Abstract
Introduction:
Hypertrophic and keloid scarring remain notoriously troublesome for patients to tolerate and frustratingly difficult for clinicians to treat. Many different treatment modalities exist, signifying the failure of any method to achieve consistently excellent results. Intralesional cryotherapy is a relatively recent development that uses a double lumen needle, placed through the core of a keloid or hypertrophic scar, to deliver nitrogen vapour, which freezes the scar from its core, outwards.
Methods:
This article provides a comprehensive review of the literature on intralesional cryotherapy for hypertrophic scars and keloids. A systematic review or meta-analysis was not possible, since the existing articles did not permit this.
Results:
A search of English language, peer-reviewed literature was carried out. The evidence base was found to be low (level 4). In addition, much of the published evidence comes from a very few groups. Despite this, consistent findings from case series suggest that the technique is safe and achieves good scar reduction with very few treatments. Adverse effects include depigmentation, recurrence and pain. Pain and recurrence appear to be uncommon and depigmentation may be temporary.
Discussion:
Well-constructed, prospectively recruited comparative trials are absent from the literature. These are strongly encouraged, in order to strengthen general confidence in this technique and in the repeatability of outcomes reported thus far.
Keywords: Cryosurgery, cryotherapy, hypertrophic scar, keloid, scar outcomes, scar reduction
Lay Summary
There are many different types of treatment for hypertrophic scars and keloid scars, but no treatment works perfectly in all individuals. Intralesional cryotherapy is a relatively new technique which freezes a scar from the centre outwards. This article provides a summary of the technique and the evidence for its usefulness in the medical literature. While no large-scale studies have been reported, the findings from small scale studies have been very encouraging. Intralesional cryotherapy appears to be safe and effective, with few adverse effects, but more research is needed in order to compare it adequately with other types of treatment
Introduction
For as long as physicians and surgeons have intervened in attempts to influence and improve hypertrophic scars and keloids, the inability of any therapeutic technique to achieve satisfactory scar reductions in all patients has been uncomfortably apparent.1–3 Numerous treatment options are available, although the quality of evidence for their efficacy is generally low, which should serve as an indication of the lack of consistency and predictability in outcomes among the various treatment modalities.
This profusion of options has led to a certain degree of confusion regarding which treatments or techniques should be regarded as first line and which second or third lines. International groups have produced treatment guidelines for hypertrophic scars and keloids. However, rather than providing specific treatment pathways, these groups have tended to produce very generalised reviews of some treatments and suggestions regarding some treatments that may be considered.4–7
Into this confusing and sometimes frustrating milieu, intralesional cryotherapy has emerged as an additional method of scar treatment. The technique, initially described by Weshahy8 in 1993 for treatment of skin lesions and by Zouboulis and Orfanos in 20009 for treatment of scars, was subsequently modified and developed Har-Shai et al. from 2003.10,11
Intralesional cryotherapy utilises an intralesional needle cryoprobe to produce rapid scar freezing from the core outwards, thus ensuring that all of the adverse scar in question is frozen. In this way, the technique differs from earlier spray, or contact cryotherapy, techniques which tend to produce more shallow patterns of freezing and often only partial freezing of the scar.10–12 Although still at a relatively early stage in the prevalence of its use, the promise of this technique has been recognised.7
This article comprises a review of the technique of intralesional cryotherapy for hypertrophic scars and keloids and the evidence for its efficacy. Additionally, the author discusses possible further measures to provide an evidence base with which to establish its role within a treatment schedule for hypertrophic scars and keloids.
Technique of intralesional cryotherapy in keloids and hypertrophic scars
Scar selection
While it is clear than any scar may be treated with this technique, certain scar types may be preferred, possibly due to better outcomes, or improved adverse effects.
Keloid scars with a narrow base may be regarded as ideal scars for this treatment. The narrow base ensures concentration of the cooling effect within the small soft tissue pedicle, thereby maximising freezing. Broader scars, with wider bases, may also be predicted to respond to treatment, but may not display as complete a response as narrow-based, pedunculated keloids.10,13
Anaesthesia
The majority of cases may be treated under local anaesthesia. Attention to anaesthetic technique in these cases is crucial, as trauma from a hypodermic needle puncture site outside the scar may stimulate formation of more keloid scarring. For this reason, translesional delivery of local anaesthetic is utilised (Figure 1). Typically, 0.5% bupivicaine with adrenaline may be used. In addition, oral analgesics may be commenced. In very large keloids, a general anaesthetic may be required.
