Abstract
Background
Colorectal cancer (CRC) is among highest prevailing cancers in the whole world, especially in western countries. For a diverse of reasons, patients prefer naturally occurring dietary substances over synthetic agents to prevent cancer. Vicenin-2 is largely available in a medicinal plant Ocimum sanctum and is an apigenin form, 6,8-di-C-glucoside, which has been reported to have a range of pharmacological values which includes antioxidant, hepatoprotective, anti-inflammatory and anti-cancer. This study was aimed to analyze the anti-proliferative effect of Vicenin-2 on human colon cancer cells via the Wnt/β-catenin signaling inhibition.
Methods
MTT assay was used to assess the cell viability at different concentrations and time point. Vicenin-2 at a concentration of 50 µM (IC50) decreased the phosphorylated (inactive) glycogen synthase kinase-3β, cyclin D1, and non-p-β-catenin expressions in HT-29 cells, which were evidenced through western blot analysis.
Results
Further, Vincenin-2 reduced the T-cell factor (TCF) / Leukocyte erythroid factor (LEF) reporter activity in HT-29 cells. Vicenin-2 also promoted substantial cell cycle arrest at the G2M phase of HT-29 cells, as well induced apoptosis in HT-29 cells, as revealed through flow cytometric analysis. Furthermore, immunoblot analysis showed that Vicenin-2 treatment enhanced the expression of Cytochrome C, Bax and caspase-3 whereas suppressed the Bcl-2 expression.
Conclusion
Together, these results revealed that Vicenin-2 can act as a potent inhibitor of HT-29 cell proliferation and can be used as an agent against CRC.
Keywords: colorectal cancer, Vicenin-2, β-catenin, apoptosis, caspase-3
Introduction
Colorectal cancer (CRC) is one of the highest prevailing cancers worldwide, especially in the western countries. Recent statistics states that, there are 6,94,000 CRC-related deaths and 1.36 million new CRC cases reported annually worldwide.1,2 Once the CRC is diagnosed, treatment at early stage is remarkable, and at metastasis stage, 5-year survival rate of the CRC patients is only 12.5%.3 Currently, there is a hike in the number of studies and research interest in identifying novel sources of bioactive compounds for colon cancer prevention.4,5 Especially, naturally occurring bioactive compounds from a dietary source are of substantial interest for the prevention of CRC.6
The development of CRC is critically influenced by the Wnt/β-catenin signaling pathway.7 β-Catenin cooperates with glycogen synthase kinase (GSK)-3β and adenomatous polyposis coli (APC) forming a complex, which is degraded by the ubiquitination process under normal circumstances. During CRC, APC is mutated and thus becomes unable to bind with β-catenin and GSK-3β, causing an accumulation of β-catenin in the cytosol, which later translocates into the nucleus.8,9 β-Catenin binds with T-cell factor (TCF)/leukocyte erythroid factor (LEF) in the nucleus and eventually transcribes downstream target genes such as C-Myc and cyclin-D1, which are responsible for cell proliferation.7,10 Due to these factors, Wnt/β-catenin signaling has become the crucial target to treat CRC.11
Vicenin-2 is a nontoxic flavonoid from the herb Ocimum sanctum Linn and Moringa oleifera, which are widely available in the region of Southern Asia.12 Vicenin-2 has been reported to possess many pharmacological properties that include antioxidant, hepatoprotective, antiinflammatory, and anticancer effects.13 Vicenin-2 inhibits angiogenesis and decreases the expression of vascular endothelial growth factor in prostate cancer, and it was proven to be effectively absorbed after oral administration in preclinical models, which was correlated with tumor regression.12
Materials and methods
Chemicals
Vicenin-2, β-actin antibody, and DMEM were obtained from Sigma-Aldrich Co., St Louis, MO, USA. Polyvinylidine difluoride (PVDF) membrane was obtained from Millipore, Burlington, MA, USA. Fetal bovine serum (FBS), trypsin-EDTA, antibiotics–antimycotics and PBS were obtained from Thermo Fisher Scientific, Waltham, MA, USA. Primary antibodies against phosphorylated (inactive) GSK-3β, cyclin D1, and non-p-β-catenin were obtained from Santa Cruz Biotechnology (Santa Cruz Biotechnology Inc., Dallas, TX, USA) and Cell Signaling (Danvers, MA, USA). The secondary antibodies, horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and rabbit anti-mouse IgG were purchased from Dako Denmark A/S, Glostrup, Denmark. Enhanced chemiluminescence kit was obtained from Thermo Fisher Scientific. All the chemicals used in this study were of highest quality and analytical grade.
