Abstract

Introduction:
Radiation therapy is a well-recognised modality for the adjuvant treatment of keloid scars. It can be conventionally delivered as external beam using a large apparatus at a distance from the lesion or as brachytherapy with specialised equipment to enable the delivery of treatment in the immediate vicinity of the keloidal tissue.
Methods:
An English literature review was performed with keywords ‘brachytherapy’ and ‘keloid’ using the databases PubMed, Embase and Web of Science from their individual dates of inception until June 2017. Studies pertinent to the field are presented in a chronological manner to depict the evolution of different brachytherapy strategies over the last decades. We also discuss considerations relating to the risk of secondary carcinogenesis, which are relevant to shared decision-making in the clinical setting.
Discussion:
Low dose rate interstitial brachytherapy was first introduced in the English literature in 1976 and currently appears to have been superseded by more modern approaches, including high dose rate interstitial brachytherapy. This modality compares favourably to more traditional modes of radiotherapy in terms of recurrence as well as rates of symptomatic relief from keloidal symptoms. Superficial brachytherapy was introduced more recently in the relevant literature and appears to be associated with favourable therapeutic outcomes compared to external beam radiation therapy.
Conclusion:
Brachytherapy is a valid modality of radiotherapy for the adjuvant treatment of keloid scars, with high dose rate interstitial and surface regimens gaining in popularity over recent years. Further research needs to focus on randomised controlled trials to further establish the role of different radiotherapy modalities in keloid scar management.
Keywords: Keloid, radiation, brachytherapy, scar, carcinogenesis, radiotherapy
Lay summary


Keloid scars are a distressing and challenging condition to manage effectively. Radiation therapy is a well-recognised modality to treat keloids after they are removed with surgery. Conventionally, treatment is given as external beam radiotherapy using a large apparatus at a distance from the scar. Another way of delivering radiation is by using a radioactive wire fed through a small plastic tube placed on top of or within the wound following scar removal, known as brachytherapy.
A detailed analysis of the relevant literature shows that brachytherapy compares favourably to the traditional external beam radiotherapy and can be even used in cases where keloids scars have recurred after external beam treatment. The risk of suffering cancerous changes in the area treated with radiotherapy appears to be very small and no reports of brachytherapy-induced malignancy exist in the literature so far.
Introduction
Adjuvant radiation therapy is a recognised option for the treatment of keloid scars. It was first described by Sequeira in 19091 and is currently considered the most efficacious modality according to the international advisory panel on scar management.2 The reported therapeutic response rates are generally in the range of 67–98%.3
Keloids are characterised by a variety of pathophysiological parameters including the accelerated proliferation of fibroblasts and an impaired balance between proliferation and apoptosis.4 Moreover, it has recently been suggested that endothelial dysfunction is one of the contributory pathophysiological mechanisms, which is mediated via the propagation of the inflammatory response in scar tissue.5,6 Following surgical excision of a scar, active blood borne repopulation of fibroblasts occurs; postoperative radiation treatment is thought to prevent recurrence by inducing fibroblastic apoptosis as well as imparting toxicity to endothelial cells.7,8
The concept of biological effective dose (BED) is important in considering appropriate radiotherapy regimens for the adjuvant treatment of keloid scars. BED represents a measure of the radiation delivered by a particular combination of dose per fraction as well as the total dose to a given lesion. Different tissues have varying degrees of radiosensitivity denoted by a tissue-specific α/β ratio. For keloid scars this value is widely accepted to be 10; nevertheless, further analysis is necessary to further confirm the validity of this value.9
A number of literature reports have examined the BED required for the successful treatment of keloid scars and concluded that a value (for both external beam as well as brachytherapy) of ⩾ 30 Gy is associated with < 10% recurrence. In simple terms, this relates to a single dose of 13–15 Gy, two fractions of 8.5–10 Gy or three fractions of 6–7.5 Gy given within two days of surgery.10,11
In conclusion, the current consensus appears to focus on delivering a relatively high dose of radiation in a limited number of fractions and a short overall treatment time (the latter defined as the time period between surgery and the last radiation dose).12
Types of radiotherapy modalities
Radiation can be delivered either as external beam radiotherapy (EBRT) or brachytherapy. EBRT involves a conventional large radiotherapy apparatus (Figure 1) and the delivery of a relatively high radiation dose due to the large distance between the scar and the delivering equipment. One of the main drawbacks is the inevitable coverage of healthy tissue in the irradiation field.
Figure 1.
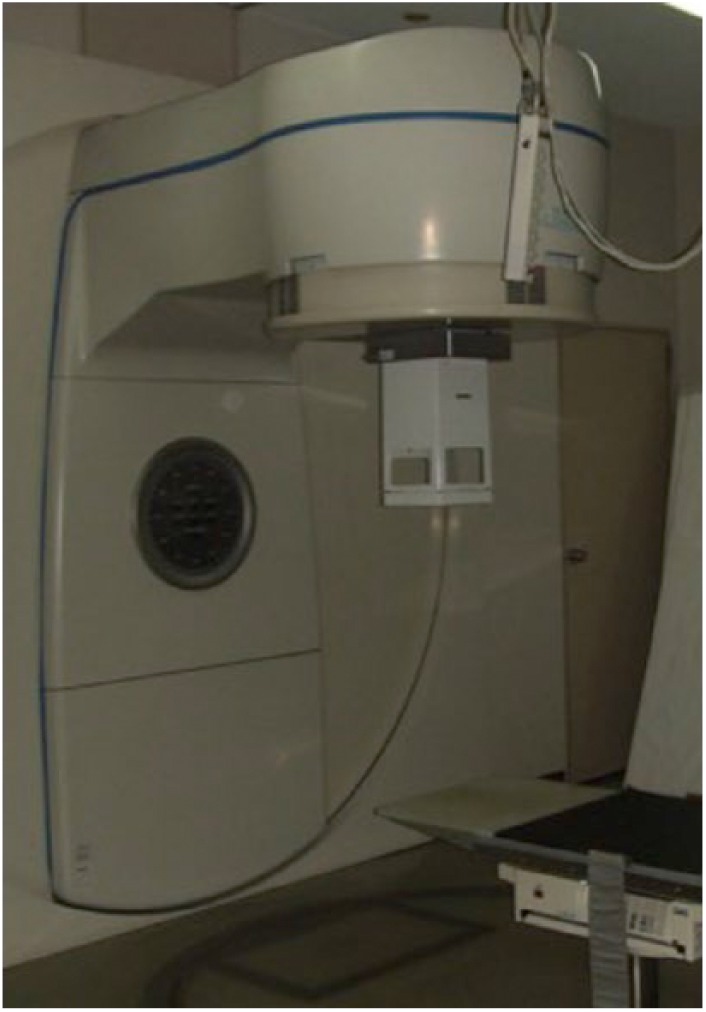
External beam radiotherapy machine.
