Abstract
Objective
To determine whether a 2-week methotrexate (MTX) discontinuation after vaccination improves the efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis (RA).
Methods
In this prospective randomised parallel-group multicentre study, patients with RA on stable dose of MTX were randomly assigned at a ratio of 1:1 to continue MTX or to hold MTX for 2 weeks after 2016–2017 quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B-Yamagata and B-Victoria. The primary outcome was frequency of satisfactory vaccine response, defined as greater than or equal to fourfold increase of haemagglutination inhibition (HI) antibody titre at 4 weeks after vaccination against ≥2 of four vaccine strains. Secondary endpoints included seroprotection (ie, HI titre ≥1:40) rate, fold change in antibody titres.
Results
The modified intention-to-treat population included 156 patients in the MTX-continue group and 160 patients in the MTX-hold group. More patients in MTX-hold group achieved satisfactory vaccine response than the MTX-continue group (75.5% vs 54.5%, p<0.001). Seroprotection rate was higher in the MTX-hold group than the MTX-continue group for all four antigens (H1N1: difference 10.7%, 95% CI 2.0% to 19.3%; H3N2: difference 15.9%, 95% CI 5.9% to 26.0%; B-Yamagata: difference13.7%, 95% CI 5.2% to 22.4%; B-Victoria: difference 14.7%, 95% CI 4.5% to 25.0%). The MTX-hold group showed higher fold increase in their antibody titres against all four influenza antigens (all p<0.05). Change in disease activity was similar between groups.
Conclusions
A temporary MTX discontinuation for 2 weeks after vaccination improves the immunogenicity of seasonal influenza vaccination in patients with RA without increasing RA disease activity.
Trial registration
Keywords: rheumatoid arthritis, methotrexate, discontinuation, vaccination, influenza
Introduction
Rheumatoid arthritis (RA) is a common chronic systemic inflammatory diseases, affecting 1% of general population.1 It requires long-term treatment with disease-modifying antirheumatic drugs (DMARDs), which as immune-suppressive agents inhibit both cellular immunity and humoral immunity. Since the underlying immune dysfunction and the treatment-associated immune suppression render patients with RA more susceptible to infections,2 vaccines are strongly recommended against preventable diseases in patients with RA.3–5 This is of particular importance when patients are confronted with new epidemics such as pandemic influenza infection.6
Methotrexate (MTX) is the most commonly prescribed DMARD for the treatment of RA due to its high efficacy and favourable safety profile. Even in the era of biologic DMARDs, MTX remains as the anchor drug because of its synergistic effect with biologic DMARDs.7 However, MTX significantly decreases vaccine response to pneumococcal and seasonal influenza vaccines, particularly response to novel strain antigens.6 8–11 We previously showed in a pilot study, where MTX was discontinued for 4 weeks in different periods with respect to trivalent seasonal influenza vaccination, that a temporary discontinuation of MTX after vaccination could significantly increase immunogenicity in patients with RA who had been on a stable dose of MTX.12 However, a 4-week discontinuation was associated with an increased risk of RA flare by up to 1.4-fold during the 16-week follow-up period, suggesting that a shorter MTX discontinuation strategy may be desirable. Based on this pilot study, we hypothesised that a 2-week discontinuation of MTX after vaccination would be as effective as the 4 weeks of discontinuation while minimising a flare risk.
Therefore, we investigated the effect of MTX discontinuation for 2 weeks after vaccination on the response to seasonal influenza vaccination in patients with RA in this randomised controlled clinical trial.
Methods
Study rationale and design
This was a prospective multicentre randomised investigator-blind parallel-group intervention study that aimed to investigate the effects of a 2-week MTX discontinuation on vaccine response to 2016–2017 seasonal influenza vaccination in patients with RA. A pilot study was conducted to estimate the efficacy and time of temporary MTX discontinuation to improve vaccine response. In the pilot study, MTX discontinuation for 4 weeks before vaccination did not improve vaccination response, whereas MTX discontinuation for 2 weeks before and after vaccination or for 4 weeks after vaccination improved vaccine response. Therefore, the period of 2 weeks after vaccination were considered a critical period, where MTX should be held in the current study (online Supplementary figure S1).
annrheumdis-2018-213222supp001.pdf (547.8KB, pdf)
Patients were recruited by their primary rheumatologists in outpatient clinic setting in three tertiary medical centres in South Korea. The first patient was recruited on 7 October 2016 and the last on 7 January 2017. The study was registered with www.clinicaltrials.gov, protocol number: NCT02897011. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all patients before enrolment in the trial.
