in durometer measurements, three months after AFG treatment in 56 patients, compared with preoperative scores (Table 7).
Table 7.
Summary of articles included in this review.
| Reference | n | Scar profile | Aims of study | Fat injection technique/method | MFU | Outcome measure | Summary of results |
|---|---|---|---|---|---|---|---|
| Byrne et al. (2016)19 |
13 | Burn scars that reduce range of motion of the hand | Use of AFG to treat burn scars and increase mobility and hand movements | 9 women and 4 men (mean age 40 years; age range 19–64
years). Fat graft taken from the abdomen in 12 patients,
lateral thigh in 1 patient. Processed according to Coleman’s
technique 1200 rpm for 3 min then injected under the scar in
multiple planes. Procedure was repeated until the amount
injected was suitable. Volume injected = not specified |
9.1 months | - DASH - POSAS - MCQ |
Statistically significant improvement in total active movement of the hand from 146° to 170°. POSAS scores included significant improvements in scar colour, thickness and stiffness. However, there was no significant difference in itch, pain and grip strength postoperatively. No change in DASH and MHQ scores |
| Admani et al. (2015)15 |
1 | Atrophic and hypertrophic (dog bite) | Use a combination of AFG, AFR and PDL to treat a scar in a 3-year-old | Fat graft taken from umbilicus. Scar was pre-treated with
AFR and PDL. Fat injected deep into the scar. Nine
treatments repeated every 8 weeks. Volume injected = 5 mL |
19 months | Photographic evidence of: - erythema - scar texture - skin contracture - volume |
Scar colour, texture and contracture improved after
treatments and follow-up. Erythema continued to be treated. A multi-modal approach should be considered due to heterogeneous nature of scars |
| Geurrissi et al. (2015)25 |
4 | Retracted scars resulting from burns | Use of AFG to treat retracted scars from burn injuries and restore function at the site | 4 patients (age range 19–27 years) had fat grafts taken and
processed according to Coleman’s technique. AFG injected
under the scar. 2 patients had 2 treatments, 2 had 1
treatment. Volume injected = range 15–80 mL |
1 month | Subjective observation by patient and
surgeon: - appearance - texture - elasticity Patients report of mobility at site |
Improvement in elasticity and mobility at the site, e.g restoration of leg extension and flexion. This improvement noted soon after first treatment. Most significant improvement was texture, least significant was appearance. Filling effect aided by multiple treatments |
| Huang et al. (2015)3 |
13 | Painful neuropathic scars | Use of AFG in treating post-traumatic neuropathic scar pain in 13 patients with scars resulting from surgery, trauma and crush injuries | 9 male and 4 female patients were included (mean age 33
years; age range 19–75 years). The mean duration of pain was
4.29 months. Fat grafts were taken from the abdomen and
processed according to the Coleman’s technique 3000 rpm for
3 min. Lipoaspirate was injected under the scar at the
dermal hypodermal junction. |
19 months | VAS and NSPI at 1, 4 and 24 weeks | VAS score decreased from 7.51 to 4.38 after 1 week of
treatment (P < 0.009); at 4 weeks, 5.38;
and at 24 weeks 5.62. NSPI score decreased from 49.38 to 25 after 1 w eek (P < 0.004). Improvement in 77% of patients using VAS |
| Klinger et al. (2015)27 |
1 | Hypertrophic | Use of AFG to treat nasolabial fold scar, repair facial nerve damage and restore mouth movement in a 45-year-old man | Fat graft taken from right flank and processed according to
Colman’s technique, 3000 rpm for 3 min. AF was injected
under the scar and again 6 months later. Needle inserted in
anterograde direction to overcome scar
retraction. Volume injected = 13 mL |
15 months | Photographic evidence. Mimic facial movements and patients’ observation of effect on pain | Full restoration of facial mimic movements and diminished pain in the region of the scar. Increased scar release attributed to needle technique. AFG is promising for improvement of nerve dysfunction, associated pain and scar appearance |
| Lisa et al. (2015)28 |
1 | - Retractile 2 cm in length |
Use of AFG to treat a postsurgical scar of the oral mucosa of a 47-year-old. Resulting pain in shoulder during mastication effecting feeding and speaking | Fat graft taken from the right flank and processed according
to Coleman’s technique, 3000 rpm for 3 min, and injected
under the scar. Volume injected = 5 mL |
12 months | POSAS MRI scan at 3 months |
Observed scar release after one treatment and improvement in the scar appearance and a halt in analgesic consumption post treatment. Volume filling effect observed with MRI scan. AFG should be used to treat pain syndromes |
| Bollero et al. (2014)17 |
19 | Burn, traumatic and surgical scars | Determine the efficacy of AFG in treating scars by assessing graft resorption and microcirculation using CEUS, in 19 patients | Mean age of patients 40 years (age range 19–59 years).