Brachytherapy, deriving from the Greek ‘brachy’ meaning short and ‘therapy’ for treatment, was first introduced in the international literature by Nicoletis and Chassagne in 1967.13 It relies on delivering radiation within the immediate target area using a smaller delivery apparatus compared to external beam therapy. The carrier sits at the level of the dermis or attached to the external surface of the skin.
Brachytherapy appears to have the following comparative advantages:7,14,15
more focused in situ delivery and distribution of radiation to the target area;
less exposure of surrounding healthy skin to radiation; and
need for lower dose of radiation to achieve the same therapeutic effect compared to EBRT.
Brachytherapy can be divided into interstitial vs. surface modalities and the former is further subdivided into low dose rate and high dose rate modalities. The ‘dose rate’ term relates to the amount of radiation delivered over an individual treatment session as well as the overall treatment time.
Interstitial or internal brachytherapy makes use of a hollow catheter, which is inserted in the wound after keloid excision (Figure 2) before closure. A radioactive wire source is fed through this to deliver the radiation at the level of the dermis.16
Figure 2.
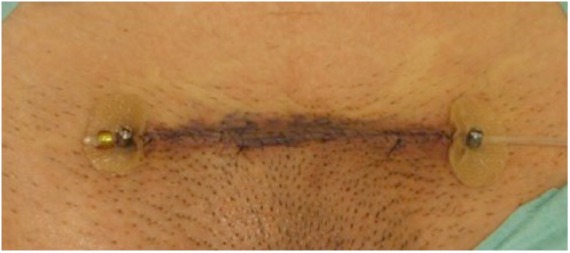
Interstitial brachytherapy for the treatment of a suprapubic keloid scar: the catheter, is placed within the dermis at the time of the extralesional scar excision.
Low dose rate (LDR) brachytherapy involves the use of a low dose radioactive source with a longer application period (typically 20–72 h) requiring hospitalisation in appropriately shielded lead chambers;14 this has now been largely replaced by high dose rate brachytherapy.
High dose rate (HDR) brachytherapy is a form of interstitial delivery characterised by the use of a highly radioactive source through the catheter applied for a short period of time (less than 10 min), making it suitable for outpatient settings.17 This modality has also been shown to be better tolerated as well as cheaper compared to the low dose rate equivalent.18
Surface brachytherapy makes use of an external applicator that attaches on the skin surface with adhesive tape to act as the medium through which the radioactive wire (attached to the source, e.g. Iridium (Ir) 192 source) is inserted to deliver the radiation (Figure 3). It is advantageous in terms of the ability to extend the treatment period without the risks of wound dehiscence that interstitial regimens have; it is also suitable for once daily administration and can adjust flexibly over the wound contour and length.19 Additionally, due to the remote afterloading system used, the exposure for healthcare staff is minimised.20 There is no LDR modality described for surface brachytherapy.
Figure 3.
Iridium 192 brachytherapy apparatus (a), surface brachytherapy carriers-Freiberg flaps (b) and their placement on the surface of the wound before the delivery of radiation.
The indications for brachytherapy are similar to EBRT but also extend to the following situations, which would necessitate jointed radiation fields:14,19
Uneven, curved surfaces to avoid including deeper structures in the irradiation field, e.g. jawline, shoulder, axilla;
Long wounds (the senior author’s institution uses a cut-off point of 13 cm for the use of brachytherapy preferably over external EBRT).
The contraindications to brachytherapy are similar to conventional external beam modalities and include:14,21 pregnant or nursing women; children and young adults; and distance of less than 4 cm from the gonads or thyroid gland.
The use of a split skin graft as wound coverage appears to be a relative contraindication for the use of brachytherapy. A case report of recurrent auricular keloid excision treated with Integra and interstitial brachytherapy and a split skin graft three weeks postoperatively has been reported.22
Methodology
A detailed literature search was performed with MESH terms [brachytherapy] and [keloid] using the databases PubMed, Embase and Web of Science from their individual dates of inception until June 2017. The inclusion criteria comprised all peer-reviewed articles referring to different modalities of adjuvant (postoperative) brachytherapy treatment for keloids in the English literature. The nature of the surgical treatment was filtered to represent complete extralesional keloid excision with direct or local flap closure; any articles referring to tangential excision were excluded.
Case reports, conference abstracts and letters to the editor were not included in the analysis and after accounting for duplicates, all eligible manuscripts were scanned for additional pertinent literature. All articles were assessed for relevance to the study objective by both authors and a small number of additional manuscripts were retrieved for inclusion in the study. We present the findings applicable to the different types of interstitial and superficial brachytherapy in chronological in order to show the evolution of this type of radiotherapy.
Results of literature analysis
There are a number of limitations in the study of adjuvant brachytherapy in the management of keloid scarring; these include the moderate to low level of evidence with most studies being prospective and retrospective case series apart from one systematic review and one meta-analysis comparing different radiotherapy modalities. Many studies fail to specify if the keloid scars treated were confirmed on histology as well as the patients’ Fitzpatrick skin type. The follow-up periods are variable and a number of confounding factors exist including different radiation sources, timing between surgery and therapy, overall treatment time and fractionation. All these parameters are elaborated upon in this work.
LDR interstitial brachytherapy
The first English literature report of brachytherapy using a low dose rate regimen was published in 1976 on 31 keloids treated with an Ir 192 source. The scars were of mixed aetiology and treated with 2000 rad (20 Gy equivalent) at 2.5 mm from the wire axis following excision starting approximately 24 h postoperatively. The follow-up for this study was over two years and a recurrence rate (defined as the reappearance of keloid or persistent itching) of 19.4% was noted. The authors commented that the majority of recurrences (4/6) were associated with complicated wound healing, namely infection, dehiscence and haematoma.16
The largest study for this modality is a retrospective review of 544 patients (855 keloids) with an average cohort age of 24 years (age range = 2–82 years). A total of 547 keloids received one session of brachytherapy and 23 received two sessions with an average dose of 19.14 Gy. Following complete surgical keloid excision and insertion of a 1.6 mm plastic tube at 5 mm depth in the wound, an Ir wire was used; the radiation was delivered in a lead chamber over a two-day hospital stay. The recurrence rate was reported as 21% after the first and 30.4% after the second treatment; the factors associated with recurrence included older age, previous treatment, earlobe location and keloid size as well as wound infection. Out of the 555 keloids treated and reviewed, 80.1% were considered to have improved functional symptoms and 75.3% had a better cosmetic appearance.14 The average follow-up for this study was 6.91 years (range = 15 months–13 years).