Participants
Patients with RA who were aged 19 years or older and had been on the same dose of MTX for 6 weeks or longer were eligible for inclusion. RA was defined according to the revised 1987 American College of Rheumatology criteria.13 The exclusion criteria were pregnant or lactating women, patients with a previous anaphylactic response to vaccine components or to an egg component, evidence of an acute infection with temperature >38°C at the time of vaccination, history of Guillain-Barré syndrome or demyelinating syndromes and previous vaccination with any live vaccine 4 weeks before or any inactivated vaccine 2 weeks before start of the study. Patients who necessitated a change in their RA treatment regimen within 4 weeks before enrolment and patients with any other additional rheumatic disease except for secondary Sjogren’s disease were also excluded.
Randomisation and masking
Medical Research Collaborating Center (MRCC) at Seoul National University Hospital generated a randomisation table that was stratified by centres. MRCC was not involved in the other processes of the trial. The eligible patients were randomly assigned to continue MTX or to discontinue MTX for 2 weeks after vaccination by a Central Interactive Web Response System (IWRS) at a 1:1 ratio according to the randomisation table. Information on the intervention was concealed from the investigators who enrolled or assessed the study patients. Investigators who performed the haemagglutination inhibition (HI) antibody titre assay were masked to the allocated treatment. Because of the nature of the study, patients were not masked to intervention. To measure the adherence to the study protocol, study participants were required to record their MTX administration in a diary.
Intervention
The 2016–2017 seasonal quadrivalent influenza vaccine (GC Flu, Green Cross, South Korea) contained four antigens: 15 µg of A/California/7/2009 Reassortant virus NYMC X-181 (H1N1), 15 µg of A/Hong Kong/4801/2014 NYMC X-263B (H3N2), 15 µg of B/Phuket/3073/2013 (B-Yamagata) and 15 µg of B/Brisbane/60/2008 in a 0.5 mL prefilled syringe. The vaccine was delivered as a single intramuscular injection in the deltoid muscle by healthcare providers.
After vaccination, patients in the MTX-continue group continued their MTX in their current dose, whereas patents in the MTX-hold group suspended it for 2 weeks and then resumed it at previous dose.
Before (week 0) and at 4 weeks after vaccination, the serum of the patients was collected. The HI antibody titres against each of the four influenza strains in the vaccine were measured in duplicate by an independent laboratory (the Vaccine Bio Research Institute of the Catholic University, Seoul, Korea) according to standard procedures. The average of the duplicate measurements for each antigen was used for analyses.
Adding or changing DMARDs were not allowed until postvaccination serum was obtained. During MTX discontinuation, acetaminophen (650 mg up to three times per day), non-steroidal anti-inflammatory drugs (in standard dosing) and/or prednisolone (or its equivalent) up to 10 mg per day were allowed to treat RA flares. Medications for other comorbid conditions were allowed.
Outcomes
The primary outcome was the frequency of satisfactory vaccine response to influenza antigens 4 weeks after vaccination. A satisfactory vaccine response was a priori defined as greater than or equal to fourfold increase in HI antibody titre at 4 weeks after vaccination relative to the baseline in two or more of four influenza vaccine antigens. Secondary endpoints included satisfactory response in ≥1 antigen, ≥3 antigens, ≥4 antigens of influenza vaccine, vaccine response to each antigen, frequency of seroprotection (defined as HI titres of ≥1:40) and fold change in postvaccination HI antibody titres against each vaccine antigen relative to baseline as well as incidence of influenza infection during influenza season 2016–2017.14 Influenza-like illness was defined clinically as the presence of fever >38°C and cough with 48 hours of symptom onset.15 Patients were interviewed using a structured questionnaire in March 2017 and between July and September 2017 during their follow-up visit in the clinic or per telephone. Adverse events that were associated with vaccination were captured from the patients at each visit. A RA flare was defined as an increase in Disease Activity Score in 28 joints (DAS28) of >1.2 (or >0.6 if the baseline DAS28 was ≥3.2).16
Statistical analysis
All analyses were conducted according to a predefined protocol. The analysis population was the modified intention-to-treat (mITT) population that included all study subjects who received the influenza vaccine and in whom both prevaccination and postvaccination HI titres were available. The safety was summarised for all participants who received the vaccination.
