Abstract
The formation of a wide range of excessive scars following various skin injuries is a natural consequence of healing. Scars resulting from surgery or trauma affect approximately 100 million people per annum in the developed world and can have profound physical, aesthetic, psychological and social consequences. Thus, scar treatment is a priority for patient and physician alike. Laser treatment plays an important role in scar management with additional support from ancillary modalities. Subsequent to part 1: Burns scars, part 2 focuses on our strategies and literature review of treatment of keloid, hypertrophic, pigmented and acne scars where lasers are used in conjunction with other measures, and illustrated with case studies.
Keywords: Corticosteroids, fluorouracil, laser, multimodality scar management, scar, silicone, surgery
Lay Summary
Scars can result as part of the normal healing process after a burn or other trauma such as surgery or injury. However, there is a range of scarring from ‘good’ to ‘bad’ depending on various features of the scars. Some can be can be lumpy and raised (hypertrophic and keloid scars), have changes in pigmentation (increased or decreased colouration) or have specific features related to the cause (for instance acne scars, burns scars). We review the senior author’s experience over twenty years in treating scars with a range of treatments in conjunction with lasers. This is the second and final article in the series looking at 4 main aspects of scarring. In part 1, the focus was burns scars.
In Part 2, we now focus on hypertrophic and keloid scars (thickened raised scars); pigmented scars and acne scars. Lasers play an important role in managing a variety of scars in our practice, which work best in combination with other treatments as described.
Introduction
Scars are a major disease burden on morbidity, mortality and quality of life.1,2 In this second manuscript, we review our centre’s multimodality approach for scar management, focusing on scar hypertrophy, keloid, acne pigmentation and vascular scarring, based upon experience and backed by evidence.
Hypertrophic scars and keloids
Hypertrophic scars are typically confined within the borders of the initial insult and present as immature, linear or widespread. Immature hypertrophic scars are red, on occasion pruritic or painful, mildly raised and are in the process of remodelling. Usually, these scars mature to flat scars. Linear scars are most commonly a result of a healed laceration, or surgical incision. They tend to improve and flatten only slowly with time and hence patients seek help to reduce the symptoms of itchiness and poor appearance. All types may regress to varying degrees spontaneously with time.3,4 Hypertrophic scar pathogenesis may be related to atypical extracellular matrix metabolism secondary to abnormal and exaggerated fibroblastic activation.5,6 This manifests histologically as well organised type III collagen but with an overexpression of both types I and III, profibrotic pyridinoline type collagenous crosslinkages, fibronectin deposition with overexpression of interleukins (IL)-4–6, -13 and -21 and underexpresion of IL-12 and interferon (IFN)-γ.5,6
Keloids are defined as excessive scars that invade beyond the borders of the initial insult and do not regress spontaneously. They recur in 45–100% of cases following excision.3,4 This is due to the fact that the new closure is exposed to the same mechanical, immunological and biochemical forces as the original scar.3–6 Areas particularly prone include the earlobes, chest, shoulders, upper back and posterior neck. Minor keloids are focally raised, pruritic scars that can occur up to one year following the initial injury. Major keloids are large, raised (>5 mm), dark red scars associated with pain and pruritis and continue to increase in size over years.
Although similar approaches to both hypertrophic and keloid scarring exist in the literature, there is no universally accepted regimen. Most commonly used techniques lack well-designed randomised controlled studies.
Prophylaxis of hypertrophic and keloid scars
Meticulous wound debridement, removal of foreign and necrotic material, tension-free closure with the least reactive suture material and every attention to avoid infection are effective preventive measures.7,8 After wound closure, methods include tension reduction either mechanically9,10 or neurotoxically mediated,11 hydrating occlusive silicone gel dressings12,13 and pressure garment therapy.7,14 The earlier this treatment occurs for abnormal immature scars with intact epithelium, the more favourable the outcome. The transition to a formal treatment regime develops when a true hypertrophic scar or keloid, and not an immature hypertrophic scar, has been diagnosed. Conventional treatment of both hypertrophic scars and keloids commonly involves massage,15–18 pressure therapy,19–23 hydrating occlusive silicone dressings,24–29 corticosteroid injection,30–32 surgical excision33–35 and radiotherapy,36–39 alone or in combination.30,31 Only hydrating occlusive silicone dressing and corticosteroid injection have been demonstrated effective in randomised controlled trials.31,40 A comprehensive review of the strength of the evidence in the other first line modalities is available elsewhere41 and is beyond the scope of this chapter.
Prophylaxis of hypertrophic and keloid scars
Corticosteroid injection. Corticosteroid injection, either alone or in combination with other agents, has become one of the most widely practised treatment modalities for hypertophic scars and keloids.42,43 These are normally administered intralesionally in the form of insoluble triamcinolone acetonide (0–40 mg mL−1). Injections are performed every four to six weeks until pruritic and pain-related symptoms subside and the scar flattens. Response rates vary (50–100% with a recurrence rate of 9–50%44,45). Complication are common and 63% experience localised dermal atrophy, ulceration, hypopigmentation or telangiectasia.46 Significant pain may be controlled by the addition of local anaesthetic. As monotherapy, coticosteroids are most effective for younger keloids, which may completely flatten while older keloids are more resistant.47 Given these limitations, other agents have been used in combination with corticosteroids to further modulate the hyperproliferative response.
Fluorouracil. The senior author advocates the combined use of 5-Florouracil (5-FU), a pyrimidine analogue, commonly used as an antimetabolite chemotherapy reagent. It inhibits thymidylate synthase activity. First used to reduce subconjunctival scarring in the context of glaucoma filtering surgery in 1984,48,49 it was later used as both a monotherapy and in combination with corticosteroids for the treatment of hypertrophic scars and keloids.32,50 Combined therapy with concentrations in the range of 40–50 mg mL−1 has been found to be more efficacious than 5-FU monotherapy,51–55 with no additional complications.56 Acting intracellularly, it promotes fibroblastic apoptosis, without necrosis, via inhibition of DNA synthesis in hyperproliferative and metabolically active cells. More recently, Huang et al. demonstrated the possibility of low dose 5-FU at 1 mg mL−1 and triamcinolone work together to inhibit fibroblastic proliferation, type I collagen deposition and matrix metalloproteinase-2 induction in vitro, and promote apoptosis.57,58 Our current approach, however, is to use conventional doses of 50 mg mL−1 injected intralesionally in combination with 10–40 mg mL−1 depending on scar resistance and extent every four to six weeks until the scar is flat, soft and symptom-free. The scar is not injected to the point of blanching to avoid ischaemia, ulceration and potential deterioration. Once a plateau is reached, the injections may be given between increasingly longer intervals titrated to response. All patients are informed of the fact that initial injections are uncomfortable due to the dense nature of the scar and subsequent injections becoming less so. In addition, local complications such as post-injection pain, ulceration and burning are outlined as is the theoretical risk of 5-FU-induced neutropenia and fetal complications if pregnant. The treatment is not performed during pregnancy or in those patients with bone marrow suppression.
Excision, combined with postoperative 5-FU and triamcinolone injection, can be effective,33 but must be considered on a case-by-case basis, for example if a patient presents with mature bulky keloid disease of the earlobe or face, or a functionally disabling immature or early-stage hypertrophic scar. It must be remembered that hypertrophic scars mature during a period of at least 12 months and demonstrate decreased contractures, thickening, softening and repigmentation quite often without treatment.59 Therefore, excision may not be warranted despite the fact recurrence may be low.60,61 For mature keloidal excision, a complete excision of all scar tissue is performed62,63 and closure is delivered with minimal tension and suture material leaving everted wound margins. Indeed, the senior author has performed this with the ablative CO2 laser. Undermining however, is not recommended and sutures should be placed multiplanar to reduce tension.33 Residual lesional injection is performed thereafter at the time of surgery and continued at four- to six-week intervals titrated to response.