Figure 1.
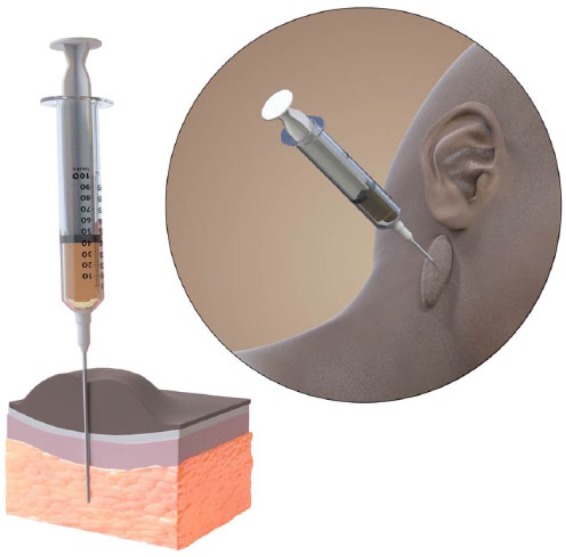
Translesional delivery of local anaesthetic for intralesional cryotherapy. Needle puncture of unscarred skin is avoided. Illustrator: Matthew Briggs (info@clinicalillustration.com)
Skin preparation
The skin is prepared with topical antiseptic and then draped.
Cryoprobe penetration of scar
A sterile double-lumen cryoprobe (CryoShape, Etgar Group International Ltd, Kfar Saba, Israel) is inserted through the middle (core) of the scar, along its long axis. Care must be exercised to prevent penetration by the cryoprobe, of unscarred adjacent skin. Sterile gauzes are placed between the patient’s skin and protruding ends of the cryoprobe, in order to protect these areas from unintended freezing.
Freezing process
The cryoprobe is connected to a canister containing liquid nitrogen. Nitrogen vapour flows through the cryoprobe and out to the atmosphere through an escape tube. Freezing is rapid and may be assessed visually and by palpation. The process continues until all the scar and a 5–10-mm margin of uninvolved skin are completely frozen (Figures 2 and 3). The extent of freezing at the margin is best assessed by palpation of a very distinct, hard, subcutaneous ‘shoulder’ around the scar base. Therefore, the treatment end-point is determined by physical features rather than a time limit. When this point is reached, nitrogen flow is stopped and the cryoprobe is allowed to thaw before being removed. A sterile non-adherent dressing is applied.
Figure 2.

Keloid scar on the helix of a 12-year-old boy. Pre-treatment.
Figure 3.
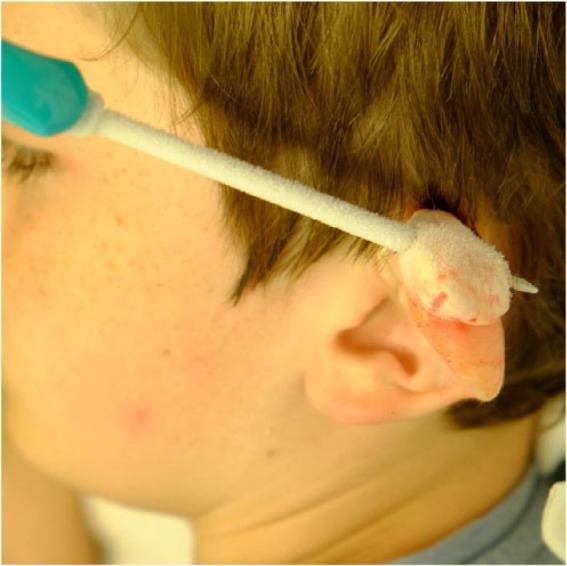
The same patient as in Figure 2 during intralesional cryotherapy to the same scar.
Postoperative management
All but the most extensive cases may be discharged on the day of treatment. Oral analgesia is provided, for either regular use, or as required. Patients may be given several dressings to apply at home, as it is expected that serous or serosanguinous discharge may be considerable in the week after treatment (Figure 4).
Figure 4.