Number of AFG sessions varied according to response. Total
of 28 procedures carried out. Fat grafts were taken from
abdomen, medial aspect of knee and hip. Injected using the
Coleman technique 3000 rpm for 3 min. Injected various
sites, breast, face, chest wall. CEUS and clinical
evaluation completed 1 month and 3 months postoperatively.
Procedure performed by 3 different people. 1 patient
operated on 4 times, 6 operated on twice. Volume injected = not declared |
3 months | - Clinical evaluation - Photographic evidence - CEUS measured vascularisation and thickness of subcutaneous tissue |
24 out of 28 procedures showed improved scar quality with restored contouring. Vascularisation increased in these compared with the 4 patients without improvement |
| Dini et al. (2014)23 |
1 | Atrophic | Use of AFG to treat alopecia areata and atrophic scarring of eyebrow in a 26-year-old | Fat graft taken from the medial knee and purified using
simple filtration. A single AFG injected under the scar in
various planes. Volume injected = 0.5 mL |
3 months | - Visual analysis | Hair growth noted at 3 months with cosmetic improvement of scar. Good filling effect observed. Promising alternative to steroids and hair transplant |
| Gentile et al. (2014)24 |
30 | Facial scar, e.g. burns and traumatic | Use of SVF-enhanced AFG vs. PRP-enhanced AFG vs. AFG | 10 patients (5 men, 5 women; age range 23–63 years) in the
SVF-enhanced group. 10 patients (5 men, 5 women; age range
21–69 years) in the PRP-enhanced group. 10 patients (5 men,
5 women) were included in the control group. AFG was
obtained and processed according to the Coleman technique at
3000 rpm for 3 min. Grafts were injected into ready-made
tunnels. Volume injected = not declared |
60 months | - Photographic documentation - MRI + US - POSAS |
Volume maintenance 63% in SVF-enhanced group compared to 39% in controls (P < 0.0001). Volume maintenance 69% in PRP-enhanced group compared with controls. Results lasted at 60 months and patients were satisfied with softness, texture and contouring |
| Pallua et al. (2014)31 |
26 | 35 facial scars, e.g. traumatic scars | Use of AFG to treat facial scars and effect on tissue microcirculation | 16 women and 10 men were included (mean age 46 years; age
range 22–64 years). Fat graft was taken from the abdomen and
processed according to Coleman’s technique 3000 rpm for 3
min. Fat injected under the scar. Follow-up was carried out
at 1, 3, 6 and 12 months. Volume injected = not declared |
12 months | Pre- and postoperative recording
of: • POSAS • photographic evidence • laser Doppler (Hb levels, microcirculation and tissue O2 sats) |
All patient and observer parameters improved
postoperatively. Parameters assessed by patients showed a
significant improvement in pain, colour, stiffness and
irregularity, P = 0.0331,
P = 0.0007, P = 0.0030
and P = 0.0039,
respectively. Parameters assessed by observers showed a significant improvement in pigmentation and pliability, P = 0.0282 and P = 0.0404, respectively. Slight increase in Hb and microcirculation postoperatively. No significant improvement in microcirculation |
| Maione et al. (2014)29 |
36 | Retractile scars post short-stature surgery | Use of AFG in improving painful and retractile scars from limb lengthening surgery in patients with short stature | 28 male and 8 female patients (mean age 16.5 years; age
range 14–18 years) and post short stature limb lengthening
surgical scars were included. Scars were divided into cases
where durometer measurements were highest and controls where
scars were thinnest. Fat grafts were processed according to
Coleman’s technique at 3000 rpm for 3 min and injected under
the scar at the dermal hypodermic junction. Fat was injected
in multiple planes in the manner of a web. Control region of
scars injected with saline. Volume injected = not declared |
3 months | Pre- and postoperative recording in cases an controls
of: • POSAS • durometer measurements |
Significant reduction in durometer measurements
postoperatively, P < 0.05. Significant
improvement in all POSAS observations, for patients and
observer parameters, except itch. Improvement first noted at 2 weeks |
| Zellner et al. (2015)33 |
35 | Primary cleft lip repair scars | Use of AFG to improve scarring post primary cleft lip repair. Compare intervention to controls | 35 patients (mean age 4.9 months; age range 2–20 months)
with cleft palates were included. 19 patients received AFG,
taken from medial thighs and was processed using Telfa
rolling. Additional scar care included vitamin E, silicone
gel and gentle massage. Both groups compared using the
t-test. Volume injected = 0.5–2 mL |
6 months | Photographic evidence (3 blinded
analysers). Five-point scale to analyse the cleft palate features of: • face • upper lip • nose • midface |
Significant improvement in the intervention group compared
to the control group in all facial areas, except the nose,
P < 0.05 and P <
0.06, respectively. Improvement in the intervention group with unilateral clefts compared with controls with unilateral clefts, with upper lip and face showing significant improvements, P < 0.05. Intervention group showed improvement at 6 months, lip changes being significant, P < 0.05. |