Cell line and culture conditions
Human colon cancer cell line HT-29 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and was maintained in DMEM, supplemented with 1% nonessential amino acid, 10% v/v FBS, 1% l-glutamine (Thermo Fisher Scientific), and 100 µg/mL penicillin/streptomycin (Sigma-Aldrich Co.) in a humidified atmosphere with 5% CO2 at 37°C. The cells were grown in T75 culture flasks comprising DMEM added with 1% antibiotics (100 µg/mL of streptomycin and 100 U/mL of penicillin) and 10% FBS. The cells were conserved in a humidified atmosphere with 5% CO2 at 37°C. The cells were trypsinized and passaged prior to achieving confluence. The cells were then collected for analyses, including immunoblotting analysis, cell viability, and flow cytometric analysis.
For the treatments of Vicenin-2 (Stock solution of 1 M of Vicenin-2 in DMSO), cells were starved for 24 hours in a serum-free DMEM and incubated with Vicenin-2 at different concentrations (0, 3.12, 6.24, 12.5, 25, 50, 100, and 150 µM) for 24 and 48 hours in a serum-free DMEM.
Cell viability
MTT assay was used to determine cell viability as described previously.14 Briefly, cells were seeded at a density of 1×104 cells/well in a 24-well plate and cultured in a serum-free DMEM for 16 hours. The cells were then treated with Vicenin-2 in serial concentrations (0, 3.12, 6.24, 12.5, 25, 50, 100, and 150 µM) for 24 or 48 hours. Each concentration treatment was performed in triplicate. The medium was aspirated after treatment, and PBS was used to wash the cells. Subsequently, the cells were incubated for 4 hours with MTT solution (5 mg/mL). The supernatant formed was removed, and the isopropanol was used to solubilize the formazan and was measured spectrophotometrically at 570 nm. The percentage of viable cells was calculated in contrast to untreated cells. Percentage viability was calculated using the following formula:
Confocal microscopic analysis
Cells were treated with and without Vicenin-2 for 3 and 6 hours and later fixed with 4% paraformaldehyde. Cells were then permeabilized with 0.3% Triton X-100, incubated with goat serum, and then stained with anti-non-p-β-catenin antibody (Abcam, Cambridge, MA, USA, 1:200 dilutions) at 4°C overnight. The cells were then incubated with secondary antibody goat anti-rabbit IgG (1:500 dilutions) for 1 hour at 37°C and 1 µM DAPI (Southern Biotech, Birmingham, AL, USA) for 10 minutes. The samples were then examined under a confocal microscope.
Determination of cell cycle distribution
Flow cytometry was used to analyze the cell cycle distribution. Cells were collected and fixed with 1 mL of ice cold 70% ethanol after treatment with Vicenin-2, incubated at −20°C for at least 24 hours, and centrifuged at 380 × g at room temperature for 5 minutes. Cell pellets obtained were treated with 1 mL of cold staining solution comprising 20 µg/mL RNase A, 20 µg/mL propidium iodide, and 1% Triton X-100, and then incubated in dark for 15 minutes at room temperature. The samples were subsequently analyzed using FACS Calibur system (version 2.0; BD Biosciences, San Jose, CA, USA) using Cell Quest software. The results represented were of at least three independent experiments.
Luciferase reporter assay
The firefly luciferase reporter plasmid M50 super 8× TOPflash or its control counterpart M51 super 8× FOPflash was used to transiently transfect the HT-29 cells. X-tremeGene HP DNA transfection agent (Hoffmann-La Roche Ltd., Basel, Switzerland) was used to transfect the HT-29 cells. Super TOPflash has seven consensus TCF/LEF-binding sites upstream of a minimal thymidine kinase promoter driving the expression of luciferase. TCF/LEF binding sites are mutated in Super FOPflash. The cells were cotransfected using sea pansy Renilla pRL-SV40 purchased from Addgene (Cambridge, MA, USA), where expression of luciferase was driven by the SV40 promoter.16 The cells were treated with vehicle, Vicenin-2, or lithium chloride (LiCl) 48 hours after transfection, and activity of luciferase reporter was examined via the Dual-Glo Luciferase Assay System (Promega Corporation, Fitchburg, WI, USA). The activity of firefly luciferase was normalized to the activity value of each sample from Renilla luciferase.
