Abstract
Background
Clinical practice guidelines focusing on judicious use of antibiotics for childhood acute otitis media (AOM) have been introduced in many countries around the world.
Objective
To systematically review the effects of these guidelines on the prescription of antibiotics and analgesics for children with AOM.
Methods
Systematic searches of PubMed, Embase and Cochrane Library from inception to 6 June 2017 using broad search terms. Studies specifically aimed at evaluating the effects of introduction of national AOM practice guidelines on type of antibiotic and/or analgesic prescriptions were included, irrespective of design, setting or language. The Risk Of Bias In Non-randomized Studies of Interventions tool was used to assess risk of bias.
Results
Of 411 unique records retrieved, seven studies conducted in six different countries (France, Italy, Spain, Sweden, UK and USA (twice)) compared data before and after guideline introduction. All studies had an observational design, using longitudinal data of children aged under 15 years (n=200–4.6 million) from either routine care, insurance databases or electronic surveys. Risk of bias of all studies was judged serious to critical.
Of the five studies reporting on antibiotic prescription rates, three showed a decline of 5%–12% up to 3 years after guideline introduction and two found no or negligible effect. In one US study, the initial 9% decline decreased to 5% after 4–6 years. The recommended first choice antibiotic was prescribed more frequently (9%–58% increase) after guideline introduction in four out of five studies reporting on this outcome. Analgesic prescription rates for AOM were reported in one US study and increased from 14% to 24% after guideline introduction.
Conclusion
Based upon what is published, the effects of introduction of national clinical practice guidelines on antibiotic and analgesic prescribing for children with AOM seem modest at the most.
Registration
PROSPERO: CRD42016050976.
Keywords: acute otitis Media, aom, guidelines, antibiotics, analgesics
Introduction
With emerging antimicrobial resistance posing a serious threat to global public health, promoting judicious use of antibiotics has become a top priority for governments worldwide. As a consequence, clinical practice guidelines for common infectious diseases, including acute otitis media (AOM), have been introduced and updated in many countries over the past decades.1 Although AOM guidelines vary regarding specific recommendations across countries, they generally emphasise the importance of accurate diagnosis and adequate analgesia as well as advocating selective antibiotic prescribing.1
It has been suggested that guideline adherence for AOM may be suboptimal2 due to a variety of factors, such as fear of serious complications and parental pressure to prescribe antibiotics.3 In daily practice, antibiotics are commonly prescribed to children with AOM, ranging from around 50% in the Netherlands4 to 80% in the USA,5 whereas analgesics are only recommended in a minority of cases.6
However, the true impact of introducing AOM guidelines on prescription of antibiotics and analgesics for children with AOM in daily practice has not been reviewed systematically. We aim to do so and provide an overview of current available studies that compare prescription data before and after national AOM clinical practice guideline introduction.
Methods
Search strategy and study selection
We performed systematic searches of the PubMed, Embase and Cochrane Library databases from inception to 6 June 2017 using database-specific syntaxes of keywords relevant to ‘acute otitis media’ and ‘guidelines’ (see online supplementary for full search strategies). After removing duplicates (RefWorks), two reviewers (YD and RTvU) independently screened titles and abstracts for inclusion. Discussion with a third and fourth reviewer (MLAdH and RPV) resolved any discrepancies. We screened reference lists of included studies for additional studies.
archdischild-2017-314103supp001.docx (14.4KB, docx)
We included all original studies, irrespective of design, setting or language, evaluating the effects of the introduction of national clinical practice guidelines on prescription of antibiotics (rate and type) and/or analgesics for children (up to the age of 16 years) with AOM by comparing data before and after guideline introduction. We only included studies in which the time between data collection before and after guideline introduction was less than 5 years; this was to minimise the impact of other factors that may affect AOM epidemiology and subsequent prescription rates, for example, the introduction of pneumococcal conjugate vaccines and anti-smoking campaigns.
Data extraction and synthesis
The primary outcome of interest was the overall antibiotic prescription rate for AOM. Secondary outcomes included type of antibiotic prescribed and analgesic prescription rate.