Another case series of LDR radiation relates to a predominantly adult cohort (only three patients aged < 20 years) of 39 patients with 46 keloids. These received 12 or 15 Gy at 2.5 or 5 mm to the wire axis and were followed up for a mean period of seven months (range = 2–104 months). The control rate in this study was 63.2% and no correlation was found between the dose variation and efficacy of the protocol.23 The importance of this report lies in presenting brachytherapy as a salvage modality following previous radiation treatment (seven patients had previous therapy with corticosteroids and interstitial radiation).
A French retrospective case series on 32 patients (55 keloid scars) used an average dose of 17.9 ± 2.2 Gy at 5 mm distance from the wire axis and irradiation time of 44.3 ± 11.3 h. The recurrence rate (defined as the reappearance of a symptomatic cutaneous tumour in all or part of the treatment area) was 23.6% with skin types 5 and 6 carrying a higher risk (P = 0.0095). In total, 97% of pruritic and 87.5% of painful symptoms totally disappeared and the authors identified that patient satisfaction was not linked to recurrence but mostly to the resolution of functional symptoms. In terms of side effects, 72% of scars were telangiectatic, 67% hypopigmented and 31% were sclerotic with no cases of neoplasia reported in the series.24 This work, represents the last manuscript in the English literature concerning the use of LDR brachytherapy. One of the main reasons for the loss of popularity of the LDR modality relates to the long overall treatment time, which necesitates a period of hospitalisation. Table 1 summarises the studies pertaining to LDR interstitial brachytherapy in chronological order.
Table 1.
Chronological summary of studies pertinent to LDR interstitial brachytherapy.
| Author | Patient/ keloid no. | Scar location | Skin type | Follow-up | Definition of recurrence | Brachythera-py regimen | Time interval between surgery and first fraction | Recurrence rate | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Malaker et al., 197616 | 30/31 | Head + neck 80.6% Limbs 12.9% Trunk 6.5% |
Unspecified | 2 years (minimum) | Obvious new keloid formation or itch symptoms | Ir 192 2000rad (20 Gy) at 2.5 mm from wire axis | Approximately 24 h postoperatively with irradiation time 20–36 h | 19.4% (5 patients recurred by 6 months and 1 by 2 years) |
Three cases (neck, wrist, abdomen) associated with haematoma
or wound infection postoperatively One case partial dehiscence post surgery and radiotherapy |
| Escarmant et al., 199314 | 544/855 | Earlobe 51.4% Abdomen 10.9% Sternum 10% Extremities 12.8% Head 12.1% |
97.2% mixed race | 6.91 years average (range = 15 months–13 years) | Scars not flat | Average 19.14 Gy (8–30) administered as a single fraction in most cases (23 keloids received 2 fractions) | Less than 6 h for 768/783 and 24–48 h for 15/783; second session within 6 h of surgery | 21% after 1st therapy 30.4% recurred once again after 2nd therapy |
Recurrence was found to be associated with older age, previous treatment, earlobe location, size, infection, bruising/loose sutures |
| Clavere et al., 199723 | 39/46 | Trunk 73.9% | 33% white | 7 months median (range = 2–104) |
Not defined | 12 Gy (7 keloids) and 15 Gy (22 keloids) at 2.5 mm (5 mm for 17 keloids) | Less than (6 h all cases apart from 1 patient) | 37% (7 patients lost to follow-up) 14 recurrences with 2 had infection or dehiscence | Seven patients had previous treatment with steroids or surgery alone and two had postoperative interstitial radiotherapy before |
| Arnault et al., 200924 | 32/55 | Earlobe 7.3% Retro-auricular 25.4% Sternum 25.5% Back /shoulders 20% Head + neck 18.2% Limb 1.8% Abdomen 1.85 *Histologically confirmed keloid scars* |
Type 1 3.6% Type 2 1.8% Type 3 36.4% Type 4 7.3% Type 5 29.1% Type 6 21.9% (Fitzpatrick classification) |
Not defined | Reappearance of symptomatic cutaneous tumour in all or part of the treatment area | Average dose 17.9 ± 2.2 Gy at 5 mm distance with average irradiation time of 44.3 ± 11.3 h | Maximum of 7 h after surgery | 23.6% with types 5 and 6 having a higher risk (P = 0.0095) | 79% of pruritus and 87.5% of pain totally
disappeared 72% scars were telangiectatic, 67% hypo-pigmented and 31% sclerotic |
HDR interstitial brachytherapy
The first English literature reference for this modality relates to a seven-year prospective study investigating the role of surgery as an adjunct to HDR brachytherapy in 169 scars. In this cohort, 147 patients underwent surgery combined with a total 12 Gy/4 fractions brachytherapy and 22 patients underwent adjuvant brachytherapy only (18 Gy/6 fractions). The median follow-up period was 37.3 months (range = 13–85 months) and the overall recurrence rate (defined as reappearance of the keloid scar in the same location) was 4.7%.
The surgical subgroup recurrence rate was 3.4% and the rate in the brachytherapy only group was 13.6% with an overall significant improvement in symptoms including pruritus, erythema and burning sensations. Telangiectasia and skin pigmentation changes were seen in twelve and ten patients respectively. The importance of this report was to affirm that the combination of surgery and brachytherapy yields better results compared to isolated modalities.18
Another case series relates to 17 keloids (previously treated with surgery and external radiation) managed with re-excision and 15 Gy /3 fractions salvage Ir 192 brachytherapy. At a median follow-up of 26 months, 12% of keloids showed recurrence. One case involved an accidentally dislodged catheter, which necessitated the use of external beam radiation with subsequent recurrence in part of the keloid site outside the treatment field. Another patient developed recurrence located at the periphery of the tube carrier site. Reported adverse effects included changes to skin pigmentation in three patients, skin toxicity in two and sternal ulceration in another two cases. The importance of this report relates to the use of the modality as salvage treatment following previous surgery and EBRT. This paper also highlights the shortcomings of interstitial modalities with dislodgment of the carrier tube being a contributory factor towards treatment failure.25
A retrospective review of 35 patients (54 keloids) employing adjuvant HDR Ir 192 brachytherapy compared the efficacy of the following different regimens (minimum follow-up of 12 months):
nine patients with a regimen of 4/3/3 Gy (BED of 13.4 Gy) showed a recurrence rate of 44%;
38 patients had 6/4/4 Gy (BED of 20.8 Gy) with a recurrence rate of 3%;
six patients had 6/6/6 Gy and one had a single dose of 16 Gy with no recurrences.