Protein extraction and Western blot analysis
HT-29 cells were cultured in 100 mm culture plates (1×106 per plate) containing growth medium. The cells (70%–80% confluent) were rinsed twice after 24 hours with serum-free medium and then incubated in 5 mL serum-free medium to starve the cells. The cells were treated with dimethyl sulfoxide (vehicle) and 50 µM of Vicenin-2 after starvation. After the appropriate treatment time, the cells were lysed in radioimmunoprecipitation assay buffer containing phosphatase inhibitor cocktail and 1× protease. Then, the cells were sonicated for 30 minutes at 4°C, and the homogenate was centrifuged for 10 minutes at 14,000 × g to collect the supernatant, which was stored at −70°C for further analysis. The protein concentration was quantitated according to Lowry’s method. The cell lysates (40 µg) were electrophoresed in a 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), which was then transferred onto PVDF membranes. The membranes were incubated along with primary antibodies (GSK-3β, p-GSK-3β, Bcl-2, Bax, Cytochrome C, cyclin D1, non-p-β-catenin, and caspase 3) and added into tris-buffered saline. The membranes were rinsed and incubated with HRP-conjugated goat anti-rabbit IgG (1:5,000 dilutions) and rabbit anti-mouse IgG (1:5,000 dilutions) secondary antibodies. The bands were visualized on autoradiographic films with the SuperSignal West-Pico Kit (Pierce, Rockford, IL, USA) and quantified by densitometry with ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All data were expressed as mean±SD. Statistical analysis was performed using windows Statistical Package for Students version 7.5 to perform one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. P-values <0.05 (P<0.05) were regarded as significant.
Results
Vicenin-2 decreased the cell viability in HT-29 colon cancer cells
HT-29 cells were treated with different concentrations of Vicenin-2 (0–150 µM) for 24 and 48 hours. MTT assay was performed to analyze the cell viability, and the results are shown in Figure 1. Increasing concentrations of Vicenin-2 treatment gradually decreased the percentage of cell survival. From these results, the IC50 value of Vicenin-2 was calculated to be 50 µM. Therefore, we chose this concentration to perform further analysis to identify the mechanism of action of Vicenin-2.
Figure 1.
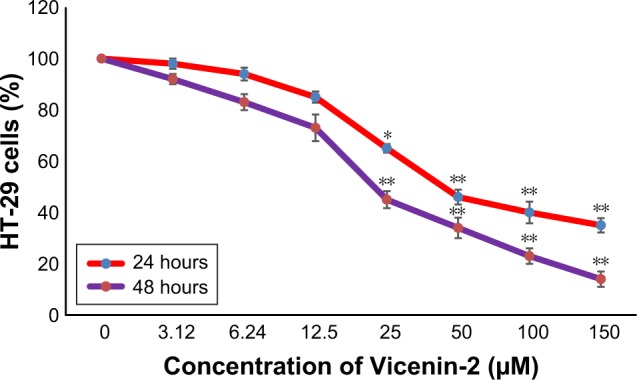
Cell viability assay of Vicenin-2.
Notes: The HT-29 colon cancer cells were treated with the doses of 0, 3.12, 6.24, 12.5, 25, 50, 100, and 150 µM concentrations of Vicenin-2. After the treatment, the cells were incubated with MTT solution for 4 hours. Vicenin-2 showed inhibition of HT-29 cell growth at the IC50 of 50 µM. “*” denotes the statistical difference at P<0.01. “**” denotes the statistical difference at P<0.05.