Intralesional cryosurgery. This technique evolved from simple cryosurgery, first introduced by Shepard and Dawber.64 Suitable for small scars only, liquid nitrogen contact or spray technique may induce vascular injury leading to anoxia, scar tissue necrosis, sloughing and thus scar flattening.65 The process may take two to ten treatment sessions with 20–30 days between each one. Success rates are in the range of 32–74% after two or more treatments, with higher response rates in hypertrophic scars compared to keloids.31,66,67 Reported complications include immediate blistering and pain with longer-term risks of dermal atrophy that can either hyper- or hypopigment.67,68 In contrast, intralesional cryosurgery involves placing a novel intralesional cryoneedle (Cryoshape™) within the long axis of the scar. The probe consists of an elongated double-lumen uninsulated needle with a safety vent and a cutting, sealed, distal tip designed to enhance the penetration of the often dense, hard scar. To the proximal end of the probe is connected liquid nitrogen that is pressurised to circulate through the needle, which leads to an ice ball forming around the cryoneedle leaving the abutting scar tissue completely frozen. There is an apparent reduction in myofibroblasts and mast cells with an accompanying normalisation of collagen structure and organisation.69 First described by Weshahy in 199370 and later popularised by Har-Shai et al.,69,71–77 the technique has shown increased efficacy over simple cryosurgery,71–77 with reports of clinical efficiency in the range of 20–75% scar volume reduction.71,78,79 Complications include pain (although less than contact cryotherapy72,80), peritreatment oedema and epidermolysis, and temporary hypopigmentation. Skin surface temperature is less effected in intralesional cryotherapy and thus melanocyte sparing is a feature accounting for a lower incidence of dyschromia.81
Radiotherapy. In a small cohort of older adult patients, in whom other treatment options are declined, ionising radiation in combination with intralesional excision, as described, can be used for resistant hypertrophic scars and keloids.39 Radiation acts to inhibit fibroblast proliferation and collagen synthesis, inducing apoptosis of proliferating cells at doses of 15–30 Gy over six sessions with precise dosimetry with appropriate shielding in the immediate postoperative period.31,36,37,82 Radiotherapy is restricted to older adults given the small but theoretical risk of carcinogenesis38,39 but is efficacious. Success rates in the literature are in the range of 25–88%,83,84 but this is complicated by the retrospective nature of studies with variable follow-up periods and poorly defined clinical assessment. Therefore, evidence remains variable and there is a need for randomised prospective studies with objective clinical evaluation and long-term follow-up.
Hypertrophic and keloid scar patient case illustrations
1. A 40-year-old woman, Fitzpatrick type II, presented two years following a mid-line laparotomy with hypertrophic scarring. She complained of pruritis, pain and discomfort from clothing. The shortened scar limited full extension and splinted the costal margin to the pelvis. She was treated with intralesional chemotherapy and pharmocotherapy. She underwent three cycles of intralesional 100 mg of 5-FU and 40 mg of Triamcinolone six weeks apart. Improvement in colour, pruritus and scar thickness.
2. A 78-year-old woman, Fitzpatrick type II, presented with a hypertrophic scar following excision of a squamous cell carcinoma from her left presternal region. She complained of itching, pain and the raised appearance. She underwent three cycles of intralesional 50 mg of 5-FU and 10 mg of Triamcinolone six weeks apart.
3. A 38-year-old man, Fitzpatrick type VI, presented with a five-year history of bilateral recalcitrant mandibular margin keloids with intense pruritus from repeated folliculitis related to shaving trauma. Failed previous pharmacotherapy with stand-alone intralesional triamcinolone and also radiotherapy. The patient underwent complete scar excision and 200 mg of 5-FU and 40 mg of Triamcinolone injection at closure which was complicated on the right by delayed wound healing secondary to a small proximal wound dehiscence. Two months postoperatively, once all wounds were healed, residual keloid margin intralesional injection of the same mixture was repeated every six weeks. Nine cycles have passed and the time between injections will now be lengthened. The keloid disease load has been controlled with no pruritus and the patient can once again shave. Pre treatment (top row) and post treatment (bottom row).
4. A 22-year-old woman, Fitzpatrick type V, presented with recalcitrant pinna keloid scars from a helical rim piercing with intense pruritus previously treated with a course of stand-alone intralesional triamcinolone. Underwent complete surgical excision of all scar tissue and 50 mg of 5-FU and 10 mg of Triamcinolone injection at closure. Two months postoperatively, once all wounds were healed, margin intralesional injection of the same mixture was repeated every six weeks The keloid disease load has been controlled with a favourable cosmetic outcome.
5. A 27-year-old woman, Fitzpatrick type II, presented with presternal recalcitrant acne vulagaris-related keloid scars with intense pruritus and tenderness previously treated with topical silicone agents. She underwent intralesional injection of 50 mg of 5-FU and 10 mg of Triamcinolone injection repeated every six weeks. The keloid disease load has been controlled after 18 injections with a favourable cosmetic outcome and resolution of symptoms. Treatment is ongoing as thickening occurs if treatment interval exceeds six weeks. Pre treatment (left) and post ninth treatment (right).
6. A 24-year-old, Fitzpatrick type II, presented with a five-year history of mature static keloids related to previous acne vulgaris inflammation in the left neck. He complained of the cosmetic appearance of the scars and difficulties shaving. A single ablative CO2 laser treatment with the Ultrapulse® 2 mm true spot hand piece 175 mJ multiple passes to ablate the keloid to the level of the surrounding epidermis was performed.
Figure 1.
Photographs are pretreatment (a), six weeks after first treatment (b) and six weeks after last treatment (c).
Figure 2.

(a) At presentation; (b) 24 9 months post treatment showing improvement in colour, pruritus and scar thickness.
Figure 3.

(a–c) Pre treatment and (d, e) post treatment.
Figure 4.
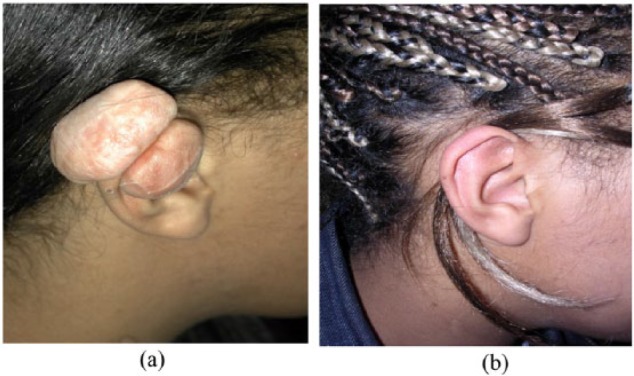
(a) Pretreatment and (b) four years post treatment, two years after last scar injection. Managment: intralesional excision and 50 mg of 5-FU and 10 mg of Triamcinolone injection at closure.
Figure 5.

Recalcitrant acne vulgaris-related keloid scarring. (a) Pre treatment and (b) post treatment.
Figure 6.

(a, b) Mature static keloids related to previous acne vulgaris inflammation. (c, d) After nine months there was no recurrence and there was a favourable cosmetic outcome.
Acne scarring
Acne vulgaris is a common cutaneous disease with a multifactorial pathogenesis that affects a significant proportion of the population. The condition may be divided into either comedonal, papulopustular or congloberate acne or depending on severity, mild moderate or severe. It has a prevalence of over 80% in adolescents and persists in 12–14% of cases into adulthood.85–91
Severe acne can lead to significant psychosocial concerns, such as ostracism and withdrawal from society.92 Pathogenesis is multifactorial and includes the proliferation and colonisation of Propionibacterium Acne within the follicles, excessive sebum production with abnormal sebum lipid profiles, androgen dysfunction and follicular hyperkeratinisation.93 As a consequence, there is associated infrainfundibular inflammation, follicular rupture and perifollicular abcess formation. The resulting dermal injury stimulates the wound-healing cascade with enzymatic degradation of collagen fibres and subcutaneous fat that leads to fibrosis and scarring.94 The severity of scarring is related to both the depth in the dermal pilosebaceous unit where inflammation and wound healing occur and the duration of inflammatory process, as well as an individuals genetic predisposition to scarring. The final result is either a net loss of collagen in the form of atrophic scar types, which are by far the most common,94–96 or a net gain in the form of hypertophic scars or keloids.
Atrophic acne scars are commonly classified into ice pick, boxcar and rolling subtypes. Ice pick are the most common and are seen in 60–70% of all patients, the remainder being either boxcar (20–30%) or rolling scars (15–20%).
Subtle differences between each of these subtypes offer a guide to management options. Such differences include scar width, depth and three-dimensional architecture, but they can be difficult to differentiate. Ice pick subtypes are narrow punctiform, very sharply demarcated epthelialised tracks with a wide opening (<2.0 mm) that tapers as a V shape, to a deeper infundibulum in the deep dermis or subcutaneous fat. In some cases these can branch and interconnect, posing difficulties for surgical excision. Boxcar scars are larger and are characterised by round, oval or angular shaped depressions (1.5–4.0 mm in diameter), which can be shallow (0.1–0.5 mm) or deep (⩾0.5 mm) with well-defined wide bases with vertical edges and with a cross-sectional U shape. Finally, rolling scars are the result of dermal tethering to the subcutis, which are greater than 4.0–5.0 mm in width and give the appearance of superficial shadowing with an underlatory M-shape appearance to otherwise texturally normal skin if seen in isolation. The Goodman and Baron qualitative scoring system is universally accepted97 is shown in Table 1.
Table 1.