The authors concluded that better results were obtained with higher BED schemes and as a result their current institutional regime comprises three fractions of 6 Gy (BED of 28.2). One case of recurrence in the 4/3/3 group was retreated with surgery and three fractions of 6 Gy brachytherapy with good cosmetic results.26
Another retrospective case series of 30 keloids treated with surgical excision and 14 Gy in two fractions demonstrated a significant difference in scar thickness before and after the treatment (5.65 ± 3.58 mm vs. 0.39 ± 0.63 mm, P < 0.001) at a mean 26.9 months follow-up. No late toxicity (erythema/hyperpigmentation) or malignancy was reported in this work.27
A retrospective case series of 25 patients investigated the management of recurrent earlobe keloids with 500 cGy Ir 192 adjuvant brachytherapy. Of the patients, 92% were recurrence-free at a mean of 35-month follow-up period; two patients suffered infective complications and one constricted ear deformity (no reference was made whether this was a surgical or a radiotherapy-related issue).21
A Dutch study investigated 28 patients with 35 keloids receiving 12 Gy / 2 fractions Ir 192 adjuvant brachytherapy. The mean age of the cohort was 36.3 years (age range = 18–68 years) with a host of different Fitzpatrick types (1–6). Three patients did not complete the radiation protocol due to a dislocated catheter/technical problem and two of these showed keloid recurrence at follow-up. As far as the rest of the protocol is concerned, the recurrence rate was 3.1% and was seen at a mean follow-up of 33.6 months. The average reduction in the scar surface area post treatment was 56.7% (P = 0.011). For patients with non-recurring scars, POSAS scores as well as the resolution of symptoms including pain and itch were favourable. Reported complications included postoperative infection (5.7%) requiring oral antibiotics as well as dyspigmentation disorders (hyper and hypo) in six out of 35 scars (21.4%). When subdividing this group into skin colour, five of these patients had Fitzpatrick type 5 to 6 skin (African-American) and one patient had type 3 to 4 skin (Mediterranean/Asian). No cases of dermatitis or cutaneous malignancy were reported.15
Another prospective study involved 24 patients with 32 recurrent keloid scars (three of which had previously been treated with EBRT) with an age range of 20–80 years. The cohort was treated with keloid excision and Ir 192 brachytherapy in three fractions of 6 Gy at 5 mm depth. Two high-risk patients were also treated with silicone gel and pressure therapy. The average follow-up period was 29.4 months (range = 7.9–72.4 months) and the local control rate was 94%. One of the patients with hypertrophic scarring at the site of brachytherapy tube insertion was treated with another dose of brachytherapy two weeks later with complete remission of symptoms and a satisfactory final appearance. Two patients suffered hypo- and one hyperpigmentation, while six had mild delay in wound healing.28
The most recent study assessed the efficacy of a single 13 Gy fraction interstitial HDR brachytherapy delivered within 2 h postoperatively to 29 keloids. The mean recurrence rate was 24.1% at a median follow-up of 53 months. The authors linked the relatively higher recurrence rate in the study to a number of factors including the stringent definition of recurrence (elevated scar with no itching), the large number of patients lost to follow-up as well as the longer follow-up period of 53 months compared to many other studies in the field.29 Table 2 summarises the studies pertaining to HDR interstitial brachytherapy in chronological order.
Table 2.
Chronological summary of studies pertinent to HDR interstitial brachytherapy.
| Author | Patient/ keloid number | Scar location | Skin type | Follow-up | Definition of recurrence | Brachytherapy regimen | Time interval between surgery and first fraction | Recurrence rate | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Guix et al., 200118 | 169/169 | Face 45.6% Trunk 43.2% Extremities 11.2% |
Caucasian 99.4% African-American 0.6% |
37.3 months median (range = 13–85) | Presence of new keloid scar in same location | Surgery and brachytherapy -12 Gy / 4 fractions
(147) Brachytherapy-18 Gy / 6 fractions (22) |
30–90 min after surgery | 4.7% overall recurrence rate (surgery + brachytherapy 3.4% vs. brachytherapy only 13.6%) | Skin pigmentation changes 10 patients Telangiectasia 12 patients |
| Garg et al., 200425 | 12/17 | Earlobe 46.7% Chest 33.3% Others 20% |
Unspecified | 26 months (range = 12–71) | Not defined | 15 Gy / 3 fractions | Start on day of surgery < 24 h; consecutive days | 12% | One recurrence post catheter dislodgement necessitating EBRT
and one at periphery of implants S/E: pigmentation changes – three patients, acute skin toxicity – two patients, two ulcerations over sternum |
| Veen et al., 200726 | 35/54 | Ear 42.6% Sternum 31.5% Others 25.9% |
Unspecified | 1 year minimum | Not defined | 6/4/4 Gy (38) 6/6/6 Gy (6) 4/3/3 Gy (9) 16 Gy (1) |
6 h postop and rest the following day with 6 h interval | 6/4/4 Gy: 3% 6/6/6 Gy: 0% 4/3/3 Gy: 44% 16 Gy: 0% |
Depigmentation in case of single 16 Gy dose One recurrence in the 4/3/3 was retreated with surgery and 6/6/6 regimen with good cosmetic result |
| DeLorenzi, 200727 | 24/30 | Ear 26.7% Trunk 63.3% Extremities 10% *Keloids vs. hypertrophic scars defined* |
Caucasian 79.25 ‘Tensed’ skin 20.8% |
26.9 months mean (range = 8–58) |
Not defined | 14 Gy / 2 fractions | 1st within 24 h; 2nd after 24 h of first dose |
No recurrence data provided Change in scar thickness from 5.65 ± 3.58 mm to 0.39 ± 0.63 mm (P < 0.001) |
43% patients had previous treatment (not
radiation) All patients had scar thickness < 2 mm post treatment; 1 patient had suture breakdown resulting in 3 cm dehiscence No late toxicity or malignancy seen |
| Arneja et al., 200821 | 25/25 | Earlobe/ inferior helix 100% | African 8% Asian 20% European 72% |
35 months (range = 24–57) | Not defined | 500 cGy / 3 fractions | Immediately post op for first dose, then days 1 and 2 | 8% | Extralesional excision of scar and direct
closure/postauricular advancement flap No keloids previously treated by irradiation; two infection related recurrences and one constricted ear |
| Van Leeuwen, 201417 | 28/35 | 37% earlobe 20% sternum | Fitzpatrick type 1+2 34.3%, type 3+4 14.3%, type 5+6 51.3% |
33.6 months mean (range = 24–96) | Growing pruritic nodular scar | 12 Gy / 2 fractions | Within 4 h of resection, 2nd dose within 24 h of 1st dose | 3.1% | No patients had previous radiation treatment 3 did not complete protocol (2 had catheter dislocation, 1 technical problem) Postoperative infection 5.7% Pigmentation 21.4% No malignancy reports POSAS scores and symptom resolution (pain/itch) reports were favourable |
| Jiang et al., 201628 | 24/32 | Sternum 52.4% Earlobe/ auricle 47.6% |
Unspecified | 29.4 months (range = 7.9–72.4) | Presence of new keloid /raised scar not growing beyond boundaries of original wound | 18 Gy / 3 fractions (one patient had single dose of 6 Gy) | Within 6 h, other two in first postoperative day with treatment interval of 6 h | 6% (2 keloid recurrences and 2 mildly hypertrophic scars appearing post treatment) | Mild pigmentation abnormalities in 3 patient and mild delay in healing in 6 patients (3 patients previously treated with EBRT did not develop recurrence) |
| Hafkamp et al., 201729 | 24/29 | Ear 43.8% Head + neck 9.4% Presternal area 15.6% Abdomen 15.6% Shoulder 15.6% |
Fitzpatrick type 2 12.5%, type 3 4.2%, type 4 29.2%, type 5 50%, type 6 4.1% |
53 months median (range = 19–95) | Elevation of the scar outside the initial wound without itch | 13 Gy / single fraction | Within 2 h | 24.1% | Only 2 patients had not received any treatment for their
keloids before inclusion in the study. Complications included: infection (n = 1), chronic wound (n = 1), dehiscence (n = 1), hypopigmentation (n = 1) |
HDR interstitial modality compares more favourably to LDR in terms of its applicability in the outpatient setting; nevertheless it has catheter dislodgment as a significant shortcoming, which accounts for the emergence of superficial modalities as an alternative brachytherapy modality.