Two review authors (YD and RTvU) independently extracted the following data from the included studies: characteristics of study (year, country, design, setting and data source), study population (number and age of children with AOM), guideline details (date of introduction, method of dissemination and management recommendations) and data on our predefined outcomes. Discussion with a third and fourth reviewer (MLAdH and RPV) resolved any discrepancies. To obtain further information on guideline dissemination strategies, we contacted authors of the original publications as well as clinical scientists involved in guideline development in countries subject to this review.
Methodological quality of the included studies was assessed by three reviewers independently (YD, RTvU and RPV) using ‘The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool’,7 and any discrepancies were resolved by discussion.
Antibiotic prescription rates, type of antibiotic and analgesic prescription rates before and after introduction of the AOM clinical practice guideline were presented for each study individually. Where before and after guideline introduction data were reported for individual years or subgroups, (such as age), we aimed to calculate averages.
Results
Search results and study characteristics
Figure 1 shows the search results; 20 of the 411 unique records were considered potentially relevant. Of these, seven studies8–14 were suitable for inclusion in this review. For detailed information of the included studies (see table 1); the seven studies were conducted in six countries: France, Italy, Spain, Sweden, UK and USA (two studies). All were observational studies using longitudinal data of children aged under 15 years; they differed substantially in terms of setting (primary vs secondary care), number of patients (n=200–4.6 million), study duration (6 months–10 years longitudinal data) and data source (routine care, insurance databases or electronic surveys).
Figure 1.
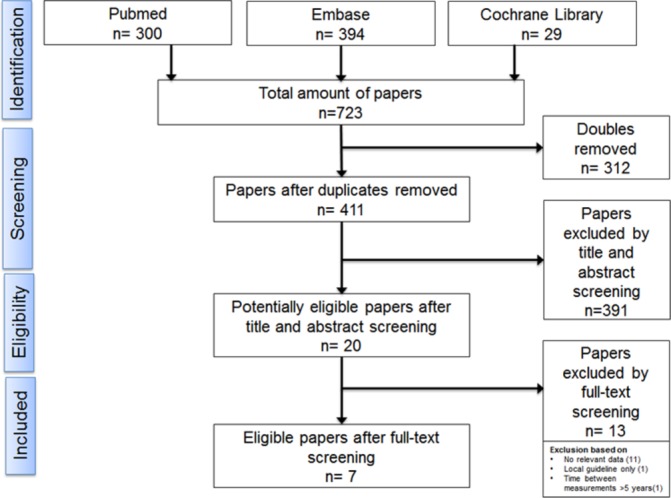
Flow chart.
Table 1.
Baseline characteristics of included studies
| Study ID | Country | Study design | Participants | Data source | Time | Outcomes reported | |||
| Study population | Setting | Age | Guideline introduction | Years of follow-up | |||||
| Tyrstrup et al 14 | Sweden | Observational | N=1 245 599* | PC | 1–12 Y | Routine care | 2010 | Pre: 2008 Post: 2013 |
Antibiotic prescription rate Type of antibiotic |
| Palma et al 11 | Italy | Observational | N=4559 Npre=2692 Npost=1867 |
SC | 0–14 Y | Routine care | 2010 | Pre: 2007–2010 Post: 2011–2013 |
Antibiotic prescription rate Type of antibiotic |
| Levy et al 9 | France | Observational | N=14 661 | SC | 6 M–2 Y | Routine care | 2011 | Pre1: November 2009–October 2010 Pre2: November 2010–October 2011 Post: November 2011–October 2012 |
Type of antibiotic |
| McGrath et al 10 | USA | Observational | N=4 629 460 | SC | 3 M–12 Y | Insurance databases | 2004 | Pre: 2000–2003 Post1: 2005–2007 Post2: 2008–2011 |
Antibiotic prescription rate |
| Coco et al 8 | USA | Observational | N=1114 Npre=584 Npost=530 |
PC+SC | 6 M–12 Y | Electronic surveys | 2004 | Pre: January 2002–June 2004 Post: July 2004–December 2006 |
Antibiotic prescription rate Type of antibiotic Analgesic prescription rate |
| Thompson et al 13 | UK | Observational | N=464 845† | PC | 3 M–15 Y | Routine care | 2003 2004 |
Pre: 1999–2001 Post: 2005–2006 |
Antibiotic prescription rate |
| Ríos et al 12 | Spain | Observational | N=200 Npre=102 Npost=98 |
PC | 2–15 Y | Routine care | 2001 | Pre: January–March 2000 Post: January–March 2002 |
Type of antibiotic |
*Number of patient years.