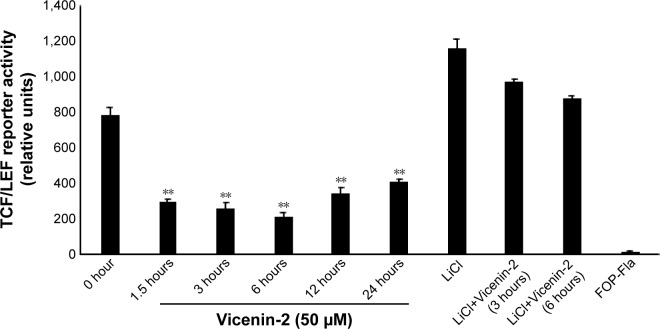
Vicenin-2 reduced the TCF/LEF reporter activity
Administration of Vicenin-2 at the dose of 50 µM concentration significantly reduced the TCF/LEF binding activity in luciferase TCF/LEF reporter assay in a time-dependent manner (Figure 2).
Figure 2.
Vicenin-2 reduces TCF/LEF reporter assay.
Notes: Vicenin-2 administration reduces the TCF/LEF reporter activity. The details of the experiment are included in the “Materials and methods” section. “**” denotes the statistical difference at P<0.05.
Abbreviations: LEF, leukocyte erythroid factor; TCF, T-cell factor.
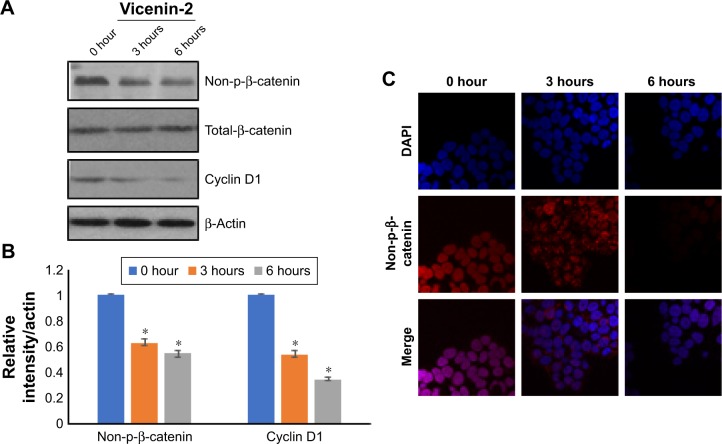
Effect of Vicenin-2 on cyclin D1 and non-p-β-catenin expressions
Western blot analysis was performed to evaluate the effects of Vicenin-2 on the non-p-β-catenin (92 kDa) and its downstream target cyclin D1 (36 kDa) expressions. Total cell lysates of HT-29 cells were treated with or without Vicenin-2 for the indicated time and were analyzed using SDS-PAGE electrophoresis, which were subsequently immunoblotted with antisera against β-actin, non-p-β-catenin, and cyclin D1. The results are shown in Figure 3A. The localization of non-p-β-catenin was evidenced by the confocal microscopic analysis. The effect of Vicenin-2 was clearly indicated by confocal microscopic analysis (Figure 3).
Figure 3.
Vicenin-2 inhibits the expression and activation of Wnt/β-catenin signaling.
Notes: (A) Western blot analysis of Wnt/β-catenin signaling proteins such as non-p-β-catenin, total-β-catenin, and cyclin D1. (B) The densitometric quantification of respective Western blot by ImageJ software (NIH). “*” denotes the statistical difference at P<0.05. (C) The nuclear localization of non-p-β-catenin was analyzed by a confocal microscope. Vicenin-2 treatment time dependently reduces the nuclear accumulation of non-p-β-catenin.