In a prior pilot study, the satisfactory vaccine response to a trivalent seasonal influenza vaccination (defined by greater than or equal to fourfold increase in HI antibody in ≥2 of three influenza vaccine antigens) in patients with RA continuing MTX and those patients holding MTX treatment for 4 weeks were 54% and 71%, respectively.12 Assuming that 2 weeks of MTX discontinuation would improve the vaccination response to that seen in patients who discontinued it for 4 weeks and assuming an alpha level of 0.05 (two-tailed), a power of 0.80 and dropout rate of 20%, 160 patients per group would be required for the study with a total target number of 320 patients.
Continuous variables were analysed by using a t-test. For vaccine titres, the reciprocal of HI titres were log-transformed for group comparisons. The binary secondary efficacy variables (frequency of satisfactory vaccine response and frequency of disease flare) were analysed by using Χ2 tests or Fisher’s exact test, as appropriate. P value <0.05 was considered to indicate statistical significance. All analyses were performed by using SPSS V.20.
Results
Baseline characteristics
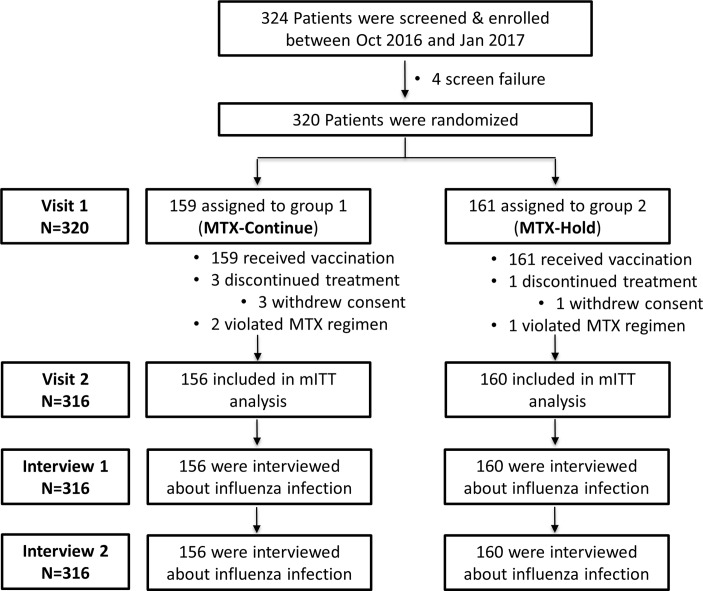
We enrolled 320 patients with RA (159 in MTX-continue group and 161 in MTX-hold group) between 7 October 2016 and 9 January 2017. All patients received the vaccination. Three patients in the MTX-continue group and one patient in MTX-hold group withdrew their consent and were excluded from analysis. Accordingly, 316 patients (156 in the MTX-continue group and 160 in the MTX-hold group) were included in the mITT population (figure 1). Patients were predominantly female (82.7% in the MTX-continue group and 87.5% in the MTX-hold group). The mean age was 52.2 years for the MTX-continue group and 53.7 years for the MTX-hold group. The two groups did not differ at baseline in terms of demographic or disease characteristics, including seropositivity for rheumatoid factor (RF) or anticyclic citrullinated peptide antibody (ACPA) or DAS28-C-reactive protein (CRP). The groups were also comparable in terms of their treatment regimen at baseline, including their use of systemic corticosteroids and MTX dose (table 1).
Figure 1.
Patient flow. MTX, methotrexate; mITT, modified intention-to-treat.
Table 1.
Baseline characteristics in the modified intention-to-treat population
| MTX continue (n=156) |
MTX hold (n=160) |
|
| Female (%) | 129 (82.7) | 140 (87.5) |
| Age, years | 52.2 (9.5) | 53.7 (10.3) |
| Duration of RA, years | 6.8 (6.5) | 6.9 (6.2) |
| Body mass index, kg/m2 | 23.3 (3.3) | 23.2 (3.3) |
| Diabetes mellitus (%) | 8 (5.1) | 8 (5.0) |
| Smoking | ||
| Never (%) | 128 (82.1) | 130 (81.3) |
| Current (%) | 11 (7.1) | 10 (6.3) |
| Former (%) | 17 (10.9) | 20 (12.5) |
| RF positivity (%) | 120/154 (77.9) | 132/157 (84.1) |
| Anti-CCP positivity (%) | 105/121 (86.8) | 111/135 (82.2) |
| DAS28-CRP | 2.2 (0.9) | 2.3 (1.1) |
| Treatment | ||
| GC (%) | 82 (52.6) | 74 (46.3) |
| Mean GC dose, mg/day | 1.8 (2.1) | 1.7 (2.1) |
| MTX (%) | 156 (100) | 160 (100) |
| MTX dose, mg/week | 13.3 (3.4) | 13.1 (3.2) |
| Sulfasalazine (%) | 8 (5.1) | 10 (6.3) |
| Hydroxychloroquine (%) | 35 (22.4) | 31 (19.4) |
| Leflunomide (%) | 33 (21.2) | 37 (23.1) |
| Tacrolimus (%) | 2 (1.3) | 2 (1.3) |
| Biological DMARDs | ||
| Tumour necrosis factor inhibitor (%) | 11 (7.1) | 13 (8.1) |
| Abatacept (%) | 1 (0.6) | 6 (3.8) |
| Tocilizumab (%) | 4 (2.6) | 7 (4.4) |
| Rituximab (%) | 1 (0.6) | 1 (0.6) |
| Tofacitinib (%) | 0 (0) | 1 (0.6) |
The data are mean (SD) or number (%).