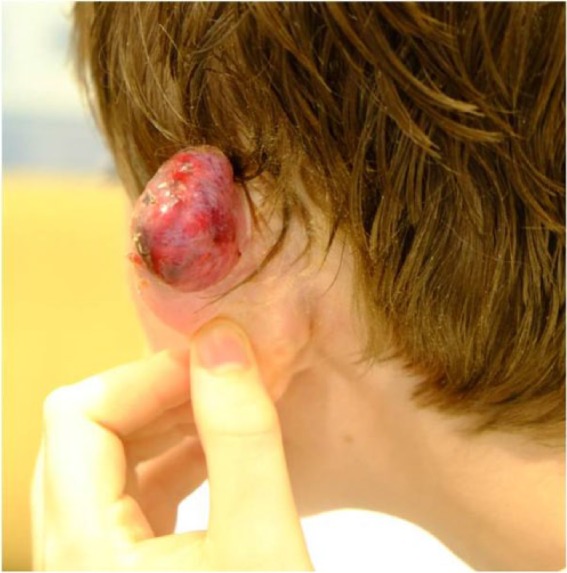
The same patient as in Figure 2 one week postoperatively, showing swollen scar exuding serous fluid.
After approximately one week, discharge usually ceases and dry necrosis becomes apparent. Shrinkage and desiccation continue for two to eight weeks postoperatively, before separation of overlying eschar, revealing a granulating wound. The wound then heals by re-epithelialisation. Typically, healing times vary from three to ten weeks (Figures 5 and 6).
Figure 5.
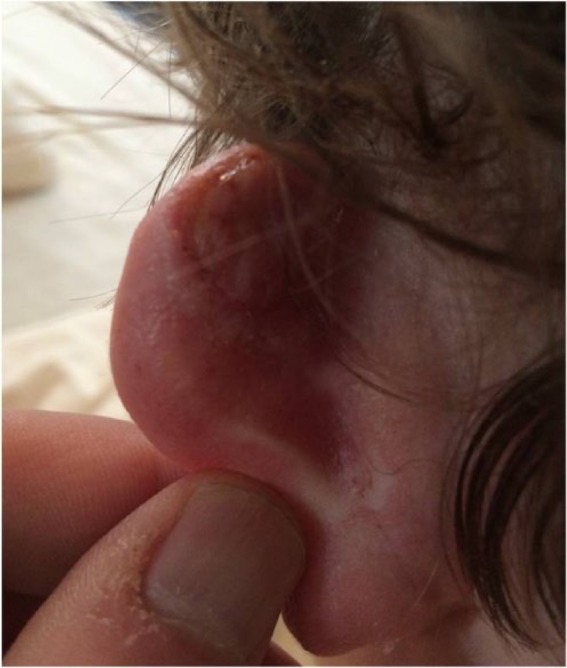
The same patient as in Figure 2 five weeks postoperatively. Scar eschar has separated, wound is re-epithelialising.
Figure 6.

The same patient as in Figure 2 11 weeks post-treatment. Healed wound. Complete resolution of keloid.
Materials and methods
This study did not constitute a systematic review. A search of peer-reviewed literature was carried out, using several databases. The methodologic flowchart is shown (Figure 7). PubMed was searched using the following search parameters: (intralesional[Title/Abstract]) AND cryotherapy[Title/Abstract]) AND keloid[Title/Abstract]) OR (intralesional[Title/Abstract] AND cryotherapy [Title/Abstract] AND hypertrophic[Title/Abstract]) OR (intralesional[Title/Abstract] AND cryosurgery[Title/Abstract] AND keloid[Title/Abstract]) OR (intralesional[Title/Abstract]) AND cryosurgery[Title/Abstract] AND hypertrophic[Title/Abstract]).
Figure 7.