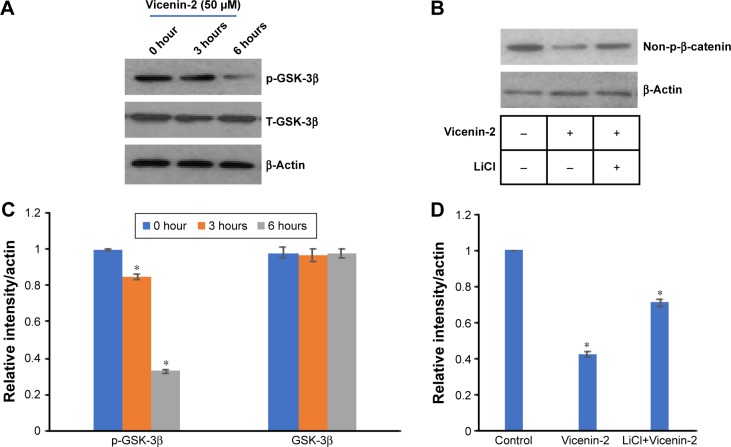
Effect of Vicenin-2 on GSK-3β in degradation of β-catenin
In order to ascertain that the downregulation of non-p-β-catenin was mediated by GSK-3β, HT-29 cells were treated with Vicenin-2 at different time intervals (Figure 3B) or in combination with LiCl, which is a GSK-3β inhibitor. Western blot analysis was used to determine the expressions of total p-GSK-3β, GSK-3β, and non-p-β-catenin. Vicenin-2 treatment alone reduced the expressions of total p-GSK-3β and non-p-β-catenin in HT-29 cells but did not show any difference in total GSK-3β (Figure 4A). Combination treatment of LiCl/Vicenin-2 showed no change in the expression of non-p-β-catenin (Figure 4B).
Figure 4.
Effect of Vicenin-2 on the expression of p-GSK-3β.
Notes: (A) Western blot expressions of p-GSK-3β and T-GSK-3β. (B) The densitometric quantification of p-GSK-3β and T-GSK-3β by ImageJ software (NIH). “*” denotes the statistical difference at P<0.05. (C) To confirm the inhibition of non-p-β-catenin by Vicenin-2 was mediated through GSK-3β, the cells were incubated with Vicenin-2 and LiCl (GSK-3β inhibitor). (D) The densitometric quantification of non-p-β-catenin by ImageJ software (NIH). “*” denotes the statistical difference at P<0.05.
Abbreviations: GSK, glycogen synthase kinase; LiCl, lithium chloride.
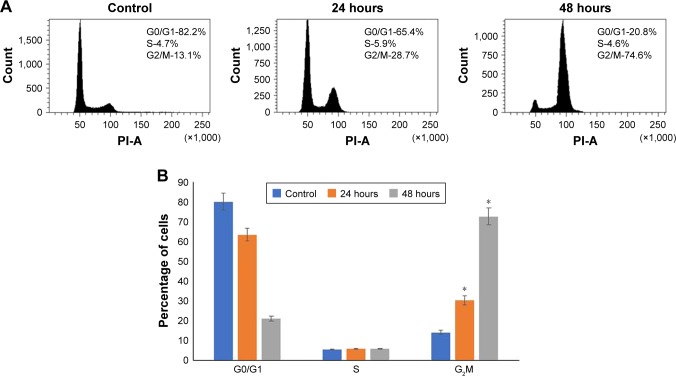
Vicenin-2 inhibits proliferation and causes G2M phase arrest through repression of cyclins
The effect of Vicenin-2 on cell cycle of HT-29 cells is represented in Figure 5 (A and B). Growth inhibitory effect of Vicenin-2 was due to cell cycle arrest, which was evidenced through accumulation of cells at the G2/M phase. Flow cytometric analysis was used to determine the effect of Vicenin-2 on the distribution of cell cycle. The results of analysis showed that the HT-29 cells were accumulated at the G2/M phase upon treatment with Vicenin-2 at both 24 and 48 hours. The percentages of cell population at the G2/M phase were increased by 28.7% at 24 hours and 74.06% at 48 hours due to Vicenin-2 treatment.
Figure 5.
Vicenin-2 arrests the cell cycle at the G2M phase.
Notes: (A) The effect of Vicenin-2 on cell cycle distribution was analyzed by flow cytometry. (B) The experiment was done in triplicate and is represented as a graph. “*” denotes the statistical difference at P<0.05.