†Number included in total study period of 17 consecutive years, no specific information on number of children over 1999–2001 and 2005–2006 periods.
M, months; N, number of patients; N/A, not available; PC, primary care setting; SC, secondary care setting; Y, years.
Table 2 summarises the key guideline recommendations of the included studies. Detailed information on guideline dissemination strategies was obtained for Italy, Sweden, UK and USA (table 3). The method of dissemination varied considerably across countries, ranging from passive dissemination through online publication or paper copies targeted at individual physicians only to extensive (public) media attention, interactive workshops and joint antibiotic stewardship campaigns.
Table 2.
Guideline recommendations in included studies
| Study ID | Country | Year | Condition | Guideline recommendation (summary) | |
| Tyrstrup et al 14 | Sweden | 2010 | Children 1–12 years with uncomplicated AOM | First line | Wait-and-see for 3 days |
| Second line | Penicillin V (first choice antibiotic) | ||||
| Palma et al 11 | Italy | 2010 | Children >2 years with uncomplicated, non-severe AOM |
First line | Analgesics, wait-and-see for 3 days |
| Second line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
||||
| Children 6 months–2 years with uncomplicated AOM Children >2 years with severe AOM* | First line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
|||
| Levy et al 9 | France | 2011 | Children >2 years with uncomplicated AOM | First line | Wait-and-see, reassessment after 48–72 hours |
| Second line | High-dose amoxicillin (80–90 mg per kg per day) | ||||
| Children <2 years with uncomplicated AOM Children >2 years with severe AOM* |
First line | High-dose amoxicillin (80–90 mg per kg per day) | |||
| Second line | Amoxicillin/clavulanic-acid or cefpodoxime in case of treatment failure | ||||
| McGrath et al 10 | USA | 2004 | Children >2 years with uncomplicated, non-severe AOM |
First line | Analgesics, wait-and-see for 3 days |
| Second line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
||||
| Children 6 months–2 years with uncomplicated AOM Children >2 years with severe AOM* | First line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
|||
| Coco et al 8 | USA | 2004 | Children >2 years with uncomplicated, non-severe AOM |
First line | Analgesics, wait-and-see for 3 days |
| Second line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
||||
| Children 6 months–2 years with uncomplicated AOM Children >2 years with severe AOM* | First line | First choice: high-dose amoxicillin (80–90 mg per kg per day) Second choice: cephalosporin |
|||
| Thompson et al 13 | UK | 2003 2004 |
Children >2 years with uncomplicated, non-severe AOM |
First line | Analgesics, wait-and-see for 24–72 hours |
| Second line | Amoxicillin thrice daily 125–250 mg, for 5 days Second choice: erythromycin, azithromycin or clarithromycin |
||||
| Children <2 years or severe AOM or recurrent infections | First line | Amoxicillin thrice daily 125–250 mg, for 5 days | |||
| Rios, et al 12 | Spain | 2001 | Children >6 months with uncomplicated AOM | First line | High-dose amoxicillin for a minimum of 5 days |
| Second line | Amoxicillin/clavulanic-acid or ceftriaxone if no response within 48–72 hours | ||||
| Children <6 months with uncomplicated AOM Children >6 months with severe AOM |
First line | Amoxicillin/clavulanic-acid or ceftriaxone | |||
| Second line | Tympanocentesis and treatment according to results of Gram staining and antibiotic sensitivity | ||||
*Severe AOM is defined as moderate to severe otalgia with fever >39°C.