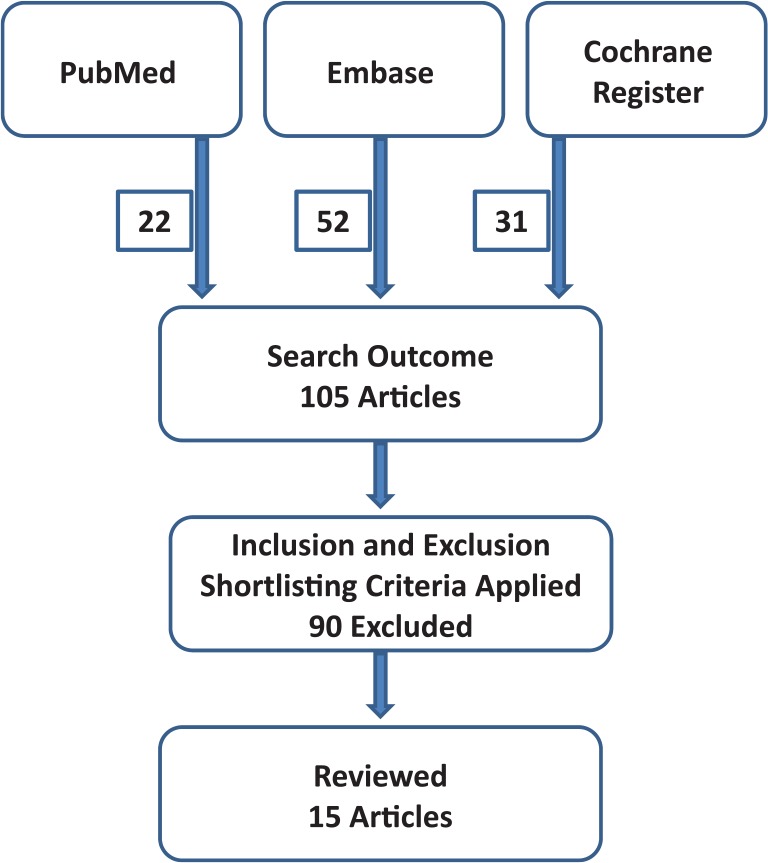
Flowchart showing review method and numbers of published articles obtained.
Ovid Embase was searched using the following parameters: (intralesional AND cryotherapy AND keloid) OR (intralesional AND cryotherapy AND hypertrophic) OR (intralesional AND cryosurgery AND keloid) OR (intralesional AND cryosurgery AND hypertrophic).
The Cochrane Register was searched using the following parameters: Intralesional – Cryosurgery – Hypertrophic Scars; Intralesional – Cyrosurgery – Keloid Scars; Intralesional – Cryotherapy – Hypertrophic Scars; Intralesional – Cryotherpay – Keloid Scars; Intralesional – Cryosurgery – Keloid; Intralesional – Cryotherapy – Keloid; Intralesional – Cryosurgery – Hypertrophic; Intralesional – Cryotherapy - Hypertrophic.
Selection criteria comprised case series, trials and abstracts from conference presentations, involving intralesional cryotherapy in hypertrophic or keloid scars, with quantified outcomes. Exclusion criteria comprised studies not involving intralesional cryotherapy; single-case reports; general reviews, practical clinical guidelines, and study protocols and descriptions without outcome data. Limited numbers of articles on this topic were anticipated. As a result, selection was not based upon types of documented outcome.
All review authors independently carried out the literature search. Duplicate articles were removed. Study validity and suitability for inclusion were discussed by the three authors, with the corresponding author (CPOB) providing adjudication in cases of difference of opinion.
Results
The search strategy yielded 105 articles, of which 15 met the selection criteria. These included studies on 334 patients, with 398 scars. The search results are summarised in Table 1.
Table 1.
Literature search results.
| Year | Article title | Authors | Citation | Study size (n) | Study duration (months) | Level of evidence | Outcome |
|---|---|---|---|---|---|---|---|
| 2001 | Intralesional cryosurgery using lumbar puncture and/or hypodermic needles for large, bulky, recalcitrant keloids | Gupta et al. | Int J Dermatol 40: 349–353 | 12 | n/a | 4 | 58% (7/12) patients showed > 75% flattening |
| 2003 | Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids | Har-Shai et al. | Plast Reconstr Surg 111(6): 1841–1852 | 10 | 18 | 4 | 51.4% mean scar volume reduction achieved after one treatment |
| 2006 | Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies | Har-Shai et al. | Wound Repair Regen 14(1): 18–27 | 9 | 18 | 4 | 67.4% scar volume reduction at 18 months after single treatment (P < 0.005) |
| 2006 | Effect of skin surface temperature on skin pigmentation during contact and intralesional cryosurgery of keloids | Har-Shai et al. | J Eur Acad Dermatol Venereol 21: 191–198 | 30 | 6 | 4 | 91.7% significant hypopigmentation rate in contact cryo. No marked hypopigmentation with intralesional cryo (P < 0.0001) |
| 2008 | Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery: the results of a prospective observational study | Har-Shai et al. | Int J Low Extrem Wounds 7(3): 169–175 | 11 | 3–96 | 4 | Significant reductions in concern scores (P = 0.001) and deformity scores (P = 0.004) after intralesional cryotherapy |
| 2012 | Intralesional cryosurgery and intralesional steroid injection: a good combination therapy for treatment of keloids and hypertrophic scars | Weshahy et al. | Dermatologic Therapy 25 (3); 273–276. | 22 | 40 | 4 | 93.5% mean volume reduction after 4 months (P < 0.01) |
