Abstract
Objectives
Delayed sleep phase syndrome (DSPS) is a chronic dysfunction of circadian rhythm of the subject that impairs functioning in social, occupational, or other spheres. High rate of depression is found among DSPS patients. Aripiprazole (APZ), a second-generation antipsychotic, is effective in treatment of depression as well as schizophrenia. Recently, few case reports show the effectiveness of APZ in treating DSPS and non-24-hour sleep–wake rhythm disorder. Therefore, we tried to treat DSPS with depression using APZ.
Methods
Twelve subjects (including four women) aged 19–64 years were included. The subjects were prescribed initially 0.5–3 mg of APZ once a day with subsequent dose adjustments.
Results
Sleep onset, midpoint of sleep, and sleep offset were significantly advanced by 1.1, 1.8, and 2.5 hours, respectively. Unexpectedly, sleep duration became significantly shorter by 1.3 hours after treatment. Their depressive moods showed an unremarkable change.
Conclusion
Low dose of APZ advanced the sleep rhythm and reduced nocturnal sleep time in the subjects with DSPS. Since it is not easy for physicians to treat prolonged sleep duration often associated with DSPS, this medication would become a new therapeutic option for these patients.
Keywords: delayed sleep phase syndrome, aripiprazole, total sleep time
Introduction
Delayed sleep phase syndrome (DSPS), a sleep disorder where a patient’s circadian rhythm is delayed from typical day/night cycle, usually begins in the second decade of life or earlier.1 DSPS is characterized by habitual sleep–wake timing that is delayed, usually by more than 2 hours, relative to conventional or socially acceptable timing (International Classification of Sleep Disorders [ICSD]-3). Affected individuals complain of difficulty falling asleep at a socially acceptable time, as required to obtain sufficient sleep duration on a school or work night. These individuals also experience difficulty arising at a socially acceptable wake time, as required to prepare for school or work. When allowed to follow his or her preferred schedule, the patient’s timing of sleep is delayed (ICSD-3). Although three types of treatments have been used for DSPS, chronotherapy, phototherapy, and exogenous melatonin administration,2,3 only strategically timed melatonin was recommended in the latest guideline.4 Considerable number of patients with DSPS were also reported to have prolonged nocturnal sleep time that also profoundly disrupted their social lives.3
Although classical antipsychotics have not been reported in the treatment of DSPS, aripiprazole (APZ), which is a second-generation antipsychotic, manifests a novel mechanism of action by serving as a partial agonist of D2 receptors.5 Depression is reported to be the most common psychopathology associated with DSPS,3 and low-dose APZ is reported to be effective in major depressive disorder (MDD) as adjunctive therapy.6 In addition, low dose of APZ was reported to be effective in the patients with DSPS7 and non-24-hour sleep–wake rhythm disorder.8 Therefore, we tried to treat DSPS with depression and unexpectedly found the reduction of prolonged total sleep time (TST) adding to the advance of circadian phase.
Methods
This study was a prospective, open-labeled, flexible-dose, 4-week design. Clinical trial registration: UMIN-CTR Clinical Trial, R000033455. We examined the efficacy and tolerability of APZ for treating patients who were previously treated but who had an inadequate response to their current DSPS symptoms. One patient was (case 1) initially diagnosed with irregular sleep–wake pattern. The study was conducted from October 2016 to May 2017 at the Department of Neuropsychiatry, Akita University Hospital. Twelve subjects (including four women) aged 19–64 years (with an average of 35.7 years) were included. We diagnosed sleep and psychiatric symptoms of the subjects using ICSD-3 and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). All of the subjects met the ICSD-3 criteria for DSPS (n=12). Comorbidities with DSPS were MDD (n=7) and dysthymia (n=3) by DSM-5. Patients were treated with a starting dose of 0.5–3 mg/day of APZ (once a day in the morning or the evening), which was increased or decreased by 0.5–1 mg/day at each visit based on clinical response and tolerability. Their sleep–wake schedules as well as depressive symptoms were assessed. Evaluations of efficacy included a 1-week pre-screening period and assessments done every 1–2 weeks. The times of sleep onset, offset and sleep period were assessed by sleep logs at each visit. The sleep parameters of 4th week were compared with those of screening periods. The patients continued to receive the same fixed dose of the previously used medications throughout the study period. The changes in depressive symptoms were assessed by general clinical impressions by physicians. Representative case 1 is presented in “Results” section. This study was approved by Ethics Committee of Akita University Graduate School of Medicine. Written informed consent was obtained from all patients. Paired t-test was used for statistical analysis.