| Azzam et al. (2013)16 |
20 | Acne vulgaris scars | Compare fractional CO2 laser treatment to AFG in 20 patients with acne scars | 10 patients received 3 sessions of fractional CO2
laser treatment and 10 patients were treated with
AFG. Volume injected = not declared |
3 months | Photographic evidence judged by 4 physicians. Patient satisfaction survey post treatment, recording scar improvement as ‘Excellent’, ‘Marked’, ‘Moderate’ or ‘Mild’ | Improved scar texture seen in AFG group. 60% of patients found an excellent or marked improvement when treated with AFG compared with 20% treated with fractional CO2 laser. No significant difference in treatment recorded |
| Bruno et al. (2013)18 |
93 | Hypertrophic burn scars | Use of AFG to treat burn scars and to histologically analyse the remodelling that occurs in scars with this treatment | Mean age of patients 43 years (age range 18–92 years). Scars
divided into case and control. Fat graft taken from the
abdomen, medial aspect of the knee, thighs and trochanteric
areas was processed using Coleman’s technique at 1250 J for
3 min. AFG was injected under the scar treated as a
case. Volume injected = not declared |
6 months | Before treatment, at 3 and 6 months: - punch biopsies - 6 histological stains - 8 antibodies |
Microscopic changes: - organised collagen deposition - reduction in melanogenic activity - epithelialisation - reduction in VEGF expression and TGF-b |
| Klinger et al. (2013)5 |
694 | Painful and retractile scars affecting daily function, i.e. joint mobility | Use of AFG in treating multiple scar types including traumatic, surgical and burn scars | 694 patients with scars were included (mean age 38.3 years;
age range 16–62 years). Fat grafts were taken from the
abdomen or trochanteric areas and processed using the
Coleman technique. AFG was injected at the dermal–hypodermal
junction with deposition in multiple planes in a web
pattern. Patients were followed up at 5 and 14 days and 1,
3, 6 and 12 months. Volume injected = 1 mL for 3.5 cm2 area |
12 months | Pre- and postoperative assessment: • subjective narratives • photographic evidence |
Improvements first noted at 14 days, restoration of function
and appearance. Relief of pain and scar elasticity. Volume
restoration, colour similar to surrounding area and improved
mobility in areas including joints and eyelids. These
improvements noted at 12 months. Improvement noted in all patients. Reduction in pain and softness of scar tissue first noted at 14 days. Volume restoration and colour similar to surrounding area |
| Klinger et al. (2013)5 |
20 | Painful and retractile scars affecting daily function, i.e. joint mobility | Use of AFG in treating multiple scar types including traumatic, surgical and burn scars. Compare this to controls | 20 patients with scars were included. Fat grafts were taken
from the abdomen or trochanteric areas and processed using
the Coleman technique. The scar was divided, part treated by
AFG (case) and part treated with saline (control). AFG was
injected at the dermal–hypodermal junction with deposition
in multiple planes in a web pattern. Patients were followed
up at 5 and 14 days and 1 and 3 months. Volume injected = 1 mL for 3.5 cm2 area |
3 months | Pre- and postoperative
assessment: • POSAS • durometer measurements |
Postoperatively scars had a significantly reduced durometer measurement compared to preoperative measures. All POSAS parameters showed a significant postoperative improvement except for itch, e.g. pain, thickness, colour and hardness |
| Mazzola et al. (2013)30 |
10 | 10 tracheostomy scars. 3 tracheostomies from head and neck cancer and 7 remaining from ITU | Use of AFG to restore function and aesthetics to tracheostomy scars | 10 patients (mean age 33.5 years; age range 20–51 years)
were included with previous tracheostomies and resulting
scars, from 4–10 years ago. Fat graft taken from abdomen and
centrifuged at 1200 J for 3 min. An 18-gauge sharp needle
was inserted under the subcutaneous tissue of the scar to
interrupt the fibrotic bands before injection of fat in
multiple planes under the scar. The area was massaged post
AFG injection. 2 sessions were carried out 6–12 months
apart. 1st session was to alter depression and the 2nd to
enhance quality of the scar. 2 patients received a 3rd
session. Volume injected 1st session = 3–10 mL; volume injected 2nd session = 3–5 mL |
21.3 months | Pre- and postoperative recording
scar: • extension • colour • pliability • thickness • relief (itch) |
After session 1, all patients reported improvement in scar appearance. After session 2, volume correction was restored for 8 patients. 2 patients achieved this after a 3rd session. Improvement was noted in itching, pain and colour for all 10 patients and a reduction in thickness of scar appearance |
| Guisantes et al. (2012)26 | 8 | Retractile and dystrophic scars, e.g. appendicectomy and cholecystectomy scars | Use of AFG in scar correction in 8 patients with retractile and dystrophic scars | 2 male and 6 female patients were included (mean age 47
years). Fat grafts were taken from the thigh or abdomen and
processed according to Coleman’s technique 3000 rpm for 3
min. 3 patients received 2 sessions, the remainder had one.