| 2012 | Intralesional cryosurgery for enhancing the involution of hypertrophic scars and keloid-A new fundamental adjunctive wound healing therapy based on experimental and clinical data | Har-Shai et al. | Wound Repair Regen 20 (5): A94 | 95 patients, 112 scars | 18 | 4 | Single treatment. 51% mean scar volume reduction. 7% scars
showed no response (conference article) |
| 2014 | Intralesional cryosurgery to treat keloid scars: results from a retrospective study | Chopinaud et al. | Dermatology 229(3): 263–270 | 10 patients, 14 scars | 10–72 | 4 | Scar surface was reduced by an average of 58.5% after intralesional cryosurgery treatment |
| 2014 | Up-to-date approach to manage keloids and hypertrophic scars: a useful guide | Arno et al. | Burns 40(7): 1255–1266. | n/a | n/a | 4 | Review |
| 2014 | A new argon gas-based device for the treatment of keloid scars with the use of intralesional cryotherapy | Leeuwen et al. | J Plast Reconstr Aesth Surg 67(12): 1703–1710 | 25 | 12 | 4 | 62% scar volume reduction (P = 0.05). Pain, itch and scar quality were improved. Scar pigmentation recovered in 62%. 17% recurrence rate |
| 2015 | Intralesional cryotherapy for treatment of keloid scars: a prospective study | Van Leeuwen et al. | Plast Reconstr Surg 135(2): 580–589 | 27 | 12 | 4 | Mean volume reduction 63%. Recurrence rate 24% |
| 2015 | Comparison of two devices for the treatment of keloid scars with the use of intralesional cryotherapy: An experimental study | Leeuwen et al. | Cryobiology 71(1): 146–150 | 8 | n/a | 4 | Argon gas device had lower recurrence rate but more hypopigmentation than to the liquid nitrogen device |
| 2015 | Intralesional vs. contact cryosurgery in treatment of keloids: A clinical and immunohistochemical study | Abdel-Meguid et al. | Int J Dermatol 54(4): 468–475. | 23 patients, 66 scars | n/a | 4 | Better excellent response rate (87% vs.60%); volume reduction (61% vs. 23%) and fewer side effects with intralesional cryosurgery than with contact cryosurgery (P < 0.05) |
| 2016 | Spray versus intralesional cryotherapy for keloids | Mourad et al. | J Dermatol Treatm 27(3): 264–269 | 50 | 6 | 4 | Intralesional cryotherapy showed greater efficacy. Fewer treatments required |
| 2016 | Intralesional cryosurgery for the treatment of keloid scars following cochlear implant surgery and removal cholesteatoma | Roitman et al. | Eur J Plastic Surg 39(4): 307–312 | 2 | 30 | 5 | Scars flattened and became paler. Symptoms reduced. No complications documented. No recurrence documented |
Quality of evidence base on outcomes
Almost all articles on intralesional cryotherapy report outcomes from small studies. The largest study reviewed contained 95 patients.19 However, the median number of patients per study was 17. The published evidence base for this technique was uniformly Level 4. Randomised controlled trials are notable by their absence, but if conducted, would greatly strengthen understanding of this treatment modality. Another striking feature of the published literature on this technique is its domination by a study group which includes the inventor of the device. While the publishing of valid findings from inventors and developers is to be strongly encouraged, it would be desirable to see more articles from entirely independent groups, as a guide to repeatability and validity.