HDR superficial brachytherapy
The first English literature report of this modality relates to a cohort of 139 patients (66 keloid scars) treated with excision and an integrated 90Sr-90Y surface applicator. Radiotherapy was commenced within 48 h of surgery with a median total dose of 14 Gy (range = 7.5–28.5). The recurrence free response rate was 80% and differed between anatomical regions with the face and neck being lowest (2%) and the thorax highest (49%, P < 0.001). Additionally, burns-related keloids had worse outcomes compared to surgical or mechanical trauma-induced lesions (P < 0.001). Regarding adverse effects, 24% patients had acute erythema and 11% hypopigmentation. No malignancy was reported at a median follow-up of 12 years.30 This study points towards anatomical factors affecting recurrence but this finding needs to be viewed in light of the small cohort size and the retrospective nature of the work.
In another study, 83 keloids were treated with four fractions of 5 Gy adjuvant irradiation using a strontium-90 surface applicator. Data were collected relating to recurrence as well as patient satisfaction (therapeutic and cosmetic outcome) on the basis of a questionnaire and an objective examination. The follow-up period for the questionnaire limb was 71 months (range = 4–109) and 31 months (range = 4–107) for the objective examination limb. The recurrence rate in the keloid scars was found to be 36% (self-reported) and 39% (objective examination); 61% patients were extremely or mainly satisfied with the therapeutic outcome and 51% with the cosmetic outcome. One of the salient findings of this work was the correlation of higher patient satisfaction reports to male gender, treatment of ear keloids as well as the post-treatment relief from keloid associated symptoms. In other words, factors other than recurrence rate or the extent of radiation side effects appear to have a significant influence on patients’ assessment of the treatment. Cutaneous changes (including telangiectasia, dyspigmentation and redness) were found in the majority of patients (slight in 17%, moderate in 33% and severe in 37%).31
A report employing an Ir 192 mould was used in 22 patients with 24 keloids after excision delivering 15 Gy in three fractions. These included two previously treated with surgery, two with surgery and steroid injections and four with steroid injections only. Sixteen keloids were followed up for a minimum of six months with a recurrence rate of 12.5%. One patient had residual keloid after treatment, one grade 1 hypopigmentation and one grade 1 fibrosis.32
In a Brazilian case series 612 patients with 892 keloids were treated with excision and a strontium-90 surface applicator (20 Gy/10 fractions). All keloids lesions were confirmed with histology and the median follow-up was 61 months (range = 6–130). The overall control rate was 87.6% and recurrence was defined as the reappearance of a new keloid in the previously treated location. Multiple regression analysis showed that factors associated with recurrence were keloid size > 5 cm, burn aetiology and previous treatment (P = 0.0001). Late adverse effects noted were telangiectasias in 10.4%, which resolved in time. No malignancies were reported in the series.33
A Japanese study used a remote superficial brachytherapy afterloading Ir 192 source to deliver a range of 15–20 Gy in 3–4 daily fractions according to the observed risk of keloid recurrence in different bodily sites. A total of 20 Gy was used to the anterior chest wall, scapular region, lower jaw and suprapubic region in four fractions and 15 Gy in three daily fractions to lesions in other areas. The work involved 36 keloids with a median follow-up of 18 months. The recurrence rate was 9.7% with a median time to failure after radiation of 12 months. Transient erythema occurred in almost all patients but there were no reports of significant side effects including skin pigmentation changes.19 The authors concluded that the superficial modality has superior efficacy compared to the interstitial equivalent based on the greater length of wound treated and recurrence rate. The results with HDR superficial brachytherapy did not differ significantly from that achieved by EBRT in the same reporting institution (3/36 vs. 17/121, P = 0.366 by chi-squared test).19 Table 3 summarises the studies pertaining to HDR superficial brachytherapy in chronological order.
Table 3.
Chronological summary of studies pertinent to HDR superficial brachytherapy.