Lipoaspirate injected under the scar in various
planes. |
18 months | Visual 4-grade scale: 1 = poor; 2 = regular; 3 = good; 4 = very good. Scored by 2 physicians |
All 8 patients reported improvement in appearance. 5 patients reported the postoperative appearance as ‘very good’ and 3 reported it as ‘good’ |
| Ulrich et al. (2012)32 |
20 | Vaginal and perineal scars post episiotomy | Use of AFG to treat chronic pain resulting from vaginal and perineal scar contracture post episiotomy | 20 patients (mean age 34 years) that had an episiotomy with
resulting scars were included. Patients’ mean time after
episiotomy was 10.3 months. All patients reported
pain-related symptoms post childbirth. All patients received
lipofilling under the scar, with 2 patients receiving a
second treatment. Mean volume injected = 12 mL (range 7–15 mL) |
6 months | Pre- and postoperative recording of: McGill pain questionnaire Present pain intensity index VAS |
Release of scar contracture resulted in immediate analgesia for 18 patients. A significant reduction in pain postoperatively compared to preoperative scores, determined by McGill Questionnaire after 1 month, P < 0.05. At 1, 3 and 6 months, there was a significant reduction in pain as shown by the Present pain intensity index and VAS, postoperatively, P < 0.05 |
| Caviggioli et al. (2011)21 | 113 | Painful post-mastectomy scars | Use of AFG to treat post-mastectomy pain syndrome and scar retraction | All patients had axillary dissection and radiotherapy and no
complications post surgery. 72 were treated with AFG, 42
were controls and not treated. Fat was taken from the
abdomen and processed according to Coleman’s technique at
3000 rpm for 5 min. Fat was injected under the scar at the
dermal hypodermic junction. Mean volume injected = 55 mL |
13 months | - Pre- and postoperative
questionnaire - VAS - Drug and analgesia intake |
Significant decrease in pain postoperatively in treated group vs. controls, P = 0.0005. 63 patients pain decreased in the treatment group and 28 out of 34 patients taking analgesia stopped post treatment. The decrease in pain in this group was greater than those that continued analgesia, 4.33 and 1.18, respectively |
| Cervelli et al. (2011)22 |
60 | Traumatic scars | AFG vs. PRP vs. Laser to treat traumatic scars | 60 patients (mean age 38 years; age range 22–45 years) with
traumatic scars were included, 20 randomly assigned to one
of the following groups: Group A, AFG + PRP; Group B, Laser;
and Group C, both treatments. PRP taken from patient’s blood
and AFG taken from the abdomen. Volume injected = 5–50 mL |
6 months | - Patient satisfaction questionnaire - Photographic evidence - MSS |
Group A = 18% improvement Group B = 30% improvement Group C = 45% improvement Both AFG + PRP with laser treatment is the best treatment for scars, with improvement in texture, colour and scar contours |
| Caviggioli et al. (2008)20 |
1 | Chemical burns | Use AFG to treat cicatricial ectropion of lower eyelid in a 43-year-old | Fat graft taken from abdomen and processed using Coleman’s
technique at 3000 rpm for 5 min. Lipoaspirate injected at
dermal–hypodermal junction of scar. Volume injected = 0.5 mL |
12 months | Photographic evidence of: - skin texture - softness - elasticity Function of eyelid: - epiphora - conjunctival continence - xerophthalmia |
Functional and cosmetic recovery. Skin softness and
elasticity seen after 1-month and 12-month follow-up.
Functional improvement in xerophthalmia and epiphora.
Conjunctival continence halted and lagoftlamus
improved. Positive results from first use of AFG in cictracial ectropian. |
| Klinger et al. (2008)11 |
3 | Hemi-facial second and third degree burn scars | Use of AFG to treat severe burn scars on the face | 3 patients (mean age 28 years; age range 16–36 years) with
severe burns were included. Age range of burns 4–33 years.