Effects upon scar volume and associated symptoms
Scar volume
Har-Shai et al. reported mean reductions in scar volumes of 51–67% after single treatments, with occasional 100% reductions.10–13 These findings were confirmed by several groups, one of which used a larger study (n = 27),14 which found similar reductions in scar size (63% mean volume reduction15 and 58.5% mean scar surface area reduction16). Occasional 100% reductions were observed again in one group.15
Arno et al. reviewed scar volume reductions with intralesional cryotherapy and with contact and spray cryotherapy and found significantly improved volume reductions associated with use of intralesional cryotherapy.17 This volume reduction does not seem confined to a particular type of intralesional cryotherapy device. Weshahy and Ebdel Hay, using a different cryoneedle to that popularised by Har-Shai, reported mean scar volume reductions of 93.5% in 20 patients at four months after treatment18 and Abdel-Meguid et al., from the same centre, observed mean scar volume reductions of 61% following intralesional cryotherapy, compared with reductions of 23% with contact cryotherapy.19 However, it is unclear if the effect persisted in the long term. Roitman et al. reported in two patients persistence of 100% keloid scar reduction at six months following single procedures.20 Gupta and Kumar reported greater than 75% scar volume reductions in 7/12 patients with keloids treated using hypodermic needles to deliver cryotherapy.21 Using a different type of cryotherapy device, utilising argon rather than nitrogen, Van Leeuwen et al. observed a mean scar volume reduction of 62%.22 In a comparative study of 50 patients, Mourad et al. found greater efficacy and requirement for fewer treatments with intralesional cryotherapy than with spray cryotherapy.23
Observed rates of non-response to intralesional cryotherapy have varied between 3%14 and 7%.11
Anxiety
One small (n = 11) prospective observational study examined patient-reported concern about the scar and clinician-reported scores on deformity from scars.12 The findings suggested that significantly reduced concern was reported from patients after receiving intralesional cryosurgery than was present before the treatment. Clinicians also reported significantly reduced deformity after intralesional cryosurgery than before treatment. While encouraging, this finding must be viewed with caution, since this was a small study with no control group and utilised a scoring system that has not been validated adequately.
Pain and pruritus
These associated symptoms may be difficult to quantify. All studies that commented upon these effects reported consistent and significant reductions in associated pain and pruritus10–13,15,16,21 following intralesional cryotherapy.
Adverse effects of intralesional cryotherapy
Pain
Pain has been reported consistently; however, in nearly all cases, this was reported as mild in nature and was easily controlled with short-course oral analgesia. The procedure has been reported as being well-tolerated by every author. Postoperative bleeding or infection have not been reported.
Prolonged healing
Slow healing times are common, as may be expected by any clinician familiar with the effects of frostbite injuries. The published evidence is far from clear regarding lengths of time to healing. This is an important omission from the published data, since, in a procedure with limited adverse effects, the amount of time spent in dressings assumes a greater degree of importance than in more complex and life- or limb-critical cases, in which dressings would be relatively minor concerns. It would seem that the healing time is of the order of several weeks, but the range is not known. We await further published evidence that will enable more solid advice to patients and strengthen the basis of informed consent.
Hypo-pigmentation (loss of colour)
Har-Shai et al. examined the cooling characteristics of intralesional cryotherapy and contact cryotherapy, in relation to subsequent pigmentary changes.24 Significantly slower cooling and significantly higher end-point temperatures were observed with intralesional cryotherapy than with contact cryotherapy. In the contact cryotherapy group, 91% (n = 19/21) developed significant depigmentation, whereas in the group treated with intralesional cryotherapy, no marked hypo-pigmentation was observed (n = 0/24). Van Leeuwen et al. observed higher rates of hypo-pigmentation, following intralesional cryotherapy with an argon gas coolant device, than with a liquid nitrogen coolant.25
Skin hypo-pigmentation would seem to be an expected adverse outcome in darker skin types treated with cryotherapy and is well-recognised following both spray and contact cryotherapy.14 This may be a temporary phenomenon, however, with reported recovery of pigmentation in 69–100% of affected individuals.15,16
Recurrence
Reporting of rates of re-growth of scars following intralesional cryotherapy is patchy in the published literature. Har-Shai et al. have reported no re-growth;10,13,14 however, van Leeuwen et al. observed recurrence in 24% (n = 7/29) treated scars. This discrepancy requires well-constructed studies, with long follow-up periods and accurate, preferably quantified reporting of pigmentation, in order to provide clearer understanding of this adverse effect.
Discussion
Intralesional cryotherapy is a valid technique for treatment of hypertrophic scars and keloids. The published evidence would suggest that it achieves reductions in scar volumes, reduces associated symptoms and does so with few adverse effects.