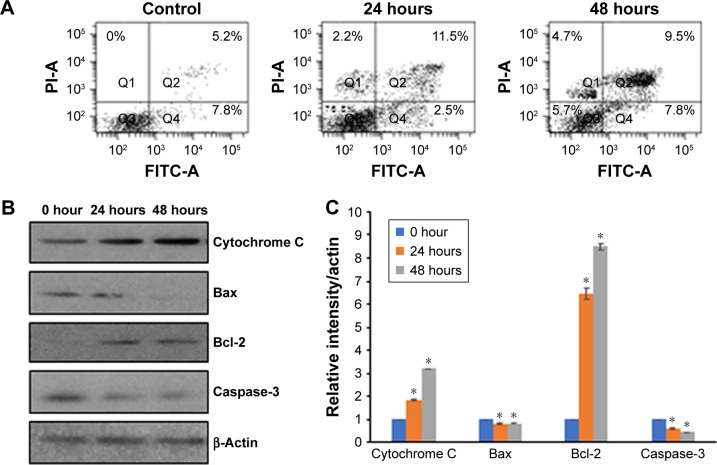
Vicenin-2 induces apoptosis by modulating the expressions of Bax, Bcl-2, Cytochrome C, and caspase-3
Since administration of Vicenin-2 was able to inhibit the Wnt/β-catenin signaling pathway and arrest the cell cycle at the G2/M phase, we analyzed the effect of Vicenin-2 on the induction of apoptosis and the underlying molecular mechanism (Figure 6). First, the effect of Vicenin-2 on annexin-V-FITC expression by flow cytometry in HT-29 cells was analyzed. It was found that Vicenin-2 treatment (50 µM) at 24 and 48 hours showed increased early apoptosis (Figure 6A). The status of apoptosis modulators such as Cytochrome C, Bcl-2, Bax, and caspase-3 was evaluated using Western blot analysis (Figure 6B). Administration of Vicenin-2, time dependently elevated the expressions of Cytochrome C, Bax, and caspase-3 and declined the anti-apoptotic Bcl-2 expression (Figure 6C).
Figure 6.
Vicenin-2 induces apoptosis in HT-29 cells by modulating the expressions of Cytochrome C, Bax, Bcl-2, and caspase-3.
Notes: (A) Vicenin-2 induces apoptosis by increasing the Annexin V-FITC expression confirmed by flow cytometry. (B) Western blot analysis of Cytochrome C, Bax, Bcl-2, and caspase-3. (C) The densitometric quantification of respective Western blot by ImageJ software (NIH). “*” denotes the statistical difference at P<0.05.
Discussion
CRC is the third most common cancer causing death in the world, demonstrating that current therapies are unable to eradicate certain cancer cells effectively.14,15 The CRC mortality rate is high due to the tendency for early metastasis and high resistance to chemotherapy and radiation.10,15 Previous reports have confirmed the existence of cytotoxic compounds in different plants that show potential anticancer effects in various cancer cells.16–19 Vicenin-2 belongs to the C-glycoside flavonoid family, which is abundantly found in the O. sanctum Linn and M. oleifera plants.20
In this study, the effects of Vicenin-2 on cell proliferation, cell cycle distribution, and apoptosis induction were evaluated in HT-29 human CRC cells. Results of this study indicated that Vicenin-2 is able to decrease proliferation of HT-29 cancer cells in a time- and concentration-dependent manner. Results of MTT assay showed that the IC50 of Vicenin-2 has significant cytotoxic effects on HT-29 cancer cells. The data also indicated that Vicenin-2 can enhance the anticancer effects at lower concentration, which in turn decreases the side effects associated with Vicenin-2 on normal cells. It was well accepted that most conventional chemotherapeutic agents target rapidly dividing tumor cells and therefore have minor effects on the slow dividing and quiescent CRCs.21,22 Furthermore, the cell cycle arrest followed by apoptosis induction in tumor cells after treatment with chemotherapeutic agents is the main efficient strategy to prevent the uncontrolled cell proliferation of cancer cells. The flow cytometric analysis of cell cycle results further support that a high percentage of HT-29 cancer cells was arrested in the G2/M phase due to treatment with Vicenin-2, which is consistent with the results of other anticancer drugs.23,24 It has been reported that Vicenin-2 induces G2/M phase accumulation due to the enhanced level of cyclin B and decreased level of cyclin D1.25
Wnt/β-catenin signaling plays an important role in the pathogenesis of CRC, and drugs that target the β-catenin pathway potentially reduce the tumor growth.26–28 Accumulation of free β-catenin in the cytosol due to activation of GSK-3β eventually translocates into the nucleus, thus trans-activating several target genes associated in tumorigenesis.29 Especially, the plant products effectively inhibit the β-catenin expression and inhibit the tumor formation. Emodin is a type of anthraquinone active substance that inhibits CRC cell invasion and migration by suppressing epithelial mesenchymal transition via the Wnt/β-catenin pathway.30 Curcumin is known as yellow gold, and it suppresses the CRC proliferation by inhibiting Wnt/β-Catenin pathways via miR-130a (Dou et al31). Telectadium dongnaiense and its constituents inhibit the CRC cell proliferation by inhibiting the Wnt/β-catenin signaling pathway. Consistent with the previous findings, treatment with Vicenin-2 downregulated the non-p-β-catenin along with a decrease in phosphorylation of GSK-3β (Kim et al32). In addition, the effect of Vicenin-2 on TCF/LEF reporter assay revealed that Vicenin-2 reduced the binding of non-p-β-catenin to TCF/LEF promoter. These results are also favorable to suggest Vicenin-2 as a potential therapeutic candidate for reversing multidrug resistance of cancer cells due to its ability to inhibit Wnt/β-catenin signaling.30