Post acne scars, qualitative global grading system (Goodman and Baron).
| Grade | Disease level | Features and tests |
|---|---|---|
| I | Macular | These scars are erythematous, hyper- or hypopigmented macules. They do not represent a problem of contour but that of colour. |
| II | Mild | Mild atrophic or hypertrophic scars that may not be obvious at social distances of 0.5 m or greater and may be covered adequately by makeup or the normal shadow of shaved beard hair in men or normal body hair if extrafacial. |
| III | Moderate | Moderate atrophic or hypertrophic scarring that is obvious at social distances of 0.5 m or greater and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial. Can be flattened by manual stretching of the skin (if atrophic). |
| VI | Severe | Severe atrophic or hypertrophic scarring that is evident at social distances greater than 0.5 m and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial. Cannot be flattened by manual stretching of the skin. |
Although acne scars cause significant concern for patients and clinicans alike, there is no standardised treatment protocol or indeed single modality used for all scar types. This is due in part to the variability in both presentation of the scars and also individual response to treatment. Furthermore, there is also variabilty seen in the resultant scarring that occurs in different patients from similar disease load. However, often the most severe scars result from severe inflammatory nodulocystic acne but may also result from more superficial lesions. Erythema and pigmentation changes represent epidermal damage whereas atrophic, hypertrophic and keloidal scars more frequently indicate dermal damage. Currently, there is no predictive tool to identify patients who are likely to develop acne scars. Despite this, there is general consensus that two key modifiable factors in acne scar formation are pivotal. These are the time delay between onset of effective treatment and the extent and duration of the inflammation. Therefore, in terms of acne scar prophylaxis, early appropriate medical treatment that is continued for as long as necessary is recommended.
Once atrophic acne scars are established and mature, a number of treatments have been advocated. Many studies used to evaluate these techniques are plagued by lack of adequate control groups, study sample size, objective outcome measures, time to follow-up and a combination of biases. Techniques with supportive level IV evidence include resurfacing manouvres that destroy the epidermis and allow for collagenous remodelling. These include dermabrasion or microdermabrasion,98–100 light or medium-depth chemical peeling with either Jessner solution, 20–35% trichloroacetic (as a spot or generalised peel) or glycolic acids99,101–103 and the use of various laser technologies.99,104–106 Ablative lasers used have included both 10,600 nm CO2 and 2940 nm erbium-doped yttrium-garnet (Er:YAG) lasers which have until recently been regarded as the gold standard.107–111 While effective in resurfacing, these modalities have been limited by prolonged downtime with oedema, serous discharge and erythema, post-inflammatory hyperpigmentation (PIH), hypopigmentation and the potential risk of worsening atrophic scars with over treatment in the form of further scarring.112–114 These complications drove the quest for less-invasive laser resurfacing methods with non-ablative lasers. The most widely used of these are the 1320 nm neodymium:yttrium-aluminium-garnet (Nd:YAG) and 1450 nm diode lasers.115–118 More recently, fractionated photothermolysis through the use of fractional ablative CO2 laser therapy has been demonstrated to be efficacious in both case reports and non-controlled trials119–125 with stronger evidence coming from two split-face randomised controlled trials.126,127
In terms of surgery, a number of approaches have been proposed. Level V evidence supports scar excision with or without elevation or grafting. Punch biopsy, hair transplant punch or elliptical excision is often performed for ice pick and deep boxcar scars.94,129 Punch elevation is used for shallow and deep boxcar scars and grafting for sharp walled or very deep ice pick scars.98,130 Subcision (percutaneously releasing fibrosis) with a tri-bevel bladed needle is supported by level VI evidence and is performed for rolling or depressed scars.131,132 An alternative to excision, is the correction of the contour deficit seen in the atrophic scars with filler. Level V evidence supports the use of synthetic and biological fillers for superficial, soft, sloping walled scars,102 but there is a risk of worsening the appearance of scars with underlying fibrotic tethering. Quite often, each of these modalities have been used in combination.88
Our unit’s approach to the treatment of atrophic acne scarring is to individually direct therapy on a case-by-case basis with the goal to improve scars rather than to deliver a complete cure resulting in a perfect complexion. Treatment depends on the extent and type of scars present and as such, the modality may vary within each facial cosmetic subunit. First, a comprehensive patient history is required which should include all pharmacological acne treatments including recent isotretinoin use and history of any keloid or hypertrophic scarring.
Surgical intervention within six months of the discontinuation of isotretinoin may result in hypertrophic scarring.133 Therefore, we perform no intervention within a 12-month period of stopping isotretinoin. Furthermore, if there is still active acne inflammation, medical treatment should be optimised in conjunction with primary care and dermatology. Such treatment can be divided into topical and systemic treatments and are often used in combination based on the current European Acne guidelines134,135 (Table 2).
Table 2.
Summary of recommendations from the current European Acne treatment guideline,138,139 adapted from Rzany and Nast.136
| Strength of evidence | Comedonal acne | Mild–moderate papulopustular acne | Severe papulopustular/ moderate nodular acne |
Severe conglobate acne |
|---|---|---|---|---|
| High | None | Benzoyl peroxide + adapalene OR clindamycin | Isotretinoin* | Isotretinoin* |
| Medium | Topical retinoid† | Azelaic acid or Benzoyl peroxide or topical retinoid† or systemic antibiotic‡ + adapalene§ | Systemic antibiotics** + adapalene§ or systemic antibiotics** + azelaic acid†† or systemic antibiotics + adapalene + Benzoyl peroxide |
Systemic antibiotics** + azelaic acid |
| Low | Azelaic acid or Benzoyl peroxide |
Blue light or oral zinc or topical erythromycin +
isotretinoin or topical erythromycin + tretinoin or systemic
antibiotic‡,**
+ Benzoyl peroxide‡‡ or systemic antibiotic‡,** + azelaic acid§ or systemic antibiotics‡,** + adapalene + Benzoyl peroxide§§ |
Systemic antibiotics** + Benzoyl peroxide‡‡ | Systemic antibiotics** + Benzoyl peroxide‡‡ or systemic antibiotics** + adapalene§,§§ or systemic antibiotics** + adapalene + Benzoyl peroxide† |
Limitations can apply that may necessitate the use of a treatment with a lower strength of recommendation as a first-line therapy.
Adapalene to be preferred over tretinoin/isotretinoin.
In case of more widespread disease/moderate severity, initiation of a systemic treatment can be recommended.
Only studies found on systemic antibiotics + adapalene, Isotretinoin and tretinoin can be considered for combination treatment based on expert opinion. Further alternatives for women include hormonal antiandrogens and topical treatments or systemic antibiotics for severe papulopustular/moderate nodular and severe nodular/conglobate acne.
Doxycycline and lymecycline.
Indirect evidence from nodular and conglobate acne and expert opinion.
Indirect evidence from a study also including chorhexidine, recommendation additionally based on expert opinion.
Indirect evidence from severe papulopustular acne.
While medical treatment is being optimised, ice pick and boxcar scars are reconstructed by punch excision, under local anaesthetic or general anasthesia. Disposable 1.0–3.0 mm punch biopsy instruments are used to excise ice pick scars, to include the shoulders of the scar, full thickness to subcutaneous fat. Boxcar scars are perpendicularly stab excised full thickness with a number 11 blade held backwards, to include the scar walls, following the shape of the scar. Polyfax ointment is used as a dressing. All boxcar and punch excisions 1.5 mm and above are closed with non-absorbable monofilament suture removed at postoperative day 5. Treated scars should be at least 5 mm apart to prevent traction on suturing and prevent adequate wound eversion. Any scars larger than 3.5 mm are excised as an ellipse and closed parallel to relaxed skin tension lines. The goal is to exchange the visibly contoured acne scarring with less visible flat scars. After a test area to access the excisional scarring response, the technique is repeated in stages until all scars have been addressed.
Once acne inflammation and concurrent infection is quiescent, patients with Goodman and Baron grade III or VI scarring, with stretch correctable contour, fractionated photothermoloysis for facial resurfacing is performed by our unit. The Lumenis Ultrapulse® fractional ablative CO2 laser (Lumenis Ltd., Yokneum, Israel) TotalFX® is used. To avoid dyschromia in Fitzpatrick phototypes ⩾ V, a simple bleaching mixture of 0.025–0.05% Retin A, 4% hydroquinone and 1% hydrocortisone cream is commenced three to four weeks prior to treatment and continued for two weeks after healing has been completed. For resistant hyperpigmentation, 0.01% Tretinoin and hydroquinone 4%, followed by a Kligmans trio preparation with 5% hydroquinone, 0.01% Fluocinolone and 0.05% Tretinoin is advised.
Up to 80% of the population will have a dormant herpes labialis or herpes zoster infection. For CO2 laser treatments near the mouth, patients are administered viral prophylaxis to avoid reactivation and antibiotics to avoid postprocedure infection.