There is some evidence of increasing usage of intralesional cryotherapy in the treatment of hypertrophic scars and keloids.15–22 In addition, the technique has been applied to other pathologies, including treatment of skin cancer, with favourable results,26 although the term ‘intralesional’ in this case is wrong and possibly dangerous. The term ‘transcutaneous’ has been suggested as an alternative.27
Obviously, an idealised treatment would be entirely non-invasive. Specialist clinicians in scarring and wound healing, who are unacquainted with the technique may view its essentially destructive nature with a degree of understandable scepticism. On the face of it, the idea that in individuals prone to adverse scarring, scars should be managed by a destructive technique and allowed to heal by secondary intention, would seem contrary to good sense. However, understanding is now growing that scar modification through surgical means that previously may have been considered inappropriate, may have a valuable role to play, if the patient fully understands and is central to the decision-making process.28 Additionally, the fact that intralesional cryotherapy achieves the degrees of scar reductions that it clearly does, demonstrates how much more is to be learned about wound healing and scarring processes.
The current evidence on intralesional cryotherapy is certainly encouraging, but objective, well-powered, comparative outcomes analysis is conspicuously absent. The issue of patient numbers and study power should not be ignored. Nor should the relatively small number of groups that have published outcomes data on intralesional cryotherapy. Adverse scars are not rare and recruitment to large-scale trials should be readily achievable. Clinicians and groups who use this technique should strive to publish their outcomes and create trials, in order to demonstrate repeatability and strengthen confidence in what may be an important advance in the treatment of such a difficult, deforming and dispiriting condition.
Intralesional cryotherapy is safe, non-toxic, well-tolerated and would seem to achieve good results in a high proportion of patients. This begs the question: where should it fit into an overall treatment pathway for adverse scars? This can only finally be answered after robust randomised controlled trials have been carried out in large numbers of patients. In addition, it is clear that investigations must also address whether isolated therapy or combination therapy will produce superior outcomes and if combination therapy, then which combination?
Crucial to any such trials and interpretation is the nature of the questions posed within them and the quality of data gathered. Ironically, given their external, exposed nature, objective assessment methods for hypertrophic and keloid scars have proved extremely difficult to implement generally. However, their use is vital to those seeking to understand scarring mechanisms and therapeutic processes. We eagerly await such studies, in anticipation of a clearer treatment strategy for this challenging pathology.
Acknowledgments
The authors gratefully acknowledge the assistance of Ms Yvonne Stubbington Clinical/Outreach Librarian, Library & Knowledge Service, St Helens & Knowsley Teaching Hospitals NHS Trust, Merseyside, for her assistance with review methodology.
Footnotes
Declaration of conflicting interests: Ciaran O’Boyle is a member of the editorial board of Scars Burns and Healing. Ciaran O’Boyle uses CryoShape intralesional cryotherapy in his clinical practice.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Peacock EE, Jr, Madden JW, Trier WC. Biologic basis for the treatment of keloids and hypertrophic scars. South Med J 1970; 63(7): 755–760. [DOI] [PubMed] [Google Scholar]
- 2. Murray JC. Scars and keloids. Dermatol Clin 1993; 11(4): 697–708. [PubMed] [Google Scholar]
- 3. Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999; 104(5): 1435–1458. [DOI] [PubMed] [Google Scholar]
- 4. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg 2002; 110(2): 560–571. [DOI] [PubMed] [Google Scholar]
- 5. Kim S, Choi TH, Liu W, et al. Update on scar management: guidelines for treating Asian patients. Plast Reconstr Surg 2013; 132(6): 1580–1589. [DOI] [PubMed] [Google Scholar]
- 6. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg 2014; 40(8): 825–831. [DOI] [PubMed] [Google Scholar]
- 7. Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg 2014; 67(8): 1017–1025. [DOI] [PubMed] [Google Scholar]