Anti-CCP, anticyclic citrullinated peptide; CRP, C-reactive protein; DAS28, Disease Activity Score in 28 joints; DMARDs, disease-modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor.
Impact of MTX discontinuation on vaccine response
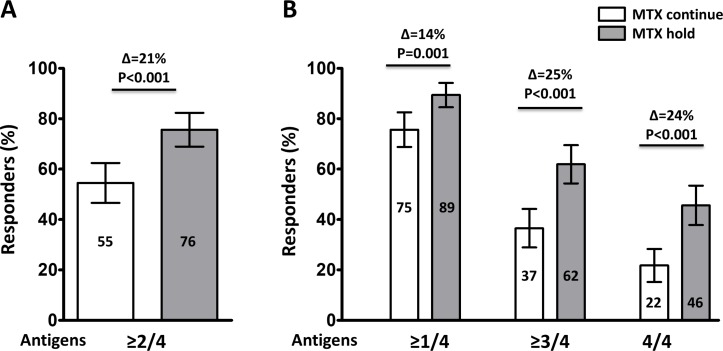
Higher proportion of patients in the MTX-hold group achieved satisfactory vaccine response, defined as greater than or equal to fourfold increase in HI antibody titre in ≥2/4 influenza antigens, compared with the MTX-continue group (75.5% vs 54.5%, p<0.001; difference 21.0%, 95% CI 10.6% to 31.7%). Similarly, the proportion achieving vaccine response (ie, greater than or equal to fourfold increase in HI antibody titre) in ≥1/4 (89.4% vs 75.6%, p=0.001; difference 13.8%, 95% CI 5.4% to 22.1%), ≥3/4 (61.9% vs 36.5%, p<0.001; difference 25.4%, 95% CI 14.3% to 36.4%) and 4/4 influenza antigens (45.6% vs 21.8%, p<0.001; difference 23.8%, 95% CI 13.4% to 34.3%) was higher in the MTX-hold group than the MTX-continue group (figure 2).
Figure 2.
Frequency of vaccination responses to the influenza antigens. Satisfactory vaccine response, defined as greater than or equal to four fold increase of haemagglutination inhibition antibody titre at 4 weeks after vaccination against ≥2 of 4 vaccine strains (A) and against ≥1 of 4, ≥3 of 4 and 4 of 4 vaccine strains (B). Numbers in the bars indicate the percentage of satisfactory responders. Error bar represents 95% CI. P values were generated by Χ2 test. MTX, methotrexate.
In terms of the responses to individual vaccine antigens, the MTX-hold group showed a higher frequency of satisfactory response to all four influenza antigens than the MTX-continue group (H1N1: difference 11.9%, 95% CI 0.9% to 22.8%, p=0.033; H3N2: difference 16.8%, 95% CI 6.1% to 27.4%, p=0.002; B-Yamagata: difference 22.7%, 95% CI 11.7% to 33.7%, p<0.001; B-Victoria: difference 32.8%, 95% CI 21.8% to 43.6%, p<0.001). Compared with the MTX-continue group, the MTX-hold group had significantly higher fold increases in their antibody titres against all four influenza antigens (table 2).
Table 2.