Results
The demographic data of cases are summarized in Table 1. Before the initiation of APZ treatment, previous treatments did not show favorable effects for DSPS.
Table 1.
Demographic data and sleep parameters of the patients with DSPS and depressive symptoms
| No | Age (years)/gender | Primary diagnosis | Comorbidity | Sleep onset (hours) | Midpoint of sleep (hours) | Sleep offset (hours) | Total sleep time (hours) | APZ (mg) | Other medications | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment (Pre-T) | Post-treatment (Post-T) | Pre-T | Post-T | Pre-T | Post-T | Pre-T | Post-T | Initial dose | Final dose | |||||
| 1 | 43/M | Irregular sleep wake pattern − → DSPS | MDD | 26 (2 am) | 25 (1 am) | 6.25 | 4.25 | 10.5 | 7.5 | 8.5 | 6.5 | 3 | 1.5 | Valproate 600 mg, mianserin 30 mg, paroxetine 20 mg, brotizolam 0.25 mg, ramelteon 8 mg, methylcobalamin 250 µg |
| 2 | 27/M | DSPS | MDD | 25 (1 am) | 23 | 6.5 | 2.5 | 12 | 6 | 11 | 7 | 1.5 | 1.5 | Mirtazapine 7.5 mg, escitalopram 20 mg, rilmazafone 1 mg, methylcobalamin 150 µg, quetiapine 150 mg, ramelteon 4 mg |
| 3 | 48/F | DSPS | MDD | 25 (1 am) | 24 (0 am) | 4.5 | 3 | 8 | 6 | 7 | 6 | 1.5 | 0.75 | Paroxetine CR 50 mg, clotiazepam 15 mg, alprazolam 2.4 mg, zolpidem 5 mg, brotizolam 0.25 mg, suvorexant 20 mg, methylcobalamin 1,500 µg |
| 4 | 50/F | DSPS | MDD | 29 (5 am) | 27 (3 am) | 8.5 | 6.5 | 12 | 10 | 7 | 7 | 3 | 1.5 | Sertraline 50 mg, alprazolam 0.8 mg, etizolam 1.5 mg, triazolam 0.25 mg |
| 5 | 55/M | DSPS | MDD | 22.5 | 24 (0 am) | 2 | 2.75 | 5.5 | 5.5 | 7 | 5.5 | 0.75 | 0.5 | Milnacipran 150 mg, paroxetine 40 mg, sulpiride 150 mg, valproate 200 mg, brotizolam 0.25 mg ramelteon 4 mg |
| 6 | 64/F | DSPS | MDD | 25 (1 am) | 23 | 5.5 | 3.5 | 10 | 8 | 9 | 9 | 1 | 1 | Sertraline 100 mg, etizolam 1 mg, flunitrazepam 2 mg, levomepromazine 25 mg, ramelteon 8 mg |
| 7 | 28/M | DSPS | MDD | 24 (0 am) | 23.5 | 4.25 | 3 | 8.5 | 6.5 | 8.5 | 7 | 0.5 | 0.5 | Duloxetine 60 mg, mirtazapine 15 mg, blomazepam 1 mg, zolpidem 5 mg |
| 8 | 44/F | DSPS | Dysthymia | 26 (2 am) | 24 (0 am) | 5.5 | 3 | 9 | 6 | 6.5 | 6 | 3 | 3 | Milnacipran 50 mg, mirtazapine 7.5 mg, triazolam 0.25 mg, etizolam 0.5 mg, diazepam 4 mg, methylcobalamin 150 µg, ramelteon 4 mg |
| 9 | 34/M | DSPS | Dysthymia | 23 | Drop out | 8 | 9 | 1.5 | Olanzapine 5 mg, rolazepam 2 mg, methylcobalamin 150 µg | |||||
| 10 | 27/M | DSPS | Dysthymia | 25 (1 am) | Drop out | 9 | 8 | 3 | Ramelteon 4 mg, methylcobalamin 150 µg | |||||
| 11 | 19/M | DSPS | (−) | 25 (1 am) | 25 (1 am) | 6 | 4.5 | 11 | 8 | 10 | 7 | 1.5 | 1 | None |
| 12 | 23/M | DSPS | (–) | 29 (5 am) | 27 (3 am) | 8.5 | 6.75 | 12 | 10.5 | 7 | 7.5 | 1 | 0.5 | None |
| DSPS mean | 38.5 | 25.7 (1.7 am) | 24.6 (0.6 am) | 5.8 | 4.0 | 9.6 | 7.4 | 8.2 | 6.9 | 1.8 | 1.2 | |||
| SD | 14.2 | 2.0 | 1.5 | 1.9 | 1.5 | 2.0 | 1.7 | 1.4 | 1.0 | 1.0 | 0.8 | |||
| Treatment difference | 1.1 | 1.8 | 2.5 | 1.3 | ||||||||||
| SD | 1.2 | 1.3 | 1.7 | 1.4 | ||||||||||
| p-value | p=0.021 | p=0.001 | p=0.0007 | p=0.018 | ||||||||||
Abbreviations: DSPS, delayed sleep phase syndrome; APZ, aripiprazole; MDD, major depressive order.