- 8. Weshahy AH. Intralesional cryosurgery, a new technique using cryoneedles. J Dermatol Surg Oncol 1993; 19: 123–126. [DOI] [PubMed] [Google Scholar]
- 9. Zouboulis CC, Orfanos CE. Cryosurgical Treatment. In: Harahap M. (editor) Surgical Treatmets for Cutaneous Scar Revision. New York: Marcel Deckler Inc, 2000, pp.185–234. [Google Scholar]
- 10. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg 2003; 111(6): 1841–1852. [DOI] [PubMed] [Google Scholar]
- 11. Har-Shai Y. Intralesional cryosurgery for enhancing the involution of hypertrophic scars and keloid - A new fundamental adjunctive wound healing therapy based on experimental and clinical data. Wound Repair Regen 2012; 20 (5): A94. [Google Scholar]
- 12. Har-Shai Y, Brown W, Labbé D, et al. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery: the results of a prospective observational study. Int J Low Extrem Wounds 2008; 7(3): 169–175. [DOI] [PubMed] [Google Scholar]
- 13. Har-Shai Y, Sabo E, Rohde E, et al. Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies. Wound Repair Regen 2006; 14(1): 18–27. [DOI] [PubMed] [Google Scholar]
- 14. Har-Shai Y, Zouboulis CC. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids. In Abramovits W, et al. (eds.) Dermatological Cryosurgery and Chemotherapy. London: Springer-Verlag, 2016, pp. 453–473. [Google Scholar]
- 15. van Leeuwen MC, van der Wal MB, Bulstra AE, et al. Intralesional cryotherapy for treatment of keloid scars: a prospective study. Plast Reconstr Surg 2015; 135(2): 580–589. [DOI] [PubMed] [Google Scholar]
- 16. Chopinaud M, Pham AD, Labbe D, et al. Intralesional cryosurgery to treat keloid scars: results from a retrospective study. Dermatology 2014; 229(3): 263–270. [DOI] [PubMed] [Google Scholar]
- 17. Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns 2014; 40(7): 1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weshahy AH, Abdel Hay R. Intralesional cryosurgery and intralesional steroid injection: a good combination therapy for treatment of keloids and hypertrophic scars. Dermatol Ther 2012; 25(3): 273–276. [DOI] [PubMed] [Google Scholar]
- 19. Abdel-Meguid AM, Weshahy AH, Sayed DS, et al. Intralesional vs. contact cryosurgery in treatment of keloids: a clinical and immunohistochemical study. Int J Dermatol 2015; 54(4): 468–475. [DOI] [PubMed] [Google Scholar]
- 20. Roitman A, Luntz M, Har-Shai Y. Intralesional cryosurgery for the treatment of keloid scars following cochlear implant surgery and removal of cholesteatoma. Eur J Plast Surg 2016; 39(4): 307–312. [Google Scholar]
- 21. Gupta S, Kumar B. Intralesional cryosurgery using lumbar puncture and/or hypodermic needles for large, bulky, recalcitrant keloids. Int J Dermatol 2001; 40: 349–353. [DOI] [PubMed] [Google Scholar]
- 22. van Leeuwen MC, Bulstra AE, van Leeuwen PA, et al. J A new argon gas-based device for the treatment of keloid scars with the use of intralesional cryotherapy. Plast Reconstr Aesthet Surg 2014; 67(12): 1703–1710. [DOI] [PubMed] [Google Scholar]
- 23. Mourad B, Elfar N, Elsheikh S. Spray versus intralesional cryotherapy for keloids. J Dermatolog Treat 2016; 27(3): 264–269. [DOI] [PubMed] [Google Scholar]
- 24. Har-Shai Y, Dujovny E, Rohde E, et al. Effect of skin surface temperature on skin pigmentation during contact and intralesional cryosurgery of keloids. J Eur Acad Dermatol Venereol 2006; 21: 191–198. [DOI] [PubMed] [Google Scholar]
- 25. van Leeuwen MC, Bulstra AE, van der Veen AJ, et al. Comparison of two devices for the treatment of keloid scars with the use of intralesional cryotherapy: An experimental study. Cryobiology 2015; 71(1): 146–150. [DOI] [PubMed] [Google Scholar]
- 26. Har-Shai Y, Sommer A, Gil T, et al. Intralesional cryosurgery for the treatment of basal cell carcinoma of the lower extremities in elderly subjects: a feasibility study. Int J Dermatol 2016; 55(3): 342–350. [DOI] [PubMed] [Google Scholar]
- 27. O’Boyle CP. A misleading procedure name may prove dangerous: cautionary note on an article by Har-Shai et al. Int J Dermatol 2016; 55(10): e557. [DOI] [PubMed] [Google Scholar]
- 28. Shokrollahi K. Making scars worse to make patients better? The role of surgery in changing the appearance of archetypal stigmatising injuries and the concept of mechanistic stigma in scar management. Scars, Burns & Healing 2015; 1: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
How to cite this article
- O’Boyle CP, Shayan-Arani H, Hamada MW. Intralesional cryotherapy for hypertrophic scars and keloids: a review. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/2059513117702162. [DOI] [PMC free article] [PubMed] [Google Scholar]