Immunogenicity of influenza vaccine
| MTX continue (n=156) | MTX hold (n=160) | P values | |
| H1N1 | |||
| Pre-vacc titre, GMT (95% CI) | 14.8 (12.7 to 17.3) | 16.2 (13.9 to 18.9) | 0.422 |
| Post-vacc titre, GMT (95% CI) | 68.4 (56.8 to 82.4) | 108.4 (90.7 to 129.5) | 0.001 |
| Fold increase, GM (95% CI) | 4.6 (3.7 to 5.7) | 6.7 (5.4 to 8.3) | 0.018 |
| Response, n (%) | 79 (50.6) | 100 (62.5) | 0.033 |
| Pre-vacc SP, n (%) | 38 (24.4) | 46 (28.8) | 0.377 |
| Post-vacc SP, n (%) | 118 (75.6) | 138 (86.3) | 0.016 |
| H3N2 | |||
| Pre-vacc titre, GMT (95% CI) | 10.2 (8.8 to 11.8) | 10.6 (9.1 to 12.3) | 0.695 |
| Post-vacc titre, GMT (95% CI) | 43.9 (36.1 to 53.4) | 84.3 (69.3 to 102.4) | <0.001 |
| Fold increase, GM (95% CI) | 4.3 (3.5 to 5.3) | 8.0 (6.4 to 9.9) | <0.001 |
| Response, n (%) | 85 (54.5) | 114 (71.3) | <0.001 |
| Pre-vacc SP, n (%) | 21 (13.5) | 21 (13.1) | 0.930 |
| Post-vacc SP, n (%) | 97 (62.2) | 125 (78.1) | 0.002 |
| B-Yamagata | |||
| Pre-vacc titre, GMT (95% CI) | 22.4 (18.7 to 26.7) | 20.8 (18.1– to 4.0) | 0.534 |
| Post-vacc titre, GMT (95% CI) | 70.4 (57.8 to 85.7) | 115.6 (97.4 to 137.3) | <0.001 |
| Fold increase, GM (95% CI) | 3.1 (2.6 to 3.8) | 5.6 (4.7 to 6.6) | <0.001 |
| Response, n (%) | 66 (42.3) | 104 (65.0) | <0.001 |
| Pre-vacc SP, n (%) | 60 (38.5) | 51 (31.9) | 0.220 |
| Post-vacc SP, n (%) | 116 (74.4) | 141 (88.1) | 0.002 |
| B-Victoria | |||
| Pre-vacc titre, GMT (95% CI) | 13.8 (12.1 to 15.8) | 11.7 (10.3 to 13.2) | 0.065 |
| Post-vacc titre, GMT (95% CI) | 39.5 (33.3 to 46.9) | 66.3 (56.8 to 77.4) | <0.001 |
| Fold increase, GM (95% CI) | 2.9 (2.4 to 3.4) | 5.7 (4.9 to 6.7) | <0.001 |
| Response, n (%) | 64 (41.0) | 118 (73.8) | <0.001 |
| Pre-vacc SP, n (%) | 33 (21.2) | 21 (13.1) | 0.058 |
| Post-vacc SP, n (%) | 95 (60.9) | 121 (75.6) | 0.005 |
Data are expressed in n (%) or value (95% CI). Antibody titres and fold increase are in GMT. Satisfactory vaccine response (ie, response=seroconversion) was defined as greater than or equal to fourfold improvement in titres relative to baseline. Seroprotection was defined as titres of ≥1:40. P values were generated by independent t-test for continuous variables and Χ2 test for categorical variables.
Antibody titres, fold changes and vaccine response before and after vaccination to individual vaccine strains.
GM, geometric mean; GMT, geometric mean titre; n, number; pre-vacc SP, prevaccination seroprotection rate; post-vacc SP, postvaccination seroprotection rate.
The overall baseline seroprotection against all four influenza antigens appeared to be similar between the both groups (table 2). Postvaccination seroprotection rate was higher in the MTX-hold group than the MTX-continue group for all four antigens (H1N1: difference 10.7%, 95% CI 2.0% to 19.3%, p=0.016; H3N2: difference 15.9%, 95% CI 5.9% to 26.0%, p=0.002; B-Yamagata: difference 13.7%, 95% CI 5.2% to 22.4%, p=0.002; B-Victoria: difference 14.7%, 95% CI 4.5% to 25.0%, p=0.005).