Fat graft taken from sub-umbilical region and injected under
the scar at the dermal–hypodermal junction. 3 procedures
were carried out at 3 months and 6 months Volume injected = not declared |
6 months | At 3 months and 6 months: • punch biopsies • haematoxylin- eosin (H&E) staining • MRI scans of the head • photographic evidence |
Improvement in skin texture, softness and elasticity. Histological staining showed architectural patterns similar to unaffected areas, including new collagen deposition and dermal hyperplasia. MRI showed scar architype including asymmetry |
Volume and contour definition
Nine articles studied the volume and contour definition of scars following AFG treatment in 794 patients.5,17,23–25,28,30,33 Scar depression is a feature reported for many cases; AFG produces a volume filling effect post treatment. Klinger et al. (2013) reported an improvement in all 694 patients following AFG treatment.5 No significant difference was reported. Mazzola et al. (2013) and Guerrissi et al. (2015) determined that multiple sessions were needed to achieve a complete fullness.30,25 Out of 14 patients, 12 received multiple AFG treatments. All 14 patients reported volume and contour restoration. Bollero et al. (2014) used contrast-enhanced ultrasound (CEUS) to detect post-treatment vascularisation in 28 patients.17 Twenty-four patients reported an improvement in scar characteristics of softness, colour and contour. CEUS confirmed the presence of increased vascularisation. However, in the remaining four patients that showed no improvement in volume post treatment, no CEUS vascularisation was detected.
Histological studies
Klinger et al. (2008) and Bruno et al. (2013) both studied the changes that occur within scar tissue following AFG treatment in a total of 96 patients (Table 8).11,18 Both studies report the morphological appearance post treatment, displaying features closer to normal tissue, compared with pre-treated scars. Lipofilling induced organised collagen deposition, vascularisation and reappearance of the papillary dermis. Bruno et al. (2013) report an altered presence of antibodies, including reduced presence of P63, responsible for diffuse differentiation and proliferation in the epithelium.18 Reduced P63 expression inhibits these processes. Alternatively, Ki67 expression increased, linked with stem cell induced cellular proliferation.
Table 8.
Summary of histological findings.
| Study | Study design | OCEBM | Histological method | Microscopic findings before | Microscopic findings after |
|---|---|---|---|---|---|
| Bruno et al. (2013)18 |
Case-control | III | H&E | - Melanocytic activity - Unorganised collagen - Presence of basal layer - Reduced granulous layer |
- Reduced melanocytic activity - Organised collagen fibres - Larger number of elastic fibres - Appearance of papillary dermis |
| Klinger et al. (2008)11 |
Case series | IV | H&E | - Cellular necrosis | - Epithelial hyperplasia - Neoangiogenesis |
H&E: Hematoxylin-eosin.
Supplementary additions
Four articles, including 101 patients, assessed the use of additional treatment alongside AFG (Table 5).15,16,22,24 These include laser treatments and enhanced AFG with SVF and PRP. Admani et al. (2015) describe a combination treatment in one case including AFG, AFR and PDL.15 A total of nine treatments were issued to address the different components of the scar. AFG treated volume contouring, AFR treated the irregularity and contracture of scar tissue, and PDL treated the erythema.
Laser treatments were used in two further studies in 80 patients, to encourage tissue tightening and remodelling.16,21 Azzam et al. (2015) determined both treatments resulted in scar improvement but the AFG treatment group had an overall higher rate of improvement compared with the fractional CO2 laser group. No significance was reported. Alternatively, Cervelli et al. (2011) determined that laser treatment, in combination with PRP-enhanced AFG, yielded the greatest improvement. The laser treated group showed a 30% improvement in 20 patients, compared with an 18% improvement in 20 patients treated with AFG. No significance was reported.21
SVF and PRP enhancement were used in two studies, on 70 patients.21,24 SVF is abundant with ADSC and PRP encourages ADSC proliferation. This was assessed by Gentile et al. (2014), comparing SVF and PRP enhanced AFG to controls.24 A significant difference was determined in contour maintenance compared to controls (treatment with AFG), for PRP and SVF preparations (P < 0.0001). An improvement of scar texture, contour and softness was reported.
Discussion
Injection of autologous fat grafts under scar tissue has resulted in a restoration of functionality and cosmetic appearance, rendering AFG a viable alternative for scar management (figure 2).5,7 This therapy has demonstrated an ability to reduce pain and improve scar colour, pliability, thickness and contouring, in a variety of scar-related diseases. It is hypothesised that ADSC are responsible for this phenomenon.7 While AFG is a promising treatment for scars, there is limited evidence due to the quality of the studies, as demonstrated in this review.
Figure 2.