AOM, acute otitis media; N/A, not available.
Table 3.
Guideline dissemination efforts in included studies
| Study ID | Country | Year | Online publication | Online self-care advice to the public | Hard copy dissemination | Public media campaigns | Newspapers | Workshops or lectures for physicians | Debates or round tables for physicians | Antibiotic stewardship campaigns* | Patient leaflet |
| Tyrstrup et al 14 | Sweden | 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Palma et al 11 | Italy | 2010 | ✓ | ✓ | ✓ | ✓ | |||||
| Levy et al 9† | France | 2011 | ✓ | ||||||||
| McGrath et al 10 | USA | 2004 | ✓ | ✓ | ✓ | ||||||
| Coco et al 8 | USA | 2004 | ✓ | ✓ | ✓ | ||||||
| Thompson et al 13 | UK | 2003 2004 |
✓ | ✓ | |||||||
| Ríos et al 12† | Spain | 2001 | ✓ |
*Antibiotic stewardship campaigns specifically set up with the aim to promote guideline awareness, through various methods (eg, lectures, educational outreach visits).
†Guideline dissemination methods solely based on article, authors not available for correspondence.
Risk of bias assessment and study findings
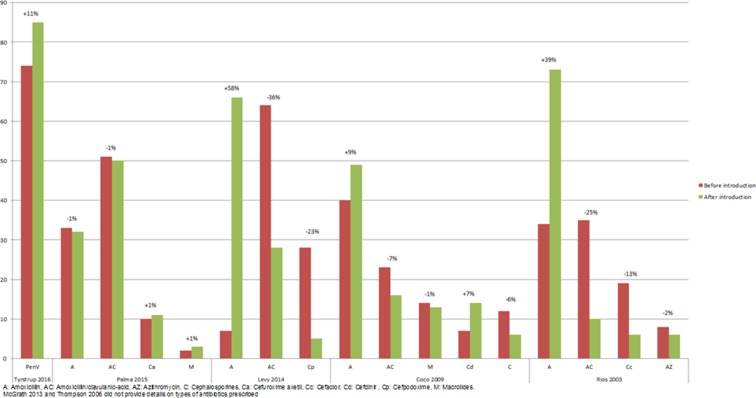
Risk of bias was judged serious in six studies and critical in one; see figure 2. The primary and secondary outcome data are illustrated in figures 3 and 4. Two of the five studies reporting antibiotic prescription rates before and after guideline introduction showed no or a negligible effect. Three studies showed a decline of 5%–12% up to 3 years after guideline introduction.
Figure 2.

Risk of bias assessment.
Figure 3.
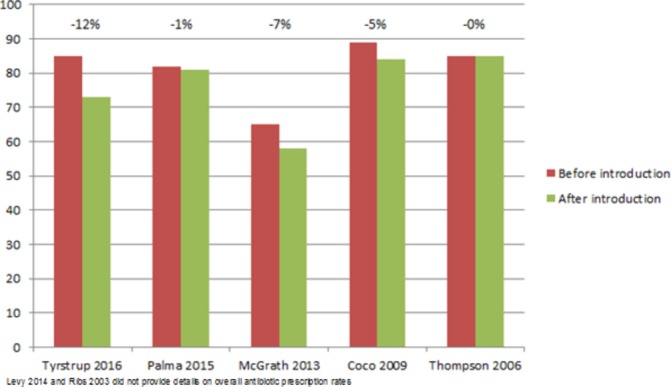
Antibiotics prescription rates.
Figure 4.
Types of antibiotics prescribed.
One US study reported both the short- and long-term impact of guideline introduction; the decline of 9% in the first 3 years decreased to 5% after four to 6 years.
In four out of five studies reporting on the type of antibiotic prescribed, prescription of the recommended first choice antibiotic, either amoxicillin or penicillin V, increased by 9%–58% after guideline introduction, with inverse trends for amoxicillin/clavulanic acid which decreased by 7%–36%.
Only one US study reported on analgesic prescription rates; this increased from 14% before to 24% after guideline introduction.