Conclusion
The present study demonstrated that Vicenin-2 inhibited cell viability, induced apoptosis, and led to cell-cycle arrest in HT-29 cells. Vicenin-2 inhibits the β-catenin expression and nuclear accumulation. In addition, this effect was mediated through the suppression of p-GSK-3β. Additionally, the expressions of apoptosis-associated proteins Bax, Cytochrome C, and caspase-3 were increased, whereas that of Bcl-2 was decreased following treatment with Vicenin-2. It was already reported that Vicenin-2 induces apoptosis in prostate cancer cells through the activation of caspase-3.25 Therefore, the Wnt/β-catenin pathway signaling axis might be a potent target for the novel treatment strategies of CRC. This study indicated that Vicenin-2 could play a beneficial role in the comprehensive clinical treatment of CRC.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Sobahi TRA, Ayyad SN, Abdel-Lateff A, Algandaby MM, Alorfi HS, Abdel-Naim AB. Cytotoxic metabolites from Callyspongia siphonella display antiproliferative activity by inducing apoptosis in HCT-116 cells. Pharmacogn Mag. 2017;13(Suppl 1):S37–S40. doi: 10.4103/0973-1296.203970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Ji Q, Deng W, et al. JianPi JieDu Recipe inhibits epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta/Smad Mediated Snail/E-Cadherin expression. Biomed Res Int. 2017;2017:2613198. doi: 10.1155/2017/2613198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafsa J, Hammi KM, Khedher MRB, et al. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed Pharmacother. 2016;84:1496–1503. doi: 10.1016/j.biopha.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Deitrick J, Pruitt WM. Wnt/beta catenin-mediated signaling commonly altered in colorectal cancer. Prog Mol Biol Transl Sci. 2016;144:49–68. doi: 10.1016/bs.pmbts.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013;14(4):2201–2205. doi: 10.7314/apjcp.2013.14.4.2201. [DOI] [PubMed] [Google Scholar]
- 9.Bourroul GM, Fragoso HJ, Gomes JW, et al. The destruction complex of beta-catenin in colorectal carcinoma and colonic adenoma. Einstein (Sao Paulo) 2016;14(2):135–142. doi: 10.1590/S1679-45082016AO3678. Portuguese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahrami A, Amerizadeh F, ShahidSales S, et al. Therapeutic potential of targeting Wnt/beta-catenin pathway in treatment of colorectal cancer: rational and progress. J Cell Biochem. 2017;118(8):1979–1983. doi: 10.1002/jcb.25903. [DOI] [PubMed] [Google Scholar]
- 11.Shang S, Hua F, Hu ZW. The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8(20):33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal SS, Jain D, Singhal P, Awasthi S, Singhal J, Horne D. Targeting the mercapturic acid pathway and vicenin-2 for prevention of prostate cancer. Biochim Biophys Acta. 2017;1868(1):167–175. doi: 10.1016/j.bbcan.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ku SK, Bae JS. Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can J PhysiolPharmacol. 2016;94(3):287–295. doi: 10.1139/cjpp-2015-0215. [DOI] [PubMed] [Google Scholar]