| Author | Patient/ keloid number | Scar location | Skin type | Follow-up | Definition of recurrence | Brachytherapy regimen | Time interval between surgery and first fraction | Recurrence rate | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Wagner et al., 200030 | 139/166 | Thorax 27.1% Face 25.3% Neck 22.3% Abdomen 10.9% Leg 6% Others 8.4% |
Unspecified | 12 years | Not defined | Median overall dose 14 Gy (7.5–28.5) with median individual
dose 3 Gy (1.5–4) 90Sr-90Y applicator |
Within 48 h of surgery | 20% | Recurrence lowest on face and neck (2%) highest thorax
(49%), P < 0.001 Burn keloids poorer success rate than surgery/mechanical trauma (P < 0.001) No malignancies reported at 12-year follow-up S/E: acute erythema 24%, hypopigmentation 11% |
| Fraunholz et al., 200531 | 66/83 | Trunk 41% Ear 23% Neck 19% Sternum 13% Extremities 4% |
Unspecified | 30 years median (range = 10–71) |
Any kind of keloidal regrowth | 5 Gy / 4 fractions at 2 mm depth 90Sr applicator |
On day of surgery (75%), following day (15%), within next 5 days (10%) |
36% (self-reported) 39% (objective examination) |
41 patients answered questionnaire and 24 participated in
the follow-up examination 61% extremely or mainly satisfied with therapeutic and 51% with cosmetic outcome Cutaneous changes (including telangiectasias, dyspigmentation, redness) were found in the majority of patients (slight in 17%, moderate in 33% and severe in 37%) |
| Narkwong et al., 200632 | 22/24 | Earlobe 100% | Unspecified | 14.8 months (range = 6–36) | Evidence of mass or obvious return of keloid | 15 Gy / 3 fractions | Start within 24 h post surgery | 12.5% (2 keloids at 6 and 15 months post treatment) | One patient had residual keloid post treatment grade 1
hypopigmentation (n = 1) and grade 3 fibrosis (n =
1) 7/22 lost to follow-up |
| Viani et al., 200933 | 612/892 | Thorax 41.4% Rest 58.6% *Histologically confirmed keloids* |
Unspecified | 61 months median (range = 6–130) |
Appearance of new keloid at site of treatment | 20 Gy / 10 fractions 90Sr-90Y beta ray applicator | Within 24 h of surgery for 36.4%; more than 24 h for rest | 12.4% | Recurrence associated with keloid size > 5 cm, burn scar
aetiology, previous treatment (P =
0.0001) S/E: telangiectasia 10.4% and resolved in all cases No malignancies reported |
| Kuribaya-shi et al., 201119 | 21/36 | Chest 44.5% Scapula 19.4% Lower jaw 16.7% Suprapubic 11.1% Others 8.3% *Histologically confirmed keloids* |
Asian (Japanese) patients | 18 months median (range = 9–29) | Elevation (even small) of treatment site | 20 Gy / 4 fractions for chest, scapula, lower jaw; 15 Gy / 3 fractions for other sites Ir 192 system |
Within 24 h of surgery | 9.7% (all on chest wall) | Transient erythema in almost all patients; no pigmentation changes |
There are some preliminary encouraging reports using superficial brachytherapy in combination with other adjuncts including intralesional triamcinolone, procaine and 5 fluorouracil for keloid scars34 as well as pretreatment with CO2 laser before brachytherapy.35 Clearly, the value of brachytherapy in combination with other non-surgical adjuvant therapies needs further research.
Comparative studies of brachytherapy regimens
A retrospective review in an Italian institution compared HDR and LDR brachytherapy for the control of 96 keloid scars in 70 patients.
The LDR subgroup of 46 keloids had a median dose of 16 Gy (range = 12–18), whereas the HDR subgroup of 50 keloids 12 Gy (range = 9–12) delivered in four fractions. The relapse rate was not statistically significant (30.4% vs. 38%, P = 0.521) but the rate of symptomatic relief was better in the HDR group (68 vs. 92%, P = 0.032) at 28-month follow-up. Aesthetic outcomes did not differ between the two modalities and in terms of late toxicity, comparison showed a mixed picture with HDR faring better over LDR in terms of telangiectasia (0% vs. 15.2%) and skin fibrosis (22% vs. 32.6%) but worse off in terms of hyperpigmentation (22% vs. 10.8%). Multivariate logistic regression analysis revealed that male sex, age < 44 years, anatomical location (arms, neck, chest wall) as well as symptomatic keloids were more likely to recur.20
A single institution retrospective analysis involving 116 histologically proven keloid patients appraised the control rate and toxicity of brachytherapy and EBRT (after surgical excision) in a variety of regimens:
HDR interstitial brachytherapy with iridium 192 (8 Gy / 1 fraction + 9 Gy / 3 fractions or 20 Gy / 4 fractions;)
HDR interstitial brachytherapy with Co 60 (20 Gy / 4 fractions or 18 Gy / 6 fractions;)
EBRT (26 Gy / 13 fractions or 30 Gy / 15 fractions.)
The authors divided patients according to the administered BED and median follow-up was 46.5 months (range = 10–120 months). The control rate for those receiving hypofractionation (> 2 Gy/fraction) vs. conventional (2 Gy/fraction) was 88.5% vs. 76.3% (P = 0.043). A BED of > 30 Gy was associated with a better control rate compared to < 30 Gy but not in a statistically significant manner (89.7% vs. 79.3%, P = 0.104). No grade 2 or higher adverse effects were reported and two cases of oesophageal cancer (one 5 years and one 6 years after treatment of neck keloids) were reported. These were, according to the authors, not definitively linked to the keloid irradiation since they affected the middle part of the oesophagus at a considerable distance from the treated area.11
Despite a single ten-year institutional retrospective report showing similar recurrence rates between EBRT and brachytherapy,36 there is strong emerging evidence that the response rate of brachytherapy is superior.
A recent systematic review of adjuvant irradiation following excision for keloid scars has concluded that HDR brachytherapy is associated with the lowest recurrence rate followed by LDR and external radiation (HDR 10.5 ± 15; range = 0–44, LDR 21.3 ± 2.1; range = 19.4–23.6; external 22.2 ± 16; range = 0–72). Additionally, in terms of the timing between surgery and radiation, the study showed that for HDR brachytherapy there is no difference in recurrence rate if radiation happened within 7 h or 24 h postoperatively; no valid conclusions could be made for LDR in this respect given the low number of included studies.15
The most recent work in the field is a meta-analysis on radiotherapy for keloids; this work has confirmed that postoperative brachytherapy yields the lowest recurrence rate (15%) compared to X-ray and EBRT (23% and 23%, respectively; P = 0.04, P = 0.1). The recurrence rate comparison between X-ray and brachytherapy was statistically significant (odds ratio [OR] = 1.94; P = 0.04) but insignificant between EBRT and brachytherapy (OR = 1.81; P = 0.10). This work also identified that the five most common complications related to pigmentation disorders with a collective total recurrence of 32.5%.37
Comparative studies of brachytherapy vs. other established regimens
A multicentre controlled open trial compared intralesional cryotherapy vs. excision with adjuvant corticosteroids or brachytherapy for keloids. Out of the 179 patients seen over the inclusion period, only 74 met the inclusion criteria and 26 gave consent for randomisation. Preliminary results showed comparable patient satisfaction between cryotherapy and excision with corticosteroids, but lower patient satisfaction in the cryotherapy treatment group (P < 0.05). Nine of the 14 patients who underwent cryotherapy asked for excision and brachytherapy due to inadequate volume reduction, hypopigmentation problems or ongoing pain complaints.38
A retrospective cohort study compared the relative effectiveness of adjuvant photodynamic therapy (PDT) and brachytherapy in a total of 45 keloidal lesions using the Patient and Observer Scar Assessment Scale (POSAS) for evaluation of final outcomes. Both patients’ and observers’ POSAS scores were more favourable for brachytherapy over PDT (P < 0.05); nevertheless, when the item on the POSAS scale was analysed separately, the observers scored higher for PDT in comparison to brachytherapy.39
Long-term risks of carcinogenesis
One of the theoretical concerns with radiotherapy administration for benign diseases is the risk of inducing secondary malignancy in the treated field. A study among international radiation oncology facilities identified that 78% of respondents found radiation therapy to be acceptable for keloid treatment; this finding betrays that a certain proportion of radiotherapy services have some reluctance in embracing this modality in the management of benign disease.40
A comprehensive search in the relevant literature identified five cases of carcinogenesis out of 6500 patients treated with EBRT for keloids. This equates to an actual risk of < 0.1%; out of those patients, only one (thigh fibrosarcoma reported in 1963) may have resulted from a malignant change in the keloidal field; in the remaining cases (breast, thyroid and basal cell carcinoma), it is doubtful if sufficient protection of the surrounding tissues and an appropriate dose of radiation were delivered.3 Another piece of work in the field has also reported the risk of secondary malignancy to be around 0.07% for keloids managed with adjuvant radiation.41
Furthermore, the additional two cases reported by Duan et al. in 2015 (after the large review by Ogawa et al.) cast significant doubts over the direct causative association between radiotherapy treatment for keloid disease and the induction of carcinogenesis.11 The authors of this work made particular reference to the distance between the irradiation field (neck) and site of carcinogenesis (oesophagus) as well as the high background incidence of oesophageal cancer in the study population; the latter might be a significant confounding factor.