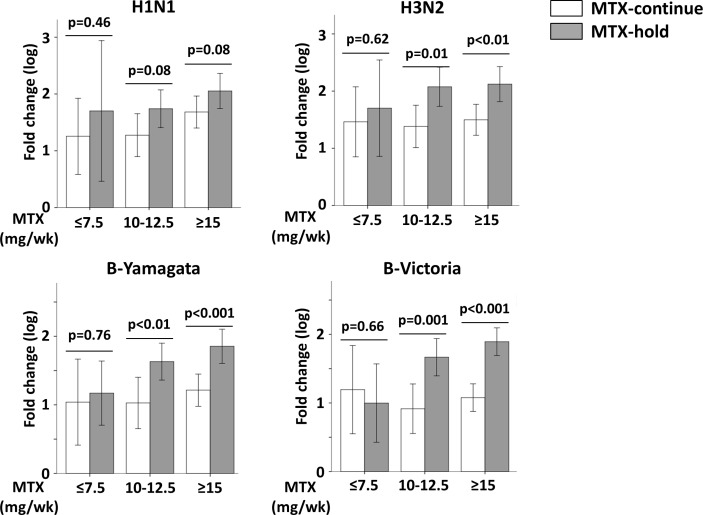
When the patients were divided into those with and without seroprotection, the response to individual antigen was higher in the MTX-hold group than the MTX-continue group in the subgroup without baseline protection (defined as HI antibody titre was <1:40 against the respective antigen), whereas the difference between both groups is less dominant in patients with seroprotection at baseline (online Supplementary table S1). The difference in vaccine response did not differ between patients in the MTX-continue group and MTX-hold group who took MTX 7.5 mg per week or less, whereas the difference was significant between the patients in the MTX-continue group and MTX-hold group who took MTX 15 mg per week or more (figure 3).
Figure 3.
Impact of baseline methotrexate (MTX) dose on vaccination responses to the influenza antigens. Log-transformed fold change in antibody titre against each vaccine strain relative to baseline was depicted according to the baseline MTX dose per week (mg/week). Bar and whiskers represent mean and SD, respectively. P values were generated by t-test.
During the follow-up period up to 1 year, one (0.6%) of 160 patients in the MTX-hold group and three (1.9%) in the MTX-continue group developed an influenza-like illness.
Safety
The vaccine was well tolerated. No serious adverse events related to the vaccination were reported during the follow-up period. In regard to RA disease activity, the mean DAS28 was only by 0.1 higher from baseline in both MTX-continue group and MTX-hold group (0.0±0.7 vs 0.1±0.8, p=0.365). However, eight (5.1%) of 156 in the MTX-continue group and 17 (10.6%) of 160 patients in the MTX-hold group experienced a flare during 4 weeks after vaccination (p=0.07). During the follow-up period, seven (4.5%) in the MTX-continue group and 10 (6.3%) patients in the MTX-hold group required rescue medications for increased joint pain (table 3). However, none (0%) of the eight patients with a flare in the MTX-continue group and three (17.6%) of 17 patients with a flare in the MTX-hold group used a rescue medication.
Table 3.
Adverse events and RA disease activity
| MTX continue (n=156) | MTX hold (n=160) | P values | |
| Any AE (%) | 34 (21.8) | 45 (28.1) | 0.194 |
| SAE (%) | 0 (0) | 0 (0) | 1.000 |
| AE occurring in >1% of patients (%) | |||
| Upper respiratory infection | 12 (7.7) | 9 (5.6) | 0.461 |
| Myalgia | 5 (3.2) | 10 (6.3) | 0.203 |
| Injection site reaction | 4 (2.6) | 6 (3.8) | 0.750 |
| Abdominal pain | 3 (1.9) | 1 (0.6) | 0.366 |
| Rash | 2 (1.3) | 2 (1.3) | 1.000 |
| Fatigue | 0 (0) | 2 (1.3) | 0.498 |
| Sore throat | 0 (0) | 2 (1.3) | 0.498 |
| Dizziness | 2 (1.3) | 0 (0) | 0.243 |
| DAS28 at visit 1 (0–100) | 2.2 (0.9) | 2.3 (1.1) | 0.517 |
| DAS28 at visit 2 (1–100) | 2.3 (0.9) | 2.4 (1.1) | 0.220 |
| Rescue medication (%) | 7 (4.5) | 10 (6.3) | 0.487 |
| RA flare at visit 2 (%) | 8 (5.1) | 17 (10.6) | 0.070 |
The data are expressed as mean (SD) or number (%). RA flare was defined as an increase in DAS28 of >1.2 (or >0.6 if the DAS28 was ≥3.2).
AE, adverse event; DAS28, Disease Activity Score in 28 joints; MTX, methotrexate; RA, rheumatoid arthritis; SAE, serious adverse event.