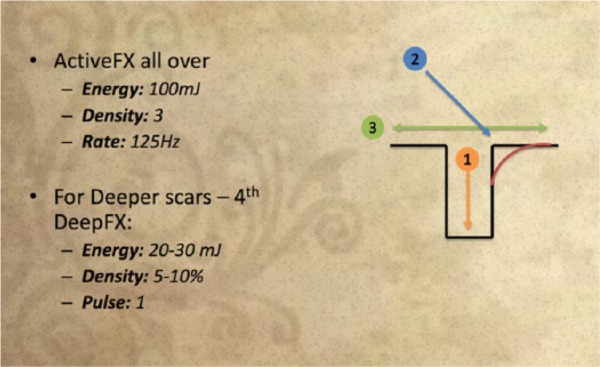
The senior author advocates a three-step approach as shown in Schedule 1. At the base of the depression of the atrophic scar, the first pass uses the DeepFX® mode with a very small beam pattern to fit the scar ‘hole’. A fluence of 15–20 mJ or up to 30 mJ in very thick and oily skin is used with a density of 10% with a single pulse. The second pass uses the ActiveFX® mode with the non-sequential array (CoolScan) with a fluence of 50–60 mJ, density of 5–7 and rate of 450 Hz with the hand piece used at 45° to contour the shoulder of the depression with a small-sized beam pattern. An intermediary scrub of this pass is then performed to reveal superficial dermal capillaries in the pink papillary dermis. ActiveFX® mode is then used as a third pass over each entire scarred facial cosmetic subunit with a fluence of 100 mJ, a density of 3 and a rate of 125 Hz. For more aggressive treatments between the second and the third pass, a further pass can be performed using the DeepFX® mode micro scanner on all the areas affected by the acne scars with a fluence of 20–30 mJ, a density of 5–10% with a single pulse. Post procedure, a thin layer of petroleum-based ointment is applied, or if a general anaesthetic has been used EMLA (eutectic mixture of lidocaine 2.5% and prilocaine 2.5%; Astra-Zeneca Pharmaceuticals, LP, London, UK) is applied followed by ice packs. Immediately post treatment erythema lasts for a few hours only and the sensation is similar to that of sunburn with a mild serous discharge. Once healed, the treated area will be red for six weeks to six months, but can be covered with makeup. The repeat treatment interval is normally three months, if required. The postoperative care regimen is the same as that used in burns scar treatment.
Scheme 1: Acne technique with the fractional ablative CO2 laser.
-
A- First pass

-
B- Second pass

-
C- Third pass
Acne scarring
7. A 46-year-old woman, Fitzpatrick type II, with Grade VI Goodman and Baron Acne Vulgaris scarring presented. She had multiple ice pick, boxcar and cobble stoning scars over bilateral cheeks and forehead. Her inflammation was quiescent with no medication and she was otherwise fit and well. She underwent two cycles of punch excision of the ice pick scars with 1.5, 2.0, 2.5, 3.0 and 4.0 mm punch biopsies. Punches over >2.0 mm were sutured with 5-0 Novafil and removed at postoperative day 5. A total of 110 ice pick scars were excised. At a third stage one year later, ablative CO2 laser therapy was perfomed. All elevated scars were treated with multiple passes of Ultrapulse® 2 mm true spot hand piece 175 mJ to flatten to the level of the surrounding epidermis. Fractional DeepFX® mode is then used at each of the scar bases 20 mL, 300 Hz, 2-10-1-10% and over each cosmetic subunit in its entirety at 20 mJ, 2-10-1-5% one pass. Finally, ActiveFX® mode was used over each scar shoulder 80 mJ, 3-5-1%.
8. A 44-year-old woman, Fitzpatrick type II, with Grade VI Goodman and Baron Acne Vulgaris scarring presented. She had multiple cobble stoning scars over bilateral cheeks and temporal regions. Her inflammation was quiescent with no medication and she was otherwise fit and well. She underwent two cycles of fractional ablative CO2 laser therapy. Fractional DeepFX® mode was used at each of the scar bases 20 mL, 300 Hz, 2-10-1-10% and over each cosmetic subunit in its entirety at 20 mJ, 2-10-1-5% one pass. Finally, ActiveFX® mode was used over each scar shoulder 80 mJ, 3-5-1%.
Figure 7.

(a) Pre treatment and (b) post treatment.
Figure 8.

(top) Pre treatment and (bottom) 12 months post last treatment.
Pigmented and traumatic tattooed scars
Dyschromia within cutaneous scars is a result of abnormal melanogenesis.137 Eumelanin is a brown-black pigment found in higher ratios in darker skin types and those chronically exposed to ultraviolet radiation, and phaeomelanin is a red-brown pigment found in people with fair skin types. Critically, loss of adnexal melanocytic populations and the fundamental melanocytic immunomodulatory, anti-oxidative and anti-inflammatory functions during wound healing is postulated to account for dyspigmentatory scarring.138–140 Most commonly, this is seen as PIH in response to a variety of cutaneous insults.141
Prevention of dyschromia is critical. Therapy in tanned and dark skin types is considered on a case-by-case basis with appropriate risk counselling. Patients are warned of a worsening of pigmentation due to the potential hyperactivation of melanocytes and post-laser inflammatory response. Collateral bulk thermal injury is avoided with topical skin cooling; conservative starting treatment parameters, often with a small test patch, are performed and only when the effects of this test patch are apparent is treatment initiated cautiously with incremental fluence and density adjustments. If dyspigmentation does arise, depigmentation can be achieved medically or mechanically. Chemical depigmentation includes a number of topical agents that work at different stages of melanogenesis to selectively bleach hyperactivated melanocytes. These stages include: (a) prior to melanin synthesis - the regulation of the transcription and activity of tyrosinase, tyrosinerelated protein (TRP)-1 or 2, or peroxidase; (b) during melanin synthesis - by regulating the uptake and distribution of melanosomes in recipient keratinocytes; (c) after melanin synthesis - through melanin and melanosome degradation and processing of pigmented keratinocytes. The senior author advocates the use of tretinoin which acts via retinoid-activated transcription factors and interferes with melanocyte development and melanogenesis. It acts to both stimulate the differentiation of melanocyte precursors, inducing transcription of tyrosinase by protein kinase C activation and microphthalmia transcription factor (MITF) expression, and removes differentiated melanocytes by promoting apoptosis via caspase-3 pathway and bcl-2 down modulation.142 In addition, it is felt that hydroquinone and azelaic acid derived from Pityrosporum Ovale, both of which inhibit tyrosinase activity during melanin synthesis, are useful additions to reduce pigmentation within a scar.
Dermabrasion, cryosurgery, chemical peeling and laser therapy143–146 as well as conventional surgical excision have been proposed to manage hyperpigmentation. In terms of laser therapy, selective melanin photothermolysis is achieved with a laser that has a wavelength in the absorption spectrum of melanin and sufficient energy levels to target melanosomes.147,148 Pulse duration should be less than the thermal relaxation of melanin. Wavelengths less than 600 nm penetrate superficially with a lower fluence and thus can be used for epidermal hyperpigmentation, leaving deeper structures intact. Wavelengths longer than 600 nm penetrate more deeply, require a greater fluence and thus can be used to target dermal pigmention. Energy delivery induces skin whitening due to thermal expansion within the melanosomes leading to local vaporisation and acoustic waves that damage the nucleus and cause melanocytic apoptosis. Melanin that is subsequently released is processed through transepidermal elimination or through phagocytosis by dermal macrophages. Our unit uses short pulsed pigment selective Q-switched alexandrite (755 nm wavelength) and Nd:YAG lasers (1064 nm) for dermally pigmented scars. For epidermal pigmentation, the frequency doubled Nd:YAG (532 nm) and the fractional ablative (10,600 nm) CO2 lasers are used.
Tattooed scars may result from commercial tattooing or traumatic injuries such as road traffic accidents or other encounters with asphault, explosives or pencil puncture wounds. In addition, they may result from surgical interventions involving inadvertent dermal inoculation. They are most frequently carbon-based and situated in the superficial dermis.149,150 Conventionally, these lesions have been treated with surgical excision or laser therapy with Q-switched systems.16 Our preference is to start treatment with either the Q-switched alexandrite (755 nm wavelength) or Nd:YAG lasers (1064 nm). The tattoo ink particles undergo photomechanical fragmentation which reduces both the dark scar colour and prominence as there is a reduction in the inflammatory and granulomatous reaction driven by the presence of the foreign body.151 In some resistant cases, formal surgical excision of the tattooed scars or combination therapy is performed.
Pigmented and tattooed scars
9. A 55-year-old woman, Fitzpatrick type V, presented with hyperpigmented facial scars to her nose, cheeks and chin following smallpox infection as a child. She was otherwise fit and well. She underwent two cycles of fractional ablative CO2 laser therapy. ActiveFX® mode was used over each scar cosmetic subunit with 175 mJ, 3-9-5 for two passes and edges were blended with 150 mJ, 3-5-1. This was repeated on the nose and anterior cheeks two years later. Pre treatment (top row) and 12 months post last treatment with a favourable resolution of the localised scar dyschromia (bottom row).