It has been reported that out of 10,000 individuals aged 18–64 years who are subjected to whole-body irradiation composed of 1 Gy, 670 (6.7%) will acquire skin cancer. In general, skin cancer kills one in 500 patients. Thus, the mortality rate associated with 1 Gy of whole-body irradiation would be 6.7% × 1/500 = 0.0134%; namely, one in 7500 people. If this reasoning is applied to earlobe keloid radiotherapy, where 0.05% of whole-body skin is irradiated with 10 Gy, the incidence of skin cancer associated with this treatment would be 6.7 × 10 × 0.05/100 = 0.0335%, namely, one in 3000 people. The mortality rate of secondary carcinogenesis of earlobe keloid treatment would be 0.0335/500 = 0.000067%, namely, one in 1,500,000 people. We believe that this risk is clinically acceptable if informed consent is obtained from the patients after they have been advised of the benefits and side effects of this type of treatment.3
Another consideration pertinent to discussions around the risk of radiation-induced carcinogenesis relates to the comparative risk of carcinogenesis following a computed tomography (CT) scanning test. It has been estimated that the risk of inducing a fatal skin tumour to a 10-cm2 area with a single dose of 13 Gy is 1.3 × 10−6, whereas the equivalent risk associated with a chest CT is 4.8 × 10−6. We believe this comparison can be a practical example to describe the risks of radiation therapy for keloids to patients. Calculations regarding the development of fatal tumours in fat and muscle have yielded similar results in terms of risk magnitude.42 There are no reports of secondary carcinogenesis in the brachytherapy literature to date.
Discussion
Treatment of keloid scars represents a challenging clinical problem and radiotherapy offers an important addendum to the armamentarium of clinical teams specialising in scar management. Adjuvant radiotherapy following extralesional excision appears to be one of the most efficacious treatment modalities; it is thought to prevent recurrence by imparting toxicity to fibroblasts as well as endothelial cells, which are both instrumental in the pathophysiology of the disease. It is clear that further research will determine the relative contribution of the different proposed mechanisms in keloid formation and recurrence.6
Brachytherapy, first introduced in 1967,13 offers clear advantages over EBRT including a more focused delivery of radiation to the target area and less exposure of surrounding skin to radiation.7,14,15
This work presents a chronological evolution of brachytherapy over the last number of decades. It becomes quite clear that HDR has largely replaced LDR strategies based on superior response rates, shorter treatment time requirements and the lack of associated hospital stay resulting in a more cost-effective solution to adjuvant brachytherapy. Furthermore, based on recent systematic review and meta-analysis work, brachytherapy offers a superior control rate compared to EBRT.15,37 Superficial brachytherapy promises to further improve patient experience by minimising shortcomings associated with catheter dislodgement and is likely to gain more momentum in the near future.
Concluding remarks
Brachytherapy provides an alternative adjuvant radiotherapy modality in keloid treatment. The current evidence suggests superior response rates associated with brachytherapy compared to EBRT for adjuvant keloid scar management and the most popular modality at present is HDR interstitial brachytherapy with the superficial modality gaining in popularity. Further work in the form of high-quality comparative clinical studies is warranted to establish the role of different radiotherapy regimens in scar management protocols worldwide.
Acknowledgments
Mr Ioannis Goutos wishes to acknowledge the Dowager Countess Eleanor Peel Trust and the HCA International Foundation for their sponsorship during his scar management fellowship scheme in Tokyo, Japan. Both authors would like to thank the Shunkosha Publishing Group, Japan for granting their permission to reproduce the content of Figures 1 and 2.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Sequeira JH. Case illustrating the effects of x-rays on scar-keloid. Proc R Soc Med 1909; 2: 96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg 2002; 297: 433–438. [DOI] [PubMed] [Google Scholar]
- 3. Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg 2009; 124: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 4. Luo S, Benathan M, Raffoul W, et al. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg 2001;107: 87–96. [DOI] [PubMed] [Google Scholar]
- 5. Huang C, Liu L, You Z, et al. Endothelial dysfunction and mechanobiology in pathological cutaneous scarring: lessons learned from soft tissue fibrosis. Br J Dermatol 2017. DOI: 10.1111/bjd.15576. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci 2017; 18: E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinh Q, Veness M, Richards S. Role of adjuvant radiotherapy in recurrent earlobe keloids. Aust J Dermatol 2004; 45: 162. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhry MR, Akhtar S, Duvalsaint F, et al. Earlobe keloids, surgical excision followed by radiation therapy: A 10-year experience. Ear Nose Throat J 1994; 73: 779–781. [PubMed] [Google Scholar]
- 9. Flickinger JC. A radiobiological analysis of multicenter data for postoperative keloid radiotherapy. Int J Radiat Oncol Biol Phys 2011; 79: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 10. Kal HB, Veen RE, Jurgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys 2009; 74: 245–251. [DOI] [PubMed] [Google Scholar]
- 11. Duan Q, Liu J, Luo Z, et al. Postoperative brachytherapy and electron beam irradiation for keloids: A single institution retrospective analysis. Mol Clin Oncol 2015; 3: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Strahlenther Onkol 2005; 181: 717–723. [DOI] [PubMed] [Google Scholar]
- 13. Nicolettis C, Cassagne D. L’irridiation interstitielle par l’iridium 192 dans la prevention des recidives après excision chirurgicalle des cicatrices cheloidiennes. Ann Chir Plast 1967; 12: 237–242. [PubMed] [Google Scholar]