Autologous fat transfer process. (Image by Info@clinicalillustration.com)
Fat stores are abundant with ADSC, which can generate epithelial hyperplasia and angiogenesis.5 Although the exact mechanism remains unclear, it is proposed that the release of growth factors is responsible for this (Figure 3). These include VEGF, PDGF, TGF-b, MMP, IGF-1, BGFG and BDNF. Growth factors stimulate endothelial cells, fibroblasts and tissue progenitor cells to remodel scar tissue.35–37 Bruno et al. (2013) and Klinger et al. (2008) demonstrated a similar mechanism with organised collagen deposition and epithelialisation following lipofilling, during histological staining.11,18 Klinger et al. (2013) demonstrated the presence of papillary dermis after AFG treatment which supports the theory of tissue architectural remodelling.5 This hypothesis is further supported by Bollero et al. (2014) who demonstrated AFG induced vascularisation with CEUS after three months.17 A significant association was detected between vascularisation and scar contouring and improved appearance.17
Figure 3.
Theories of mesenchymal stem cell’s regenerative and anti-inflammatory properties responsible for scar healing. (Image by-info@clinicalillustration.com)
Analgesic effects are produced by nerve repair, mediated by BDNF and scar entrapment release. The injection procedure itself is responsible for making space under the scar and releasing fibrotic tissue, following needle insertion.3,4,38 Ulrich et al. (2012) demonstrated that 18 out of 20 patients had immediate relief of pain following cannula insertion and release of fibrotic tissue.32 Lisa et al. (2016) suggests that the ADSC create a microenvironment, following tissue regeneration, which encourages nerve release.4 This includes neo-angiogenesis and increased hydration. ADSC possess anti-inflammatory properties that have been demonstrated in murine studies. TGF-β initiates immunosuppression by acting on T-cells and may be responsible for an analgesic effect. Huang et al. (2015) describe damage to nociceptors and peripheral nerves as the cause of neuropathic pain experienced from scar tissue causing an inflammatory response.39 It is also hypothesised that the actions of cytokine interleukin 10 (IL-10) are responsible for the inhibiting CD4 and CD8 and thus generating an anti-inflammatory response.39 ADSC are an abundant source of IL-10.
A significant improvement was detected in studies regarding pain relief following AFG. Huang et al. (2015) demonstrated a rapid analgesic effect of neuropathic pain, just one week after treatment.3 This was a significant improvement (P < 0.05), using the VAS and NPSI scores. Ulrich et al. (2012) and Caviggioli et al. (2011) both reported long-term analgesic effects at six months and 13 months, respectively, indicating AFG sustainable effects.21,32
This systematic review assessed the efficacy of AFG in treating scar tissue, in 23 articles. Positive outcomes were reported, including restoration of function and aesthetics. This included colour, thickness, pigmentation and relief of pain and itch. Scars ranged from cleft palates to post-mastectomy painful scars, all showing improvement following one or more treatments. This suggests AFG can be used as an alternative to surgical scar excision, in scar-related diseases.
Surgical excision of scars results in increased scar length and potential of worsening scar tissue. AFG has the advantage of being easily available and can be injected in multiple sites of the body. Furthermore, it is not immunogenic, is easily available and, according to Piccolo et al., is a procedure that has a short learning curve for surgeons.34 The volume filling capabilities allow the natural contours of skin to be regained while counteracting the depressive and retracted nature of scar tissue. AFG is therefore a viable alternative to surgical excision.
This review demonstrated the consistency of the procedure and technique used, when lipofilling. 16 studies followed Coleman’s technique and ensured a maximum surface area between the grafted fat and recipient site. This was displayed by the deposition of fat in multiple planes which enhances graft viability for long-term benefit. Bircoll et al. (1987) recommend increments of 1-mL fat should be deposited in passages in different planes until the full volume has been reached.40 This was adopted by Byrne et al. (2016), with significant improvement in range of motion in 13 patients with hand scars.19
The volume injected was in the range of 0.5–80 mL in this review, with variable reasoning in each study. Guisantes et al. (2012) suggests that most clinicians are guided by the size of the scar and states that < 200 mL for any scar means even thin individuals would be eligible for treatment.26 This is supported by Klinger et al. (2013) and Piccolo et al. (2015), who describe 1 mL per 3.5 cm2 and 1.6–2.0 mL per 10 cm2, respectively.5,34 The majority of studies were guided by volume restoration and degree of retraction rather than scar size when considering the need for a greater volume of fat.
Despite the advantages of AFG, complications were noted in 14 patients. This included postoperative haematoma and infection, need for further AFG sessions and surgical excision of scar tissue following unsuccessful treatment. However, the natural and rapid healing process, in combination with high levels of patient satisfaction, poses promising potential for AFG.