10. A 43-year-old woman, Fitzpatrick type II, presented having developed hypertrophic scarring within a tattoo on the right breast following Rejuvi topical tattoo removal treatment. She was otherwise fit and well. She complained of intense pruritus, pain and of its unaesthetic appearance. She underwent two cycles of intralesional injection of 50 mg of 5-FU and 10 mg of Triamcinolone six weeks apart with good response. Thereafter, she underwent laser therapy with the Q-switched Alexandrite (755 nm) system with a fluence of 7.5 Jcm–2, 10 Hz with a 2 mm spot. After three sessions over 20 months, the patient was satisfied with its appearance and resolution of symptoms.
Figure 9.
(top) Pre treatment and (bottom) 24 months post last treatment.
Figure 10.

(a) Pre treatment and (b) after intralesional injection and laser treatment.
Vascular and contoured scars
Cutaneous scars may exhibit persisting hypervascularity as erythema or telangiectasia. During the proliferative phase of wound healing, VEGF stimulates migratory endothelial cells with subsequent neoangiogenesis. This may persist for many months and can be treated with a variety of vascular lasers and non-coherent intense pulsed light (IPL) sources, all of which work on the principle of selective photothermolysis. The chromophore in a hypervascular scar is haemoglobin which in its erthrocytic oxyhaemoglobin state has a maximum peak absorption of 542 nm (α peak) and 577 nm (β peak). This can be selectively targeted and damaged with minimal collateral damage to surrounding tissues if the appropriate wavelength of light is used.152 The photothermolysis induces a selective vascular injury with thrombosis, vessel wall necrosis and perivascular collagen damage with relatively little associated thermal injury in either the epidermis or surrounding dermis.153
Vascular lasers include pulsed dye (585 and 595 nm wavelength) and KTP-systems (532 nm), followed by longer wavelength lasers such as alexandrite lasers (755 nm), diode lasers (800–900 nm) and the Nd:YAG lasers (1064 nm).154–158 IPL systems differ from lasers in that they simultaneously deliver multiple light wavelengths (500–1200 nm) at different intensities. Lasers in contrast are monochromatic and offer the advantage of high precision and peak power intensity.
Components: Gemini laser: Nd-YAG 1064 for deep, KTP 532 for superficial, IPL
Special cases: tethered scars, pin cushioned scars, webs.
Microbes in scar manipulation
Scar quality and scar burden differ significantly with the presence of infective entities. Infection is a well-established contributor to tissue volume loss, inflammation, destruction and organisation.156 Streptococcal species are associated with skin graft loss and consequently increased scar burden. For this reason, strategies dealing with prophylaxis of infection, early and effective treatment are paramount to reducing the scar burden, especially in the case of extensive disease processes such as large burns. Early diagnostics specific to invasive infection is an area of high clincal demand.157 Furthermore, an inadvertent source of delayed wound healing may be conventional topical solutions applied to prevent or treat infection.162
It is also worth noting that some bacterial products are increasingly being recognised as potential therapeutics in multimodality scar management. Clostridium histolyticum collagenase is now established for the treatment of Dupuytren’s disease.163 Intralesional collagenase followed by compression has been described for ear keloids with some success.164
Conclusion
Scar management presents a significant challenge to the plastic surgeon. This two-part study summarised our Unit’s experience in multimodality treatment, with particular reference to the hypertrophic spectrum, acne, pigmented and vascular scarring, based on the senior author’s experience spanning nearly 30 years and backed by evidence.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Ernest Azzopardi is supported by the Welsh Assembly Government through a SHIPP Innovation award and the Welsh Clinical Academic Training Scheme.
References
- 1. Sund B. New Developments in Wound Care, vol. 86 London: PJB Publications CBS, 2000. [Google Scholar]
- 2. Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med 2011; 17: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol 2003; 4: 235–243. [DOI] [PubMed] [Google Scholar]
- 4. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician 2009; 80: 253–260. [PubMed] [Google Scholar]
- 5. Armour A, Scott PG, Tredget EE. Cellular and molecular pathology of HTS: basis of treatment. Wound Repair Egen 2007; 15 (Suppl. 1): S6–17. [DOI] [PubMed] [Google Scholar]
- 6. Van der Veen WM, Bloe en MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009; 35: 15–29. [DOI] [PubMed] [Google Scholar]
- 7. Arno AI, Gauglistz GG, Barret JP, et al. New molecular medicine-based scar management strategies. Burns 2014; 40: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih B, Garside E, McGrouther DA, et al. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 2010; 18: 139–153. [DOI] [PubMed] [Google Scholar]
- 9. Bloemen MC, van der Veer WM, Ulrich MM, et al. Prevention and curative management of hypertrophic scar formation. Burns 2009; 35: 463–475. [DOI] [PubMed] [Google Scholar]
- 10. Middelkoop E, Monstrey S, Teot L, et al. (eds.). Scar Management Practical Guidelines. Brussels: Maca-Cloetens, 2011: 100–109. [Google Scholar]
- 11. Reiffel RS. Prevention of hypertrophic scars by long-term paper tape application. Plast Reconstr Surg 1995; 96: 1715–1718. [DOI] [PubMed] [Google Scholar]
- 12. Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011; 254: 217–225. [DOI] [PubMed] [Google Scholar]
- 13. Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plas Recon Surg 2000; 105: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 14. Suetake T, Sasai S, Zhen YX, et al. Functional analyses of the stratum corneum in scars. Sequential studies after injury and comparison among keloids, hypertrophic scars, and atrophic scars. Arch Dermatol 1996; 132: 1453. [PubMed] [Google Scholar]
- 15. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg 2008; 32: 82–92. [DOI] [PubMed] [Google Scholar]
- 16. Engrav LH, Heimbach DM, Rivara FP, et al. 12-Year within wound study of the effectiveness of custom pressure garment therapy. Burns 2010; 36: 975–983. [DOI] [PubMed] [Google Scholar]
- 17. Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatol Surg 2012; 38: 414–423. [DOI] [PubMed] [Google Scholar]
- 18. Roques C. Massage applied to scars. Wound Repair Regen 2002; 10: 126–128. [DOI] [PubMed] [Google Scholar]
- 19. Field T, Peck M, Hernandez-Rief M, et al. Postburn itching: pain, and psychological symptoms are reduced with massage therapy. J Burn Care Rehabil 2000; 21: 189–193. [DOI] [PubMed] [Google Scholar]
- 20. Patino O, Novick C, Merlo A, et al. Massage in hypertrophic scars. J Burn Care Rehabil 1999; 20: 268–271. [PubMed] [Google Scholar]
- 21. Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesth Plast Surg 2007; 31: 468–492. [DOI] [PubMed] [Google Scholar]
- 22. Anzarut A, Olson J, Singh P, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg 2009; 62: 77–84. [DOI] [PubMed] [Google Scholar]
- 23. Macintyre L, Baird M. Pressure garments for use in the treatment of hypertrophic scars: a review of the problems associated with their use. Burns 2006; 32: 10–15. [DOI] [PubMed] [Google Scholar]
- 24. Reno F, Grazianetti P, Cannas M. Effects of mechanical compression on hypertrophic scars: prostaglandin E2 release. Burns 2001; 27: 215–218. [DOI] [PubMed] [Google Scholar]
- 25. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg 2008; 32: 82–92. [DOI] [PubMed] [Google Scholar]
- 26. Baisch A, Riedel F. Hyperplastische Narben un Keloide. HNO 2006; 54: 981–994. [DOI] [PubMed] [Google Scholar]
- 27. Steinstraesser L, Flak E, Witte B, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intra-individual comparison. Plast Reconstr Surg 2011; 128: 306e–313e. [DOI] [PubMed] [Google Scholar]