Discussion
The introduction of national AOM clinical practice guidelines seems to have at best a modest impact on antibiotic and analgesic prescribing; antibiotic prescription rates decrease by a maximum of 12% and analgesic rates increase by 10%. Its effect on the type of antibiotic is more substantial with an increase of up to 58% for the recommended first choice antibiotic.
In line with available literature,15 16 results from the study of Tyrstrup et al 14 suggest that tailored guideline dissemination may have a larger impact on antibiotic prescription rates than passive dissemination only. Our findings also indicate that physicians find it easier to substitute rather than refrain from antibiotic prescribing. Reasons include their concerns about the risk of the child falling seriously ill when not prescribing antibiotics, or missing a diagnosis which would have been adequately treated with antibiotics.17 This is especially the case when dealing with young children, or in consultations in which physicians perceive parental pressure to prescribe antibiotics.17 Apparently, many physicians are either not convinced of, or unfamiliar with, the literature that refutes the risks of restrictive prescribing18 and parental expectations of antibiotics.3 17 19
Our findings should be interpreted with some caution. Despite our efforts to minimise the impact of external factors affecting childhood AOM epidemiology and prescribing patterns, such as anti-smoking campaigns, pneumococcal conjugate vaccination and strategies to promote breast-feeding,20 21 we cannot rule out this has influenced our results. Also, we were not able to account for ongoing prescribing trends prior to the introduction of the guideline; none of the studies applied interrupted time-series analysis.22 Importantly, dissemination of the guideline to the general audience suggesting that parents can manage milder cases of AOM themselves can lead to fewer overall AOM consultations and subsequent antibiotic prescriptions.23 Nevertheless, only two out of the seven studies reported on annual fluctuations in AOM consultation rates and none of them accounted for this in their analyses.13 14 Besides, when parents do self-manage these milder cases of AOM, physicians may be faced with more severe AOM and thus prescribe antibiotics more frequently (leading to a relative increase over time). These aforementioned trends are not captured in the studies. Neither are the phenomena that, with explicit diagnostic guidance, physicians may diagnose AOM more accurately, leading to fewer overall diagnoses and antibiotic prescriptions, but at the same time a higher prescription rate per diagnosis.
Finally, the vast majority of analgesics for AOM are obtained over-the-counter rather than prescribed. This implies that our results regarding analgesic prescriptions for AOM are incomplete and preclude strong conclusions.
Conclusion
Based on what is published, the introduction of national AOM clinical practice guidelines seems to have at best a modest impact on antibiotics and analgesics prescription rates for childhood AOM. Future studies evaluating the impact of clinical guidelines using longitudinal observational data should use a quasi-experimental approach, and take fluctuations in AOM consultation rates into account, to provide more meaningful estimates on the impact on antibiotic and analgesic prescribing.
Acknowledgments
We are grateful to our colleagues P Little (UK), P Marchisio (Italy), R Rosenfeld (USA) and M Tyrstrup (Sweden) for providing information on guideline dissemination in their respective countries.
Footnotes
YD and RTU contributed equally.
Contributors: YD, RTvU and RPV collected and reviewed primary data. YD and RTvU drafted the first version of the manuscript. All authors revised the manuscript and accepted the final manuscript for publication.