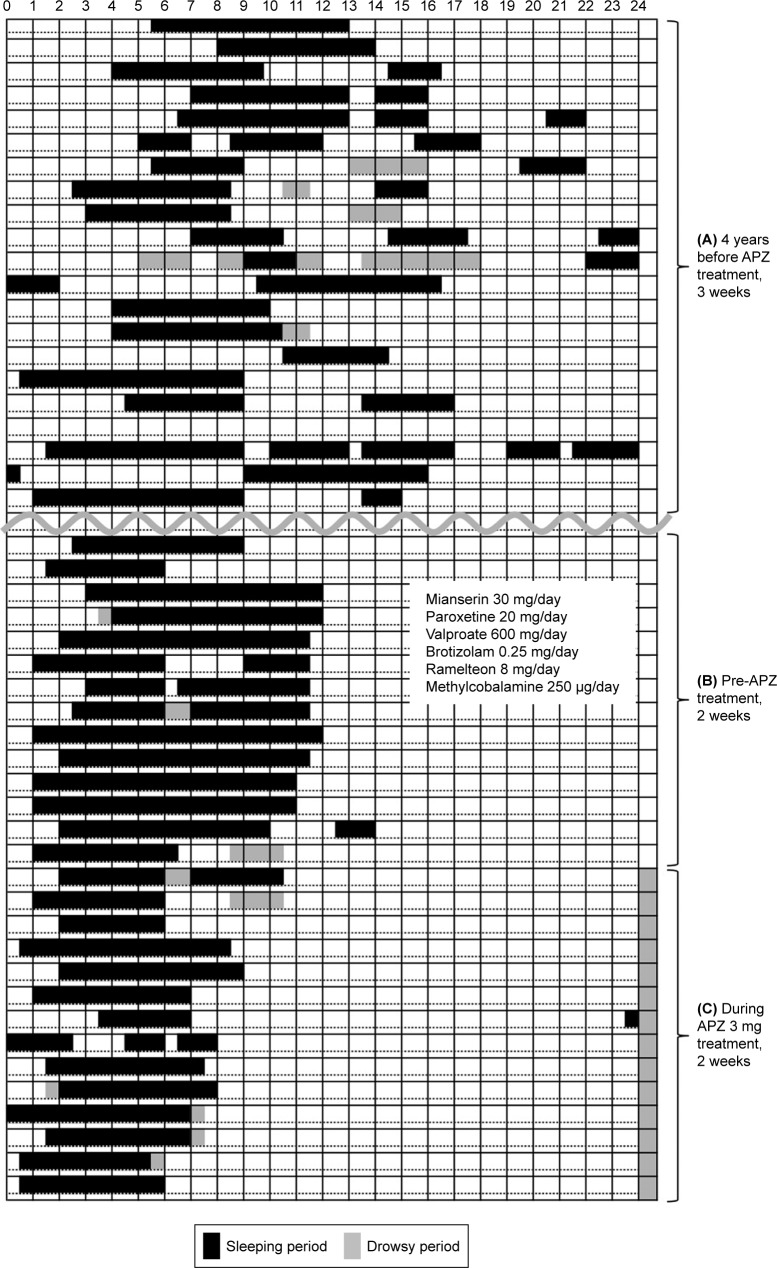
Representative case 1 was a male who was in his 40s (Table 1, Figure 1). He had an irregular sleep–wake schedule. He was prescribed 8 mg ramelteon (melatonin agonist), 250 µg methylcobalamin, 20 mg paroxetin, 600 mg valproate, 30 mg mianserin, and 0.25 mg brotizolam. His irregular sleep–wake pattern had led to DSPS with 8.5 hours sleep time. After treatment with 3 mg of APZ at evening, he started to sleep at around 1:00 am and woke up around 7:30 am with 6.5 hours sleep time.