Sensitivity analysis to assess the primary and secondary endpoints in all patients who had no MTX-protocol deviation (the per-protocol population) provide almost identical observations to the main analysis (online Supplementary figure S2 and table S2).
Discussion
Here, we demonstrated that MTX discontinuation for 2 weeks after vaccination significantly increases the immunogenicity of a seasonal influenza vaccine in patients with RA, who had been on a stable dose of MTX, without significantly increasing risk of disease activity.
Patients with RA experience more infection disease burden due to immune dysfunction associated with the underlying autoimmunity and immunosuppressive treatment than the general population; the rate of the infection causing hospital admission and death is 1.5–2.0 times higher in patients with RA than in general population.2 17 Therefore, the increased susceptibility to infection urges that patients with RA be vaccinated for preventable infectious agents.4 5 7
MTX with its established efficacy and safety is commonly used as an anchor DMARD in the treatment of RA alone or in combination with the conventional or biologic DMARDs. While MTX is therapeutically used to prevent formation of antibodies against biologic DMARDs, it also significantly reduces the immunogenicity of various vaccines, including seasonal influenza vaccine.13 14 It is recommended that vaccination should be done before a DMARD is started.7 However, most patients with RA are already on MTX at the time when vaccination is considered. In a study, a second (booster) dose of adjuvant vaccine improves vaccine response but this approach is associated with a delay by 3–4 weeks to reach a protective immune status.18 Therefore, a novel vaccination strategy is required to restore rapid and robust immunogenicity in patients with RA on MTX, especially when confronted with a devastating pandemic threat.19
In our previous pilot work, vaccine immunogenicity of trivalent influenza vaccine was significantly improved in patients with RA on stable dose of MTX, when MTX was suspended for 2 weeks before and 2 weeks after vaccination or 4 weeks after vaccination but not when it was suspended for 4 weeks before the vaccination.12 A significant beneficial effect of MTX discontinuation could be definitively shown only for H3N2 strain and B/Yamagata but not for H1N1 due to the low number of the enrolled patients. This current trial done with higher subject number clearly demonstrated that even a 2-week discontinuation of MTX after vaccination significantly improves immunogenicity in all four strains of the quadrivalent influenza vaccine (H1N1, H3N2, B-Yamagata and B-Victoria) (table 2). In addition, holding MTX improved the vaccine response especially in those patients taking higher MTX dose (figure 2), indicating inhibition of vaccine response by MTX is dose dependent. Strikingly, improvement in satisfactory vaccine response was more prominent for the less exposed type B viral strains; response increased by 11.9% for H1N1% and 16.8% for H3N2 strains while it increased by up to 22.7% for B-Yamagata and 32.8% for B-Victoria strain (table 2). These results suggest that this MTX discontinuation strategy might be more crucial for response to influenza viruses relatively new to humans. Influenza pandemics occur when influenza strain undergoes major antigenic changes. Currently, influenza infections with avian H5N1 and H7N9 viruses with a reported mortality of 40%–60% have been rising in Asian countries.20 21 The MTX discontinuation strategy therefore could be even more important when patients with RA should be vaccinated against these novel influenza strains. Further studies are needed to evaluate whether this short-term MTX discontinuation can be applied to other vaccines which has low immunogenicity such as herpes zoster.
Patients tolerated the influenza vaccination well without a major complication. The profile of adverse events was similar between the MTX-continue group and the MTX-hold group. In regard to disease activity, the mean disease activity remained stable. However, at the individual level, 10.6% of the patients in the MTX-hold group and 5.1% in the MTX-continue group experienced a flare (table 3). All patients with a flare returned to their baseline disease activity when MTX was resumed. The higher flare rate relative to stable DAS28 change might be, in part, due to the flare definition which defines as an increase in DAS28 of >1.2 or >0.6 if the baseline DAS28 was ≥3.2. Therefore, smaller change in DAS28 in patients with baseline DAS28 >3.2 was considered a flare, although patients might not feel a clinical difference. Accordingly, none (0%) of the eight patients with a flare in the MTX-continue group and three (17.6%) of 17 patients with a flare in the MTX-hold group used a rescue medication, reflecting the relatively stable DAS28 during the study duration. However, the patients in this study had very low activity at baseline and the risk of flare could be higher in patients with higher activity.