Limitations
There is a paucity of high-quality randomised controlled trials in the literature. This review demonstrates the abundance of evidence levels IV and V with case reports and case series. Furthermore, this review is limited by completeness of data including declarations of loss to follow-up. Observer bias was not reduced with blinding, therefore qualitative assessment of cases and controls by physicians should be interpreted with caution. This can be considered in future studies. Selection bias was noted with the exclusion of confounders, i.e. keloid scars and patients on steroids. Although this heterogeneity is to be expected, study populations varied with the number of treatable and non-treatable scars. Future consideration must be taken of age as a confounder. Huang et al. (2015) demonstrated unsuccessful treatments in patients aged > 50 years.3 They required multiple sessions and scored low on patient satisfaction surveys. Dini et al.’s (2014) recent work, assessing age and adipogenetic capability of fat, demonstrated reduced ADSC activity. This can be assessed further in future projects.17
Conclusion
This systematic review evaluates the existing literature and evidence regarding AFG in treating various scars. The documented description of its benefits in improving scar tissue is promising, indicating its ability to reduce functional limitations and enhance cosmetic appearance. Analgesic effects are caused by nerve repair (mediated by BDNF) and scar entrapment release. The injection procedure itself is responsible by making space under scar tissue and it is hypothesised that grafts containing TGF-β play a role in immunosuppression by acting on T-cells, resulting in an analgesic effect. However, there is a limited number of studies that report significant results and a lack of high quality randomised control trials. It is therefore difficult to conclude that AFG significantly improves scar tissue. What can be taken from this study is the potential of this treatment to be explored further in future, with an appropriate sample population and follow-up period. Detailed study of ADSC would be of great interest to support the current hypotheses that are indicated in this review. AFG is a safe treatment method with few complications and it poses promising benefits in future treatment of scar-related conditions.
Footnotes
Declaration of conflicting interests: KS is the editor of Scars, Burns and Healing but was excluded from the peer review process. This work has been presented as a Poster Presentation at the British Association of Plastic, Reconstructive and Aesthetic Surgeons Undergraduate Day in May 2016.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Gold MH, Berman B, Clementoni MT, et al. Updated international clinical recommendations on scar management: Part 1. Evaluating the evidence. Dermatol Surg 2014; 40: 825–831. [DOI] [PubMed] [Google Scholar]
- 2. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendation on scar management: Part 2. Algorithms for scar prevention and treatment. Dermatol Surg 2014; 40(8): 825–831. [DOI] [PubMed] [Google Scholar]
- 3. Huang S, Wu S, Chang K, et al. Alleviation of neuropathic scar pain using autologous fat grafting. Ann Plast Surg 2015; 74: S99–S104. [DOI] [PubMed] [Google Scholar]
- 4. Lisa A, Murolo M, Vinci V, et al. Alleviation of neuropathic scar pain using autologous fat grafting. Ann Plast Surg 2016; 76(4): 474. [DOI] [PubMed] [Google Scholar]
- 5. Klinger M, Caviggioli F, Klinger F, et al. Autologous fat graft in scar treatment. J Craniofac Surg 2013; 24(5): 1610–1615. [DOI] [PubMed] [Google Scholar]
- 6. Billings E, May J. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 1989; 83(2): 368–381. [DOI] [PubMed] [Google Scholar]
- 7. Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007; 119(5): 1409–1422. [DOI] [PubMed] [Google Scholar]
- 8. Fontdevila J, Serrarenom J, Raigosa M, et al. Assessing the long-term viability of facial fat grafts: an objective measure using computed tomography. Aesthet Surg J 2008; 28(4): 380–386. [DOI] [PubMed] [Google Scholar]
- 9. Perén P, Gómez J, Guerrerosantos J, et al. Gluteus augmentation with fat grafting. Aesthetic Plast Surg 2000; 24(6): 412–417. [DOI] [PubMed] [Google Scholar]
- 10. Coleman S. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 1995; 19(5): 421–425. [DOI] [PubMed] [Google Scholar]
- 11. Klinger M, Marazzi M, Vigo D, et al. Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthetic Plast Surg 2008; 32(3): 465–469. [DOI] [PubMed] [Google Scholar]
- 12. Negenborn V, Groen J, Smit J, et al. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions. Plast Reconstr Surg 2016; 137(1): 31e–43e. [DOI] [PubMed] [Google Scholar]
- 13. Condé-Green A, Marano A, Lee E, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring. Plast Reconstr Surg 2016; 137(1): 302–312. [DOI] [PubMed] [Google Scholar]
- 14. OCEBM Levels of Evidence - CEBM. CEBM. 2016. Available at: http://www.cebm.net/ocebm-levels-of-evidence/ (accessed 6 May 2016).