Figure 1.
The sleep diary of case 1. (A) Upper part is 3 weeks period of initial visit about 4 years before. (B) Middle part is 2 weeks period of pretreatment with APZ. (C) Lower part is 2 weeks period during treatment. The left and right ends are 0 am. Sleep time during 24 hours is indicated by black bars and drowsy period is indicated by shadow bars. (A) In the period of pretreatment, the sleep diary showed irregular sleep–wake pattern in the 3–4 years before. We started ramelteon (melatonin analog), methylcobalamin, paroxetine, etc. (B) His sleep pattern became DSPS with 8.5 hours sleep time with abovementioned medications. (C) After treatment with 3 mg of APZ at evening, he started to sleep around 1 am and got up around 7:30 am.
Abbreviations: DSPS, delayed sleep phase syndrome; APZ, aripiprazole.
As shown in Table 1, we prescribed 0.5–3 mg/day of APZ (initial average: 1.8 mg). Sleep onset, midpoint of sleep, and sleep offset became significantly advanced by 1.1, 1.8, and 2.5 hours, respectively. TST was significantly reduced by 1.3 hours (p=0.017). The reduction of sleep time and the advancements of sleep offset occurred in a couple of days, and advance in sleep phase occurred within a week. Patients depressive moods showed unremarkable change. APZ was stopped in two cases because of daytime sleepiness (case 9) or akathisia (case 10). Since TST in some cases became too short and they experienced daytime sleepiness (n=6), we carefully reduced the dose of APZ to achieve adequate TST (final average: 1.2 mg).
Discussion
With APZ treatment, sleep onset, midpoint of sleep, and sleep offset were significantly advanced by 1.1, 1.8, and 2.5 hours, respectively. As a result, TST was significantly reduced by 1.3 hours. Patients depressive moods showed unremarkable change.
So far, there are only two case reports on the effects of low dose of APZ on sleep–wake phase advancements,7,8 while reduction in TST was not focused in these cases.
The mechanism that caused a reduction in TST would be: 1) APZ directly reduced TST and advanced circadian rhythm, 2) APZ advanced circadian rhythm and thereby reduced TST, and 3) APZ improved depression and thus reduced TST.
Since there was no remarkable change in the depressive symptoms with low doses of APZ, the hypothesis (3) was denied. Considering the course of APZ effects, the early waking initially occurred during the period of high photosensitivity in a couple of days, and thereafter the advancement of circadian rhythm would be a secondary phenomenon. Therefore, hypothesis (2) was also denied. Consequently, the hypothesis (1) would be the most reasonable, because shortened nocturnal sleep time and early waking occurred soon after the beginning of APZ. Patients sleep phases seemed to be advanced as a secondary event. The current phenomenon of shortened TST was a novel and an important finding. Patients with DSPS often showed prolonged TST, and their activities of daily living (ADL) were largely disturbed.
Melatonin has been used for the treatment of DSPS.1,2,4 Since the subjects with DSPS were reported to have prolonged sleep time3 and even the sleep onset becomes advanced by strategically timed melatonin, subjects daily active periods would continuously be shortened. Although treatments have not been known for the prolonged TST, low dose of APZ would be a new therapeutic option. Additionally, APZ is no need for considering the administration timing.
The majority of antipsychotics have been reported to increase TST in the patients with schizophrenia and healthy controls,9 while reduction of TST has not been reported when using these dopamine antagonists.
APZ was known as a dopamine partial agonist, which means activation at low dopaminergic tone and inhibition at high dopaminergic tone, thus stabilizing dopamine output from either direction.10 In this point of view, APZ activates dopaminergic output in the DSPS patients whose dopaminergic tone would be low. On the other hand, before the concept of APZ being a dopamine partial agonist was introduced, this compound was reported as having both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity.11 It was also reported that low dose of APZ may show the effects of an antidepressant similar to the agonistic activity of dopamine D3 receptors.12 Since we used a low dose of APZ, dopamine D3 receptors’ agonistic activity would be predominant. Therefore, the effect of dopaminergic upregulation would reduce subjects’ TST and advance sleep–wake phase.