The study has several limitations. First, all patients are Korean ethnicity. However, the clinical characteristics are similar to those in RA populations, and vaccine efficacy in our population is similar to that in other influenza vaccine studies.8 12 22 Therefore, the result might be generalised to other ethnic groups. Second, the current population was composed of stable patients with RA with a mean baseline DAS28-CRP of 2.2 in the MTX-continue group and 2.3 in the MTX-hold group. This low disease activity might be a result of target-to-treat approach in routine clinical practice.23 Therefore, the study population might be more similar to the general RA population than patients in clinical trials which include patients with higher disease activity. However, further studies testing the generalisability of our results to patients with moderate to high disease activity or with other ethnicities are warranted. Third, our study was not powered to detect a difference of influenza incidence between the two groups. A large-scale prospective study is needed to confirm whether the improved immunogenicity of MTX discontinuation can be translated into a decreased influenza incidence.
Conclusions
In conclusion, a temporary discontinuation of MTX for 2 weeks after vaccination improves the immunogenicity of a seasonal influenza vaccine in patients with RA on stable dose of MTX without appreciably increasing disease activity.
Acknowledgments
We deeply thank Professor Roy Fleischmann for his critical comments on the manuscript and Ji Hyun Kim for her excellent assistance in conducting this trial. We also thank all the study participants for their support of the study.
Footnotes
Handling editor: Josef S Smolen
Contributors: All authors contributed to the acquisition, analysis or interpretation of data and critical revision of the manuscript for important intellectual content. EBL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. EBL, KLW and JKP were responsible for the study concept and design and drafting of the manuscript. EBL, JKP, YC and KLW were responsible for the statistical analysis.
Funding: This study was sponsored by GC Pharma (formerly known as Green Cross Corporation) Yongin-si, South Korea. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Competing interests: EBL has acted as a consultant to Pfizer and received research grants from Green Cross Corporation and Hanmi Pharm.
Patient consent: Obtained.
Ethics approval: The study was approved by the institutional review board of the Seoul National University Hospital (IRB 1608-158-787).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2. Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- 3. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. 10.1002/art.23721 [DOI] [PubMed] [Google Scholar]
- 4. van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. 10.1136/ard.2010.137216 [DOI] [PubMed] [Google Scholar]
- 5. Perry LM, Winthrop KL, Curtis JR. Vaccinations for rheumatoid arthritis. Curr Rheumatol Rep 2014;16:431 10.1007/s11926-014-0431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribeiro AC, Guedes LK, Moraes JC, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis 2011;70:2144–7. 10.1136/ard.2011.152983 [DOI] [PubMed] [Google Scholar]
- 7. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 8. Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. 10.1136/annrheumdis-2014-207191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapetanovic MC, Kristensen LE, Saxne T, et al. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther 2014;16:R2 10.1186/ar4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMahan ZH, Bingham CO. Effects of biological and non-biological immunomodulatory therapies on the immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res Ther 2014;16:506 10.1186/s13075-014-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. 10.1002/acr.22246 [DOI] [PubMed] [Google Scholar]
- 12. Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017;76:1559–65. 10.1136/annrheumdis-2017-211128 [DOI] [PubMed] [Google Scholar]
- 13. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 14. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133–8. 10.1016/j.virusres.2004.02.025 [DOI] [PubMed] [Google Scholar]
- 15. Monto AS, Gravenstein S, Elliott M, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000;160:3243–7. 10.1001/archinte.160.21.3243 [DOI] [PubMed] [Google Scholar]
- 16. van der Maas A, Lie E, Christensen R, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800–5. 10.1136/annrheumdis-2012-202281 [DOI] [PubMed] [Google Scholar]
- 17. Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481–94. 10.1002/art.1780370408 [DOI] [PubMed] [Google Scholar]
- 18. Gabay C, Bel M, Combescure C, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum 2011;63:1486–96. 10.1002/art.30325 [DOI] [PubMed] [Google Scholar]
- 19. Gatherer D. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol 2009;45:174–8. 10.1016/j.jcv.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Asian Lineage Avian Influenza A (H7N9) virus. 2017. https://www.cdc.gov/flu/avianflu/h7n9-virus.htm.
- 21. Prevention CfDCa. Highly pathogenic Asian Avian Influenza (H5N1) in people. 2017. https://www.cdc.gov/flu/avianflu/h5n1-people.htm.
- 22. Elkayam O, Amir S, Mendelson E, et al. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res 2011;63:1062–7. 10.1002/acr.20465 [DOI] [PubMed] [Google Scholar]
- 23. Stoffer MA, Schoels MM, Smolen JS, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. 10.1136/annrheumdis-2015-207526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-213222supp001.pdf (547.8KB, pdf)