- 28. Atkinson JA, McKenna KT, Barnett AG, et al. Randomized: controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incision that traverse Langer’s skin tension lines. Plast Reconstr Surg 2005; 116: 1648–1656. [DOI] [PubMed] [Google Scholar]
- 29. O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013; 9: CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg 1989; 84: 827–837. [DOI] [PubMed] [Google Scholar]
- 31. Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg 2002; 110: 560–571. [DOI] [PubMed] [Google Scholar]
- 32. Urioste SS, Arndt KA, Dover JS. Keloids and hypertrophic scars: review and treatment strategies. Semin Cutan Med Surg 1999; 18: 159–171. [DOI] [PubMed] [Google Scholar]
- 33. Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch 2011; 78: 68–76. [DOI] [PubMed] [Google Scholar]
- 34. Lee KK, Mehrany K, Swanson NA. Surgical revision. Dermatol Clin 2005; 23: 141–150. [DOI] [PubMed] [Google Scholar]
- 35. Engrav LH, Gottlieb JR, Millard SP, et al. A comparison of intramarginal and extramarginal excision of hypertrophic burn scars. Plast Reconstr Surg 1988; 81: 40–45. [DOI] [PubMed] [Google Scholar]
- 36. Guix B, Henriquez I, Andres A. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. Int J Radiat Oncol Biol Phys 2001; 50: 167–172. [DOI] [PubMed] [Google Scholar]
- 37. Yossi S, Krhili S, Mesgouez-Nebout N, et al. Adjuvant treatment of keloid scars: electrons or brachytherapy? Cancer Radiother 2013; 17: 21–25. [DOI] [PubMed] [Google Scholar]
- 38. Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg 2009; 124: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 39. Ogawa R, Mitsuhashi K, Kyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg 2003; 111: 547–553. [DOI] [PubMed] [Google Scholar]
- 40. Poston J. The use of silicone gel sheeting in the management of hypertrophic and keloid scars. J Wound Care 2000; 9: 10–16. [DOI] [PubMed] [Google Scholar]
- 41. Mustoe Thomas A., et al. International clinical recommendations on scar management. Plastic and reconstructive surgery 110.2 (2002): 560-571. [DOI] [PubMed] [Google Scholar]
- 42. Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg 2010; 125: 557–568. [DOI] [PubMed] [Google Scholar]
- 43. Burd A, Keloid Huang L. In Best practice monographs in the clinical evidence series. London: BMJ Publishing, 2012. [Google Scholar]
- 44. Wang XQ, Liu YK, Qing C, et al. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg 2009; 63: 688–692. [DOI] [PubMed] [Google Scholar]
- 45. Niessen FB, Spauwen PHM, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999; 104: 1435–1458. [DOI] [PubMed] [Google Scholar]
- 46. Wong Thian-Sze, et al. “The Efficacy of Triamcinolone Acetonide in Keloid Treatment: A Systematic Review and Meta-analysis.” Frontiers in Medicine 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sproat JE, Dalcin A, Weitauer N, et al. Hypertrophic sternal scars: silicone gel sheeting versus kenalog injection treatment. Plast Reconstr Surg 1992; 90: 988–992. [PubMed] [Google Scholar]
- 48. Gressel MG, Parrish RK, 2nd, Folberg R. 5-fluorouracil and glaucoma filtering surgery: I. An animal model. Ophthalmology 1984; 91: 378–383. [DOI] [PubMed] [Google Scholar]
- 49. Heuer DK, Parrish RK, 2nd, Gressel MG, et al. 5-fluorouracil and glaucoma filtering surgery. II. A pilot study. Ophthalmology 1984; 91: 384–394. [DOI] [PubMed] [Google Scholar]
- 50. Fitzpatrick RE. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg 1999; 25: 224–232. [DOI] [PubMed] [Google Scholar]
- 51. Apikian M, Goodman G. Intralesional 5-fluorouracil in the treatment of keloid scars. Australas J Dermatol 2004; 45: 140–143. [DOI] [PubMed] [Google Scholar]
- 52. Darougheh A, Asilian A, Shariati F. Intralesional triamcinolone or in combination with 5-fluorouracil for treatment of keloid and hypertrophic scars. Clin Exp Dermatol 2007; 34: 219–223. [DOI] [PubMed] [Google Scholar]
- 53. Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology 2002; 204: 130–132. [DOI] [PubMed] [Google Scholar]
- 54. Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathologic study. J Am Acad Dermatol 2005; 52: 474–479. [DOI] [PubMed] [Google Scholar]
- 55. Uppal RS, Khan U, Kakar S, et al. The effects of a single dose of 5-fluorouracil on keloid scars: a clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg 2001; 108: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 56. Davison SP, Dayan JH, Clemens MW, et al. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet Surg J 2009; 29: 40–46. [DOI] [PubMed] [Google Scholar]
- 57. Huang L, Cai Y, Lung I, et al. A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. JPRAS 2013; 66: e251–259. [DOI] [PubMed] [Google Scholar]
- 58. Liu W, Wu X, Gao Z, et al. Remodelling of keloid tissue into normal-looking skin. J Plast Reconstr Aesthet Surg 2008; 61: 1553–1554. [DOI] [PubMed] [Google Scholar]
- 59. Reish RG, Eriksson E. Scar treatments: preclinical and clinical studies. J Am Coll Surg 2008; 206: 719–730. [DOI] [PubMed] [Google Scholar]
- 60. Muir IF. On the nature of keloid and hypertrophic scars. Br J Plast Surg 1990; 43: 61–69. [DOI] [PubMed] [Google Scholar]
- 61. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg 2006; 8: 362–368. [DOI] [PubMed] [Google Scholar]
- 62. Syed F, Ahmadi E, Iqbal SA, et al. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol 2011; 164: 83–96. [DOI] [PubMed] [Google Scholar]
- 63. Gailloud-Mathieu M, Raffoul W, Egloff DV. Citrices hypertrophiques et cheloides: quelles options therapeutiques aujourdhui? Revue Medicale de la Suisse Romande 1999; 119: 721–728. [PubMed] [Google Scholar]
- 64. Shepherd J, Dawber RPR. Historical and scientific basis of cryosurgery. Clin Exp Dermatol 1982; 7: 321–328. [DOI] [PubMed] [Google Scholar]
- 65. van Leeuwen Michiel CE, et al. “Intralesional cryotherapy for treatment of keloid scars: a prospective study.” Plastic and reconstructive surgery 135.2 (2015): 580-589. [DOI] [PubMed] [Google Scholar]
- 66. Rusciani L, Rossi G, Bono R. Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol 1993; 19: 529–534. [DOI] [PubMed] [Google Scholar]
- 67. Zouboulis CC, Blume U, Buttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars: a prospective consecutive trial of case series. Arch Dermatol 1993; 129: 1146–1151. [PubMed] [Google Scholar]
- 68. Har-Shai Yaron, Zouboulis Christos C. “Intralesional cryotherapy for the treatment of keloid scars: a prospective study.” Plastic and reconstructive surgery 136.3 (2015): 397e-398e. [DOI] [PubMed] [Google Scholar]
- 69. Har-Shai Y, Mettanes I, Genin O, et al. Keloid histopathology after intralesional cryoneedle treatment. J Eur Acad Dermatol Venereol 2011; 25: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 70. Weshahy AH. Intralesional cryosurgery. A new technique using cryoneedles. J Dermatol Surg 1993; 19: 123–126. [DOI] [PubMed] [Google Scholar]
- 71. Har-Shai Y, Sabo E, Rohde E, et al. Intralesional cryosurgery markedly enhances the involution of recalcitrant auricular keloids – A new clinical approach supported by experimental studies. Wound Repair Regen 2006; 14: 18–27. [DOI] [PubMed] [Google Scholar]
- 72. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plastic Reconstr Surgery 2003; 111: 1841–1852. [DOI] [PubMed] [Google Scholar]
- 73. Har-Shai Y, Brown W, Labbe’ D, et al. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery. Int J Lower Extrem Wounds 2008; 7: 169–175. [DOI] [PubMed] [Google Scholar]
- 74. Har-Shai Y, Brown W, Pallua N, et al. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids. Plast Reconstr Surg 2010; 126: 1798–1800. [DOI] [PubMed] [Google Scholar]
- 75. Har-Shai Y. Intralesional cryosurgery for enhancing the involution of hypertrophic scars and keloids. A new effective technology based on experimental and clinical data. Journal of Wound Technology 2012; 15: 8–9. [Google Scholar]
- 76. Har-Shai Y, Har-Shai L. Intralesional cryosurgery for the treatment of upper lip keloid following deep chemical peeling. Eur J Plastic Surgery 2014; 37: 679–682. [Google Scholar]
- 77. Har-Shai Y, Weidman MJ. The treatment of keloids and hypertrophic scars through intralesional cryotherapy. Kosmetische Medizin 2012; 33: 48–52 (In German). [Google Scholar]