APZ would reduce sleep time and advance circadian rhythm not only through dopaminergic system but also via 5-HT-1A receptors, histamine system, GSK-3, Reverb, and Bmal1.8,13
At the beginning of this study, we used 1.5–3 mg APZ; however, this dose resulted in a greater shorting of nocturnal TST with daytime sleepiness in some cases. In the latter half of the study, we used 0.5–1 mg APZ as an initial dose. Low to middle (5–11 mg) dose of APZ is reported to be effective in MDD as adjunctive therapy.6 Compared with the patients with MDD, those with DSPS would be more vulnerable to APZ in experiencing daytime sleepiness; therefore, they might need lower dose of APZ. The daytime sleepiness in these patients would be induced by this medication due to its postsynaptic D2 receptor antagonistic activity.
Although APZ was introduced in 2000, its usage for the DSPS patients was not recognized. Several case reports had been made,7,8,13 but the dose of medication was complicated for each subject. Owing to these two-stage effects, it became difficult for the physicians to find out the efficacy of reducing prolonged TST and sleep–wake phase advancements.
There are several limitations in this study. First, this study was a prospective, open-labeled clinical observation, and not a randomized placebo-controlled double-blinded study. Therefore, some bias would exist in this type of study with a small sample size and short duration of study period. Second, neither the plasma concentrations of APZ nor nocturnal polysomnography were assessed in the subjects. Third, our DSPS subjects were suffering from mental disorders, such as depression and dysthymia. The depressive symptoms were assessed by physicians based on the general clinical impressions, and did not include the scores of mental disorders. Further research on the availability of APZ would be needed for the subjects with hypersomnolence disorders or long sleepers using polysomnography.
Conclusion
Low dose of APZ reduced prolonged TST and advanced sleep–wake phase in the subjects who had prolonged TST and DSPS. The mechanism of action would be by dopaminergic upregulation. Since it is difficult for physicians to treat prolonged TST and DSPS symptoms, this medication would become a new therapeutic option for these patients.
Acknowledgments
Preliminary version of this study was presented at APSS 2017 and published in Japanese (Seishinka Chiryogaku).
Footnotes
Disclosure
Takashi Kanbayashi has received speaker’s honoraria from Otsuka Pharmaceutical, MSD, and Eisai. Manabu Takaki has received speaker’s honoraria from Otsuka Pharmaceutical. Tetsuo Shimizu has received research grants from Eisai and MSD, and speaker’s honoraria from MSD, Takeda Pharmaceutical, Pfizer, and Yoshitomi Pharmaceutical. The authors report no other conflicts of interest in this work.
References
- 1.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988;9(1):22–27. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 2.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Okawa M, Uchiyama M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndrome. Sleep Med Rev. 2007;11(6):485–496. doi: 10.1016/j.smrv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 6.Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- 7.Takaki M, Ujike H. Aripiprazole is effective for treatment of delayed sleep phase syndrome. Clin Neuropharmacol. 2014;37(4):123–124. doi: 10.1097/WNF.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 8.Matsui K, Takaesu Y, Inoue T, Inada K, Nishimura K. Effect of aripiprazole on non-24-hour sleep-wake rhythm disorder comorbid with major depressive disorder: a case report. Neuropsychiatr Dis Treat. 2017;13:1367–1371. doi: 10.2147/NDT.S136628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51–77. doi: 10.1016/j.smrv.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J Clin Psychiatry. 2001;62(11):841–842. doi: 10.4088/jcp.v62n1101. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi T, Tottori K, Uwahodo Y, et al. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinon e (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274(1):329–336. [PubMed] [Google Scholar]
- 12.Tadori Y, Forbes RA, McQuade RD, Kikuchi T. Functional potencies of dopamine agonists and antagonists at human dopamine D(2) and D(3) receptors. Eur J Pharmacol. 2011;666(1–3):43–52. doi: 10.1016/j.ejphar.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro T. Improvement of a patient’s circadian rhythm sleep disorders by aripiprazole was associated with stabilization of his bipolar illness. J Sleep Res. 2017;26(2):247–250. doi: 10.1111/jsr.12496. [DOI] [PubMed] [Google Scholar]