Funding: This review was supported by a grant from the Netherlands Organisation for Health Research and Development (ZonMw)—HGOG subprogramme.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ovnat Tamir S, Shemesh S, Oron Y, et al. Acute otitis media guidelines in selected developed and developing countries: uniformity and diversity. Arch Dis Child 2017;102:450–7. 10.1136/archdischild-2016-310729 [DOI] [PubMed] [Google Scholar]
- 2. Haggard M. Poor adherence to antibiotic prescribing guidelines in acute otitis media--obstacles, implications, and possible solutions. Eur J Pediatr 2011;170:323–32. 10.1007/s00431-010-1286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lucas PJ, Cabral C, Hay AD, et al. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015;33:11–20. 10.3109/02813432.2015.1001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Broek d’Obrenan J, Verheij TJ, Numans ME, et al. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014;69:1701–7. 10.1093/jac/dku005 [DOI] [PubMed] [Google Scholar]
- 5. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009;302:758–66. 10.1001/jama.2009.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pulkki J, Huikko S, Rautakorpi UM, et al. Management of pain in acute otitis media in Finnish primary care. Scand J Infect Dis 2006;38:265–7. 10.1080/00365540500434679 [DOI] [PubMed] [Google Scholar]
- 7. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coco A, Vernacchio L, Horst M, et al. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics 2010;125:214–20. 10.1542/peds.2009-1115 [DOI] [PubMed] [Google Scholar]
- 9. Levy C, Pereira M, Guedj R, et al. Impact of 2011 French guidelines on antibiotic prescription for acute otitis media in infants. Med Mal Infect 2014;44:102–6. 10.1016/j.medmal.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 10. McGrath LJ, Becker-Dreps S, Pate V, et al. Trends in antibiotic treatment of acute otitis media and treatment failure in children, 2000-2011. PLoS One 2013;8:e81210 10.1371/journal.pone.0081210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palma S, Rosafio C, Del Giovane C, et al. The impact of the Italian guidelines on antibiotic prescription practices for acute otitis media in a paediatric emergency setting. Ital J Pediatr 2015;41:37 10.1186/s13052-015-0144-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ríos L, Mallafré M, González-Hidalgo RM, et al. Aplicación de una pauta terapéutica revisada de otitis media en una consulta pediátrica. Revista de Calidad Asistencial 2003;18:5–8. 10.1016/S1134-282X(03)77566-6 [DOI] [Google Scholar]
- 13. Thompson PL, Gilbert RE, Long PF, et al. Has UK guidance affected general practitioner antibiotic prescribing for otitis media in children? J Public Health 2008;30:479–86. 10.1093/pubmed/fdn072 [DOI] [PubMed] [Google Scholar]
- 14. Tyrstrup M, Beckman A, Mölstad S, et al. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care- a retrospective study of electronic patient records. BMC Infect Dis 2016;16:709 10.1186/s12879-016-2018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis DA, Thomson MA, Oxman AD, et al. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995;274:700–5. [DOI] [PubMed] [Google Scholar]
- 16. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:1–72. 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
- 17. Cabral C, Lucas PJ, Ingram J, et al. "It’s safer to …" parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: An analysis across four qualitative studies. Soc Sci Med 2015;136-137:156–64. 10.1016/j.socscimed.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 18. Van Zuijlen DA, Schilder AG, Van Balen FA, et al. National differences in incidence of acute mastoiditis: relationship to prescribing patterns of antibiotics for acute otitis media? Pediatr Infect Dis J 2001;20:140–4. 10.1097/00006454-200102000-00004 [DOI] [PubMed] [Google Scholar]
- 19. Butler CC, Rollnick S, Pill R, et al. Understanding the culture of prescribing: qualitative study of general practitioners' and patients' perceptions of antibiotics for sore throats. BMJ 1998;317:637–42. 10.1136/bmj.317.7159.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yilmaz G, Hizli S, Karacan C, et al. Effect of passive smoking on growth and infection rates of breast-fed and non-breast-fed infants. Pediatr Int 2009;51:352–8. 10.1111/j.1442-200X.2008.02757.x [DOI] [PubMed] [Google Scholar]
- 21. Fortanier AC, Venekamp RP, Boonacker CW, et al. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev 2014:CD001480 10.1002/14651858.CD001480.pub4 [DOI] [PubMed] [Google Scholar]
- 22. Kontopantelis E, Doran T, Springate DA, et al. Interrupted time-series analysis: a regression based quasi-experimental approach for when randomisation is not an option. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McWilliams DB, Jacobson RM, Van Houten HK, et al. A program of anticipatory guidance for the prevention of emergency department visits for ear pain. Arch Pediatr Adolesc Med 2008;162:151–6. 10.1001/archpediatrics.2007.30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2017-314103supp001.docx (14.4KB, docx)