- 15. Admani S, Gertner J, Gosman A, et al. Multidisciplinary, multimodal approach for a child with a traumatic facial scar. Semin Cutan Med Surg 2015; 34(1): 24–27. [DOI] [PubMed] [Google Scholar]
- 16. Azzam OA, Atta AT, Sobhi RM, et al. Fractional CO(2) laser treatment vs autologous fat transfer in the treatment of acne scars: a comparative study. J Drugs Dermatol 2013; 12(1): e7–e13. [PubMed] [Google Scholar]
- 17. Bollero D, Pozza S, Gangemi EN, et al. Contrast enhanced US evaluation after autologous fat grafting scar revision. J Surg 2014; 35(11–12): 266–273. [PMC free article] [PubMed] [Google Scholar]
- 18. Bruno A, delli Santi G, Fasciani L, et al. Burn scar lipofilling. J Craniofac Surg 2013; 24(5): 1806–1814. [DOI] [PubMed] [Google Scholar]
- 19. Byrne M, O’Donnell M, Fitzgerald L, et al. Early experience with fat grafting as an adjunct for secondary burn reconstruction in the hand: Technique, hand function assessment and aesthetic outcomes. Burns 2016; 42(2): 356–365. [DOI] [PubMed] [Google Scholar]
- 20. Caviggioli F, Klinger F, Villani F, et al. Correction of cicatricial ectropion by autologous fat graft. Aesthetic Plast Surg 2008; 32(3): 555–557. [DOI] [PubMed] [Google Scholar]
- 21. Caviggioli F, Maione L, Forcellini D, et al. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg 2011; 128(2): 349–52. [DOI] [PubMed] [Google Scholar]
- 22. Cervelli V, Nicoli F, Spallone D, et al. Treatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol 2011; 37(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 23. Dini M, Mori A, Quattrini Li A. Eyebrow regrowth in a patient with alopecia atrophic scarring treated with autologous fat grafting. Dermatol Surg 2014; 4(8): 926–928. [DOI] [PubMed] [Google Scholar]
- 24. Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma. J Craniofac Surg 2014; 25(1): 267–272. [DOI] [PubMed] [Google Scholar]
- 25. Guerrissi JO, Siniger TB, Di Lisio MG. Retraction scar treatment with fat injection. Austin J Surg 2015; 2(5): 1068. [Google Scholar]
- 26. Guisantes E, Fontdevila J, Rodríguez G. Autologous fat grafting for correction of unaesthetic scars. Ann Plast Surg 2012; 69(5): 550–554. [DOI] [PubMed] [Google Scholar]
- 27. Klinger M, Lisa A, Caviggioli F, et al. Autologous fat grafting improves facial nerve function. Case Rep Surg 2015; 2015: 520746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lisa A, Summo V, Bandi V, et al. Autologous fat grafting in the treatment of painful postsurgical scar of the oral mucosa. Case Rep Med 2015; 2015: 842854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maione L, Memeo A, Pedretti L, et al. Autologous fat graft as treatment of post short stature surgical correction scars. Injury 2014; 45: S126–S132. [DOI] [PubMed] [Google Scholar]
- 30. Mazzola I, Cantarella G, Mazzola R. Management of tracheostomy scar by autologous fat transplantation. J Craniofac Surg 2013; 24(4): 1361–1364. [DOI] [PubMed] [Google Scholar]
- 31. Pallua N, Baroncini A, Alharbi Z, et al. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg 2014; 67(8): 1033–1037. [DOI] [PubMed] [Google Scholar]
- 32. Ulrich D, Ulrich F, van Doorn L, et al. Lipofilling of perineal and vaginal scars. Plast Reconstr Surg 2012; 129(3): 593e–594e. [DOI] [PubMed] [Google Scholar]
- 33. Zellner E, Pfaff M, Steinbacher D. Fat grafting in primary cleft lip repair. Plast Reconstr Surg 2015; 135(5): 1449–1453. [DOI] [PubMed] [Google Scholar]
- 34. Piccolo N, Piccolo M, Piccolo M. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin Plast Surg 2015; 42(2): 263–283. [DOI] [PubMed] [Google Scholar]
- 35. Zuk P. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. ISRN Stem Cells 2013; 2013: 713959. [Google Scholar]
- 36. Tsuji W. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells 2014; 6(3): 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunol 2014; 15(11): 1009–1016. [DOI] [PubMed] [Google Scholar]
- 38. De Benito J, Fernández I, Nanda V. Treatment of depressed scars with a dissecting cannula and an autologous fat graft. Aesth Plast Surg 1999; 23(5): 367–370. [DOI] [PubMed] [Google Scholar]
- 39. Huang S, Wu S, Lee S, et al. Fat grafting in burn scar alleviates neuropathic pain via anti-inflammation effect in scar and spinal cord. PLoS ONE 2015; 10(9): e0137563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bircoll M, Novack B. Autologous fat transplantation employing liposuction techniques. Ann Plast Surg 1987; 18(4): 327–329. [DOI] [PubMed] [Google Scholar]
How to cite this article
- Riyat H, Touil LL, Briggs M, Shokrollahi K. Autologous fat grafting for scars, healing and pain: a review. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/2059513117728200 [DOI] [PMC free article] [PubMed] [Google Scholar]