- 78. Zouboulis CC, Rosenberger AD, Forster T, et al. Modification of a device and its application for intralesional cryosurgery of old recalcitrant keloids. Arch Dermatol 2004; 140: 1293–1294. [DOI] [PubMed] [Google Scholar]
- 79. Gupta S, Kumar B. Intralesional cryosurgery using lumbar puncture and/or hypodermic needles for large, bulky, recalcitrant keloids. Int J Dermatol 2001; 40: 349–353. [DOI] [PubMed] [Google Scholar]
- 80. Mirmovich O, Gil T, Goldin I, et al. Pain evaluation and control during and following the treatment of hypertrophic scars and keloids by contact and intralesional cryosurgery—a preliminary study. J Eur Acad Dermatol Venereol 2012; 26: 440–447. [DOI] [PubMed] [Google Scholar]
- 81. Har-Shai Y, Dujovny E, Rohde E, et al. Effect of skin surface temperature on skin pigmentation during contact and intralesional cryosurgery of keloids. J Eur Acad Dermatol Venereol 2007; 21: 191–198. [DOI] [PubMed] [Google Scholar]
- 82. Berman Brian, Andrea Maderal, Brian Raphael. “Keloids and hypertrophic scars: pathophysiology, classification, and treatment.” Dermatologic Surgery 43 (2017): S3-S18. [DOI] [PubMed] [Google Scholar]
- 83. Niessen FB, Spauwen PHM, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 1999; 104: 1435–1458. [DOI] [PubMed] [Google Scholar]
- 84. Levy DS, Salter MM, Roth RE. Postoperative irradiation in the prevention of keloids. AJR Am J Roentgenol 1976; 50: 457. [DOI] [PubMed] [Google Scholar]
- 85. Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol 2009; 129: 2136–2141. [DOI] [PubMed] [Google Scholar]
- 86. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol 2010; 63: 782–788. [DOI] [PubMed] [Google Scholar]
- 87. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol 2006; 7: 281–290. [DOI] [PubMed] [Google Scholar]
- 88. Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol 2001; 45: 109–117. [DOI] [PubMed] [Google Scholar]
- 89. Stathakis V, Kilkenny M, Marks R. Descriptive epidemiology of acne vulgaris in the community. Australas J Dermatol 1997; 38: 115–123. [DOI] [PubMed] [Google Scholar]
- 90. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999; 41: 577–580. [PubMed] [Google Scholar]
- 91. Jemec GBE, Linneberg A, Nielsen NH, et al. Have oral contraceptives reduced the prevalence of acne? A population-based study of acne vulgaris and tobacco smoking and oral contraceptives. Dermatology 2002; 204: 179–184. [DOI] [PubMed] [Google Scholar]
- 92. Koo JY, Smith LL. Psychologic aspects of acne. Pediatr Dermatol 1991; 8: 185–188. [DOI] [PubMed] [Google Scholar]
- 93. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol 2009; 18: 821–832. [DOI] [PubMed] [Google Scholar]
- 94. Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol 2011; 4: 50–57. [PMC free article] [PubMed] [Google Scholar]
- 95. Fife D, Zachary CB. Combining techniques for treating acne scars. Curr Dermatol Rep 2012; 1: 82–88. [Google Scholar]
- 96. Thiboutot D, Gollnick H. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne Group. J Am Acad Dermatol 2009; 60: S1–S50. [DOI] [PubMed] [Google Scholar]
- 97. Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg 2006; 32: 1458–1466. [DOI] [PubMed] [Google Scholar]
- 98. Goodman G. Post-acne scarring: a review. J Cosmet Laser Ther 2003; 5: 77–95. [DOI] [PubMed] [Google Scholar]
- 99. Fulton JE, Jr, Silverton K. Resurfacing the acne-scarred face. Dermatol Surg 1999; 25: 353–359. [DOI] [PubMed] [Google Scholar]
- 100. Bhalla M, Thami GP. Microdermabrasion: reappraisal and brief review of literature. Dermatol Surg 2006; 32: 809–814. [DOI] [PubMed] [Google Scholar]
- 101. Al-Waiz MM, Al-Sharqi AI. Medium-depth chemical peels in the treatment of acne scars in dark-skinned individuals. Dematol Surg 2002; 28: 383–387. [DOI] [PubMed] [Google Scholar]
- 102. Goodman GJ. Management of post-acne scarring: what are the options for treatment? Am J Clin Dermatol 2000; 1: 3–17. [DOI] [PubMed] [Google Scholar]
- 103. Lee JB, Chung WG, Kwahck H, et al. Focal treatment of acne scars with trichloroacetic acid: chemical reconstruction of skin scars method. Dermatol Surg 2002; 28: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 104. Rogachefsky AS, Hussain M, Goldberg DJ. Atrophic and a mixed pattern of acne scars improved with a 1320-nm Nd:YAG laser. Dermatol Surg 2003; 29: 904–908. [DOI] [PubMed] [Google Scholar]
- 105. Jeong JT, Park JH, Kye YC. Resurfacing of pitted facial acne scars using Er:Yag laser with ablation and coagulation mode. Aesthetic Plast Surg 2003; 27: 130–134. [DOI] [PubMed] [Google Scholar]
- 106. Sawcer D, Lee HR, Lowe NJ. Lasers and adjunctive treatment for facial scars: a review. J Cutan Laser Ther 1999; 1: 77–85. [DOI] [PubMed] [Google Scholar]
- 107. Alster TS, Zaulyanov-Scanlon L. Laser scar revision: A review. Dermatol Surg 2007; 33: 131–140. [DOI] [PubMed] [Google Scholar]
- 108. Tanzi EL, Alster TS. Treatment of atrophic facial scars with a dual-mode Er:YAG laser. Dermatol Surg 2002; 28: 551–555. [DOI] [PubMed] [Google Scholar]
- 109. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg 1996; 22: 151–154. [DOI] [PubMed] [Google Scholar]
- 110. Alster TS, Nanni CA, Williams CM. Comparison of four carbon dioxide resurfacing lasers. A clinical and histopathologic evaluation. Dermatol Surg 1999; 25: 153–158. [DOI] [PubMed] [Google Scholar]
- 111. Alster TS. Clinical and histologic evaluation of six erbium: YAG lasers for cutaneous resurfacing. Lasers Surg Med 1999; 24: 87–92. [DOI] [PubMed] [Google Scholar]
- 112. Khatri KA, Ross V, Grevelink JM, et al. Comparison of erbium:YAG and carbon dioxide lasers in resurfacing of facial rhytides. Arch Dermatol 1999; 135: 391–397. [DOI] [PubMed] [Google Scholar]
- 113. Ross EV, Miller C, Meehan KP, et al. One-pass CO2 versus multiple-pass Er:YAG laser resurfacing in the treatment of rhytides: A comparison side-by-side study of pulsed CO2 and Er:YAG lasers. Dermatol Surg 2001; 27: 709–715. [DOI] [PubMed] [Google Scholar]
- 114. Alster TS, Lupton JR. Prevention and treatment of side effects and complications of cutaneous laser resurfacing. Plast Reconstr Surg 2002; 109: 308–316. [DOI] [PubMed] [Google Scholar]
- 115. Tanzi EL, Alster TS. Comparison of a 1450-nm diode laser and a 1320-nm Nd:YAG laser in the treatment of atrophic facial scars: a prospective clinical and histologic study. Dermatol Surg 2004; 30: 152–157. [DOI] [PubMed] [Google Scholar]
- 116. Chan HH, Lam LK, Wong DS, et al. Use of 1,320 nm Nd:YAG laser for wrinkle reduction and the treatment of atrophic acne scarring in Asians. Lasers Surg Med 2004; 34: 98–103. [DOI] [PubMed] [Google Scholar]
- 117. Sadick NS, Schecter AK. A preliminary study of utilization of the 1320-nm Nd:YAG laser for the treatment of acne scarring. Dermatol Surg 2004; 30: 995–1000. [DOI] [PubMed] [Google Scholar]
- 118. Bellew SG, Lee C, Weiss MA, et al. Improvement of atrophic acne scars with a 1,320 nm Nd:YAG laser: retrospective study. Dermatol Surg 2005; 31: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 119. Chapas AM, Brightman L, Sukal S, et al. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med 2008; 40: 381–386. [DOI] [PubMed] [Google Scholar]
- 120. Walgrave SE, Ortiz AE, MacFalls HT, et al. Evaluation of a novel fractional resurfacing device for treatment of acne scarring. Lasers Surg Med 2009; 41: 122–127. [DOI] [PubMed] [Google Scholar]
- 121. Cho SB, Lee SJ, Kang JM, et al. The efficacy and safety of 10,600-nm carbon dioxide fractional laser for acne scars in Asian patients. Dermatol Surg 2009; 35: 1955–1961. [DOI] [PubMed] [Google Scholar]
- 122. Chan NP, Ho SG, Yeung CK, et al. Fractional ablative carbon dioxide laser resurfacing for skin rejuvenation and acne scars in Asians. Lasers Surg Med 2010; 42: 615–623. [DOI] [PubMed] [Google Scholar]
- 123. Wang YS, Tay YK, Kwok C. Fractional ablative carbon dioxide laser in the treatment of atrophic acne scarring in Asian patients: A pilot study. J Cosmet Laser Ther 2010; 12: 61–64. [DOI] [PubMed] [Google Scholar]
- 124. Cho SB, Lee SJ, Cho S, et al. Non-ablative 1,550-nm erbium-glass and ablative 10,600-nm carbon dioxide fractional lasers for acne scars: A randomized split-face study with blinded response evaluation. Acad Dermatol Venereol 2010; 24: 921–925. [DOI] [PubMed] [Google Scholar]