- 14.Veettil SK, Teerawattanapong N, Ching SM, et al. Effects of chemo-preventive agents on the incidence of recurrent colorectal adenomas: a systematic review with network meta-analysis of randomized controlled trials. Onco Targets Ther. 2017;10:2689–2700. doi: 10.2147/OTT.S127335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atreya CE, Yaeger R, Chu E. Systemic therapy for metastatic colorectal cancer: from current standards to future molecular targeted approaches. Am Soc Clin Oncol Educ Book. 2017;37:246–256. doi: 10.1200/EDBK_175679. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Song M, Gao Z, et al. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J Nutr Biochem. 2017;42:17–25. doi: 10.1016/j.jnutbio.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Xu DP, Zheng J, Zhou Y, Li Y, Li S, Li HB. Extraction of natural antioxidants from the Thelephora ganbajun Mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int J Mol Sci. 2016;17(10):E1664. doi: 10.3390/ijms17101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiau JY, Nakagawa-Goto K, Lee KH, Shyur LF. Phytoagent deoxyele-phantopin derivative inhibits triple negative breast cancer cell activity by inducing oxidative stress-mediated paraptosis-like cell death. Oncotarget. 2017;8(34):56942–56958. doi: 10.18632/oncotarget.18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav NK, Arya RK, Dev K, et al. Alcoholic extract of eclipta alba shows in vitro antioxidant and anticancer activity without exhibiting toxicological effects. Oxid Med Cell Longev. 2017;2017:9094641. doi: 10.1155/2017/9094641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhammad AA, Arulselvan P, Cheah PS, Abas F, Fakurazi S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Devel Ther. 2016;10:1715–1730. doi: 10.2147/DDDT.S96968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fesler A, Guo S, Liu H, Wu N, Ju J. Overcoming chemoresistance in cancer stem cells with the help of microRNAs in colorectal cancer. Epigenomics. 2017;9(6):793–796. doi: 10.2217/epi-2017-0041. [DOI] [PubMed] [Google Scholar]
- 22.Bommer UA, Vine KL, Puri P, et al. Translationally controlled tumour protein TCTP is induced early in human colorectal tumours and contributes to the resistance of HCT116 colon cancer cells to 5-FU and oxaliplatin. Cell Commun Signal. 2017;15(1):9. doi: 10.1186/s12964-017-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen K, He S, He Y. Effects of proglumide, a gastrin receptor antagonist, on human large intestine carcinoma SW480 cell line. Chin Med J (Engl) 1998;111(12):1075–1078. [PubMed] [Google Scholar]
- 24.Steen NV, Potze L, Giovannetti E, et al. Molecular mechanism underlying the pharmacological interactions of the protein kinase C-beta inhibitor enzastaurin and erlotinib in non-small cell lung cancer cells. Am J Cancer Res. 2017;7(4):816–830. [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaprashantha LD, Vatsyayan R, Singhal J, et al. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem Pharmacol. 2011;82(9):1100–1109. doi: 10.1016/j.bcp.2011.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egashira I, Takahashi-Yanaga F, Nishida R, et al. Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/beta-catenin signaling pathway. Cancer Sci. 2017;108(1):108–115. doi: 10.1111/cas.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddivari L, Charepalli V, Radhakrishnan S, et al. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complement Altern Med. 2016;16:278. doi: 10.1186/s12906-016-1254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manigandan K, Manimaran D, Jayaraj RL, Elangovan N, Dhivya V, Kaphle A. Taxifolin curbs NF-kappaB-mediated Wnt/beta-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie. 2015;119:103–112. doi: 10.1016/j.biochi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Pandurangan AK, Lu F, et al. Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3beta pathway in colon cancer. Carcinogenesis. 2012;33(9):1726–1735. doi: 10.1093/carcin/bgs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J, Cui CF, Yang L, Wang L, Jiang XH. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial mesenchymal transition via the Wnt/β-catenin pathway. Oncol Res. 2018 Jan 4; doi: 10.3727/096504018X15150662230295. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Dou H, Shen R, Tao J, et al. Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/β-Catenin pathways via miR-130a. Front Pharmacol. 2017;8:877. doi: 10.3389/fphar.2017.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim WK, Bach DH, Ryu HW, et al. Cytotoxic activities of Telectadium dongnaiense and its constituents by inhibition of the Wnt/β-catenin signaling pathway. Phytomedicine. 2017;34:136–142. doi: 10.1016/j.phymed.2017.08.008. [DOI] [PubMed] [Google Scholar]