- 14. Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys 1993; 26: 245–251. [DOI] [PubMed] [Google Scholar]
- 15. Van Leeuwen MCE, Stockmans SC, Bulstra AEJ, et al. Surgical excision with adjuvant irradiation for treatment of keloid scars: A systematic review. PRS 2015; 3: e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malaker K, Ellis F, Paine CH. Keloid scars: A new method of treatment combining surgery with interstitial radiotherapy. Clin Radiol 1976; 27: 179–183. [DOI] [PubMed] [Google Scholar]
- 17. Van Leeuwen MCE, Stokmans SC, Bulstra AEJ, et al. High-dose-rate brachytherapy for the treatment of recalcitrant keloids: a unique, effective treatment protocol. PRS 2014; 134: 527–534. [DOI] [PubMed] [Google Scholar]
- 18. Guix B, Henriquez I, Andres A, et al. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. Int J Radiat Oncol Biol Phys 2001; 50: 167–172. [DOI] [PubMed] [Google Scholar]
- 19. Kuribayashi S, Miyashita T, Ozawa Y, et al. Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res 2011; 52: 365–368. [DOI] [PubMed] [Google Scholar]
- 20. DeCicco L, Vischioni B, Vavassori A, et al. Postoperative management of keloids: low dose rate and high dose rate brachytherapy. Brachytherapy 2014; 13: 508–513. [DOI] [PubMed] [Google Scholar]
- 21. Arneja JS, Singh GB, Dolynchuk KN, et al. Treatment of recurrent earlobe keloids with surgery and high dose rate brachytherapy. PRS 2008; 121: 95–99. [DOI] [PubMed] [Google Scholar]
- 22. Reiffel AJ, Sohn AM, Henderson PW, et al. Use of Integra and interval brachytherapy in a 2-stage auricular reconstruction after excision of a recurrent keloid. J Craniof Surg 2012; 23: e379–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clavere P, Bedane C, Bonnetblanc JM, et al. Postoperative interstitial radiotherapy of keloids by Iridium 192. Dermatology 1997; 195: 349–352. [DOI] [PubMed] [Google Scholar]
- 24. Arnault JP, Peiffert D, Latarche C, et al. Keloids treated with postoperative Iridium 192 brachytherapy treatment: a retrospective study. J Eur Acad Dermatol Venereol 2009; 23: 807–813. [DOI] [PubMed] [Google Scholar]
- 25. Garg MK, Weiss P, Sharma AK, et al. Adjuvant high dose rate brachytherapy (Ir-192) in the management of keloids, which have recurred after surgical excision and external radiation. Radiother Oncol 2004; 73: 233–236. [DOI] [PubMed] [Google Scholar]
- 26. Veen RE, Kal HB. Postoperative high dose rate brachytherapy in the prevention of keloids. Int J Radiat Oncol Biol Phys 2007; 69: 1205–1208. [DOI] [PubMed] [Google Scholar]
- 27. DeLorenzi F, Tielemans HJP, Van der Hulst RRWJ, et al. Is the treatment of keloid scars still a challenge in 2006? Ann Plast Surg 2007; 58: 186–192. [DOI] [PubMed] [Google Scholar]
- 28. Jiang P, Baumann R, Dunst J, et al. Perioperative interstitial high dose rate brachytherapy for the treatment of recurrent keloids. Int J Radiat Oncol Biol Phys 2016; 94: 532–536. [DOI] [PubMed] [Google Scholar]
- 29. Hafkamp CJH, Lapid O, Davila Fajardo R, et al. Postoperative single-dose interstitial high-dose-rate brachytherapy in therapy-resistant keloids. Brachytherapy 2017; 16: 415–420. [DOI] [PubMed] [Google Scholar]
- 30. Wagner W, Alfrink M, Micke O, et al. Results of prophylactic irradiation in patients with resected keloids. Acta Oncol 2000; 39: 217–220. [DOI] [PubMed] [Google Scholar]
- 31. Fraunholz IB, Gerstenhauer A, Boettcher HD. Results of postoperative 90Sr radiotherapy of keloids in view of patients’ subjective assessment. Strahlenther Onkol 2005; 181: 724–729. [DOI] [PubMed] [Google Scholar]
- 32. Narkwong L, Thirakhupt P. Postoperative radiation with high dose rate Iridium 192 mould for prevention of earlobe keloids. J Med Assoc Thai 2006; 89: 428–433. [PubMed] [Google Scholar]
- 33. Viani GA, Stefano EJ, Afonso SL, et al. Postoperative strontium-90 brachytherapy in the prevention of keloids. Results and prognostic factors. Int J Radiat Oncol Biol Phys 2009; 73: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 34. Vivante H, Salgueiro MJ, Ughetti R, et al. 32P-patch contact brachyradiotherapy in the management of recalcitrant keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol 2007; 73: 336–339. [DOI] [PubMed] [Google Scholar]
- 35. Yan D, Zhao B, Yang H, et al. A combination of non operative treatment modalities used for the treatment of keloids. Dermatol Ther 2014; 27: 48–51. [DOI] [PubMed] [Google Scholar]
- 36. Hoang D, Reznic R, Orgel M, et al. Surgical excision and adjuvant brachytherapy vs external beam radiation for the effective treatment of keloids: 10-year institutional retrospective analysis. Aesthet Surg J 2017; 37: 212–225. [DOI] [PubMed] [Google Scholar]
- 37. Mankowski P, Kanevsky J, Tomlinson J, et al. Otpimising radiotherapy for keloids. Ann Plast Surg 2017; 78: 403–411. [DOI] [PubMed] [Google Scholar]
- 38. Bijlard E, Timman R, Verduijn G, et al. Intralesional cryotherapy versus excision with corticosteroid or brachytherapy for keloid treatment: preliminary results of a randomized controlled trial. PRS 2015; 136(4 Suppl): 149–150. [Google Scholar]
- 39. Basdew H, Mehilal R, Al-Mamgani A, et al. Adjuvant treatment of keloids: comparison of photodynamic therapy with brachytherapy. Eur J Plast Surg 2013; 36: 289–294. [Google Scholar]
- 40. Leer JW, van Houtte P, Davelaar J. Indications and treatment schedules for irradiation of benign diseases: A survey. Radiother Oncol 1998; 48: 249–257. [DOI] [PubMed] [Google Scholar]
- 41. Ragoowansi R, Cornes PGS, Moss AL, et al. Treatment of keloids by surgical excision and immediate post- operative single-fraction radiotherapy. Plast Reconstr Surg 2003; 111: 1853. [DOI] [PubMed] [Google Scholar]
- 42. 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 1991; 21: 1–201. [PubMed] [Google Scholar]
How to cite this article
- Goutos I, Ogawa R. Brachytherapy in the adjuvant management of keloid scars: literature review. Scars, Burns & Healing, Volume 3, 2017. DOI: 10.1177/2059513117735483 [DOI] [PMC free article] [PubMed] [Google Scholar]