- 125. Jung JY, Lee JH, Ryu DJ, et al. Lowerfluence, higher-density versus higher-fluence, lower-density treatment with a 10,600-nm carbon dioxide fractional laser system: A split-face, evaluator-blinded study. Dermatol Surg 2010; 36: 2022–2029. [DOI] [PubMed] [Google Scholar]
- 126. Hedelund L, Haak CS, Togsverd-Bo K, et al. Fractional CO2 laser resurfacing for atrophic acne scars: a randomized controlled trial with blinded response evaluation. Lasers in Surgery and Medicine 2012; 44: 447–452. [DOI] [PubMed] [Google Scholar]
- 127. Bjørn M, Stausbøl-Grøn B, Olesen AB, et al. Treatment of acne scars with fractional CO2 laser at 1-month versus 3-month intervals: an intra-individual randomized control trial. Lasers in Surgery and Medicine 2014; 46: 89–93. [DOI] [PubMed] [Google Scholar]
- 128. Bhatia N, David CV, Hazany S, et al. Acne scarring. In: Zeichner JA. (ed.). Acneiform Eruptions in Dermatology: A Differential Diagnosis. New York: Springer; 2014: 237–243. [Google Scholar]
- 129. Sardana K, Manjhi M, Garg VK, et al. Which type of atrophic acne scar (ice-pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg 2014; 40: 288–300. [DOI] [PubMed] [Google Scholar]
- 130. Goodman GJ. Postacne scarring: a review of its pathophysiology and treatment. Dermatol Surg 2000; 26: 857–871. [DOI] [PubMed] [Google Scholar]
- 131. Batra RS. Surgical techniques for scar revision. Skin Therapy Lett 2005; 10: 4–7. [PubMed] [Google Scholar]
- 132. Alam M, Omura N, Kaminer MS. Subcision for acne scarring: technique and outcomes in 40 patients. Dermatol Surg 2005; 31: 310–317. [DOI] [PubMed] [Google Scholar]
- 133. Roenigk HH., Jr. Dermabrasion: state of the art. J Dermatol Surg Oncol 1985; 11: 306–314. [DOI] [PubMed] [Google Scholar]
- 134. Nast A, Dreno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol 2012; 26 (Suppl. 1): 1–29. [DOI] [PubMed] [Google Scholar]
- 135. Nast A, Rosumeck S, Sammain A, et al. Methods report on the development of the European S3 guidelines for the treatment of acne. J Eur Acad Dermatol Venereol 2012; 26 (Suppl. 1): e1–e41. [DOI] [PubMed] [Google Scholar]
- 136. Razny B, Nast A. Acne treatment in the field: how guidelines and other sources can be included in daily practice. JEADV 2013; 27 (Suppl. 2): 2–5. [DOI] [PubMed] [Google Scholar]
- 137. Tyack ZF, Pegg S, Ziviani J. Postburn dyspigmentation: its assessment, management, and relationship to scarring–a review of the literature. J Burn Care Rehabil 1997; 18: 435–440. [DOI] [PubMed] [Google Scholar]
- 138. Plonka PM, Passeron T, Brenner M, et al. What are melanocytes really doing all day long? Exp Dermatol 2009; 18: 799–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Randhawa M, Huff T, Valencia JC, et al. Evidence for the ectopic synthesis of melanin in human adipose tissue. Faseb J 2009; 23: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Breathnach AS, Chadwick S. Melanocytes in early regenerated human epidermis. J Invest Dermatol 1960; 35: 245–251. [Google Scholar]
- 141. Coelho SG, Zhou Y, Bushar HF, et al. Long-lasting pigmentation of human skin, a new look at an overlooked response to UV. Pigment Cell Melanoma Res 2009; 22: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Watabe H, Soma Y, Ito M, et al. All-transretinoic acid induces differentiation and apoptosis of murine melanocyte precursors with induction of the microphtalmia-associated transcription factor. J Inv Dermatol 2002; 118: 35–42. [DOI] [PubMed] [Google Scholar]
- 143. Li YT, Yang KC. Comparison of the frequency-doubled Q-switched Nd:YAG laser and 35% trichloroacetic acid for the treatment of face lentigines. Dermatol Surg 1999; 25: 202–204. [DOI] [PubMed] [Google Scholar]
- 144. Kunachak S, Leelaudomlipi P, Wongwaisayawan S. Dermabrasion: a curative treatment for melasma. Aesthetic Plast Surg 2001; 25: 114–117. [DOI] [PubMed] [Google Scholar]
- 145. Stratigos AJ, Dover JS, Arndt KD. Laser treatment of pigmented lesions-2000. Arch Dermatol 2000; 136: 915–921. [DOI] [PubMed] [Google Scholar]
- 146. Anderson RR. Laser in dermatology-a critical update. J Dermatol 2000; 27: 700–705. [DOI] [PubMed] [Google Scholar]
- 147. Anderson RR, Parish JA. Selective photothermolysis. Precise microsurgery by selective absorption of pulsed radiation. Science 1983; 220: 524–527. [DOI] [PubMed] [Google Scholar]
- 148. Brazzini B, Hautmann G, Ghersetich I, et al. Laser tissue interaction in epidermal pigmented lesions. J Eur Acad Dermatol Venereol 2001; 15: 388–391. [DOI] [PubMed] [Google Scholar]
- 149. Bowes LE, Nouri K, Berman B, et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled Nd:YAG laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg 2002; 28: 714–719. [DOI] [PubMed] [Google Scholar]
- 150. Kauvar ANB, Hruza GJ. Principles and Practices in Cutaneous Laser Surgery. Boca Raton, FL: Taylor & Francis, 2005. [Google Scholar]
- 151. Kuperman-Beade M, Levine VJ, Ashinoff R. Laser removal of tattoos. Am J Clin Dermatol 2001; 2: 21–25. [DOI] [PubMed] [Google Scholar]
- 152. Altshuler GB, Anderson RR, Manstein D, et al. Smirnov MZ Extended theory of selective photothermolysis. Lasers Surg Med 2001; 29: 416–432. [DOI] [PubMed] [Google Scholar]
- 153. Reddy KK, Brauer JA, Idriss MH, et al. Treatment of port-wine stains with a short pulse width 532-nm Nd:YAG laser. J Drugs Dermatol 2013; 12: 66–71. [PubMed] [Google Scholar]
- 154. Mordon S, Brisot D, Fournier N. Using a ‘non uniform pulse sequence’ can improve selective coagulation with a Nd:YAG laser (1.06 microm) thanks to Met-hemoglobin absorption: a clinical study on blue leg veins. Lasers Surg Med 2003; 32: 160–170. [DOI] [PubMed] [Google Scholar]
- 155. Black JF, Wade N, Barton JK. Mechanistic comparison of blood undergoing laser photocoagulation at 532 and 1,064 nm. Lasers Surg Med 2005; 36: 55–65. [DOI] [PubMed] [Google Scholar]
- 156. Rose AE, Goldberg DJ. Successful treatment of facial telangiectasias using amicropulse 1,064-nm neodymium-doped yttrium aluminum garnet laser. Dermatol Surg 2013; 39: 1062–1066. [DOI] [PubMed] [Google Scholar]
- 157. Glaich AS, Goldberg LH, Dai T, et al. Fractional photothermolysis for the treatment of telangiectatic matting: a case report. J Cosmet Laser Ther 2007; 9: 101–103. [DOI] [PubMed] [Google Scholar]
- 158. Bencini PL, Tourlaki A, De Giorgi V, et al. Laser use for cutaneous vascular alterations of cosmetic interest. Dermatol Ther 2012; 25: 340–351. [DOI] [PubMed] [Google Scholar]
- 159. Borges da, Costa J, Boixeda P, Moreno C, et al. Treatment of resistant port-wine stains with a pulsed dual wavelength 595 and 1064 nmlaser: a histochemical evaluation of the vessel wall destruction and selectivity. Photomed Laser Surg 2009; 27: 599–605. [DOI] [PubMed] [Google Scholar]
- 160. Azzopardi E, Boyce DE. Clostridium histolyticum collagenase in the treatment of Dupuytren’s contracture. Br J Hosp Med (Lond) 2012; 73: 432–436. [DOI] [PubMed] [Google Scholar]
- 161. Azzopardi EA, Azzopardi E, Camillieri L, et al. Gram negative wound infection in hospitalised adult burn patients-systematic review and metanalysis. PLoS One 2014; 9: e95042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bae-Harboe YSC, Harboe-Schmidt JE, Graber E, et al. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol Surg 2014; 40: 519–524. [DOI] [PubMed] [Google Scholar]
- 163. Heyneman A, Hoeksema H, Vandekerckhove D, et al. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016; 42: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 164. Marshall CD, Hu MS, Leavitt T, et al. Cutaneous scarring: Basic science, current treatments, and future directions. Advances in Wound Care 2016. DOI: 10.1089/wound.2016.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
How to cite this article
- McGoldrick RB, Theodorakopoulou E, Azzopardi E, et al. Lasers and ancillary treatments for scar management Part 2: Keloid, hypertrophic, pigmented and acne scars. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/205951311689805. [DOI] [PMC free article] [PubMed] [Google Scholar]




