Abstract
We report the imaging appearances of a case of pathologically proven, neonatal neuroblastoma 4S with diffuse hepatic metastatic involvement at presentation. Patient had an abnormal appearing liver both by ultrasound and MR. There was no evidence for associated adrenal tumor by imaging. Lack of an associated adrenal mass led to initial misinterpretation of diffuse hepatic accumulation of MIBG seen with radionuclide scintigraphy. To the best our knowledge, this is the first report of metastatic neonatal 4S neuroblastoma without an adrenal (or extra-adrenal) primary identified either on pre- or post-natal imaging.
Keywords: Adrenal gland, Neuroblastoma, Liver, Neonatal, Stage 4s
CASE REPORT
A one- day old female, born at 39 weeks to a healthy mother did not have any abnormalities found on pre-natal imaging. Shortly after delivery, she was tachypneic and had an enlarged abdomen. This prompted lab work, which was significant for thrombocytopenia and coagulopathy. At this point, she was transferred to our institution.
Lab work on arrival to our hospital was significant for total bilirubin 5.7 mg/dL (normal range 0.3 – 1.9 mg/dL), indirect bilirubin 4.9 mg/dL (normal range 0 – 1.9 mg/dL), INR 2.0 (normal range 0.9–1.2), WBC 21,500/micro liter (normal range in a newborn 9.000–30,000/micro liter), platelets 69,000/micro liter (normal range 140,000 – 450,000/micro liter). Tumor markers were as follows: Alpha fetoprotein (AFP) 97,873 IU/mL (normal range below 10 IU/mL), Urine Vanillyl mandelic acid/Creatinine 540 mg/g and peaked at 809 mg/g, Urine homovanillic acid/Creatinine 554 mg/g and peaked at 723 mg/g.
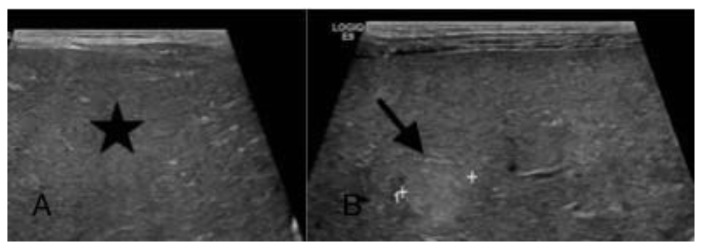
On imaging, ultrasound revealed a diffusely enlarged, markedly heterogeneous liver with patent vasculature [Figure 1]. Ultrasound appearances were interpreted as being most consistent with a hepatic primary neoplasm such as a diffuse hepatoblastoma. Given her elevated tumor markers, hepatic metastatic neuroblastoma was also offered as a differential diagnosis, although the confounding factor was her normal adrenals.
Figure 1.
A one-day-old female with neuroblastoma stage 4s.
Sonogram findings: Gray scale sonogram of the liver (performed with a convex transducer, 3.5 MHz) in transverse view (A) shows a diffusely coarse liver (star) and (B) reveals an echogenic nodule (arrow) against the background of diffusely abnormal hepatic echogenicity.
Follow-up abdominal MR images revealed a markedly enlarged and diffusely heterogeneous liver, with few T2 hyperintense foci interspersed through a background of an overall abnormal hepatic signal. On contrast-enhanced T1-weighted MR images [Figure 2], patchy heterogeneous contrast-enhancement was present. Further work up for malignancy ensued, and bone marrow biopsy was negative. The presence of abnormal tumor markers prompted an MIBG scintigraphy [Figures 3, 4], which showed diffuse hepatic accumulation of MIBG. No additional foci of abnormal uptake were identified. Adrenals were normal sized.
Figure 2.
A one-day-old female with neuroblastoma stage 4s.
MRI findings:
(A) Coronal FIESTA (1.5T, TR - 5.292, TE - 2.088) shows diffusely enlarged, heterogeneous appearing liver with at least two distinctly nodular foci of hyper-intensity (arrows).
(B) Axial T1 post intravenous administration of Gadolinium based contrast agent (0.16 cc of gadoversetamide, 1.5 T, TR - 4.104, TE - 1.284) taken through the mid liver shows patchy, heterogeneous contrast enhancement through the liver.
(C) and (D) are SSFSE (1.5T, TR - 1106.87, TE - 202.24) in axial views that show normal appearing, thin normal adrenal glands bilaterally (arrows).
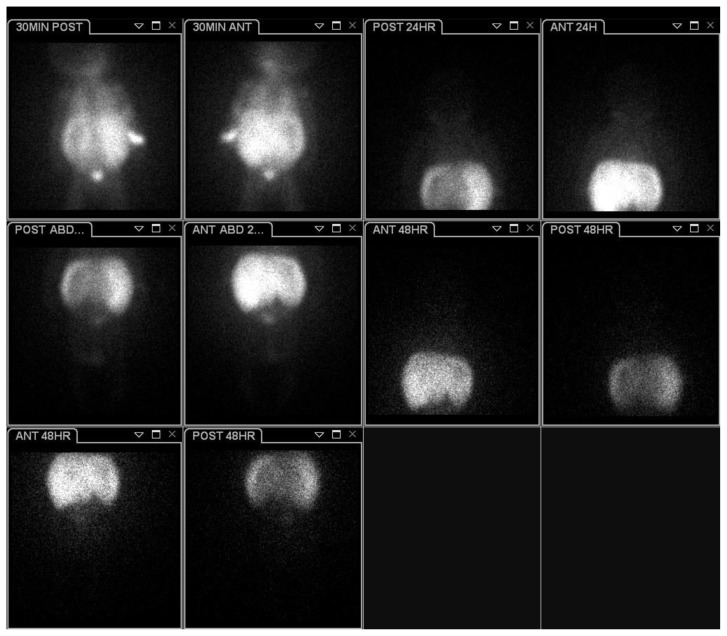
Figure 3.
A one-day-old female with neuroblastoma stage 4s.
Planar anterior scintigraphic image acquired at 48 hours following the intravenous administration of 1.6 mCi of I-123-MIBG reveals marked diffuse hepatic accumulation of the radiopharmaceutical (white star). No additional MIBG-avid masses were noted on this study. Also, there was no bone marrow uptake of the radiotracer (marrow aspirate and biopsy were negative).
Figure 4.
A one-day-old female with neuroblastoma stage 4s.
Planar and sequential single photon emission computed tomography (SPECT) images acquired at 30 minutes, 24 hours, and 48 hours after the intravenous administration of 1.6 mCi of I-123-MIBG demonstrate diffuse hepatic uptake with no additional radiotracer uptake.
Ultimately, a liver biopsy was performed [Figure 5], which was consistent with poorly differentiated neuroblastoma, low MKI, favorable histology per Shimada classification. No n-myc amplification was identified in the tumor by FISH analysis. She was diagnosed with neuroblastoma stage 4s.
Figure 5.
A one-day-old female with neuroblastoma stage 4s.
Pathologic findings: (A) Two wedge biopsies (one shown here, with capsule present - arrows) and two core biopsies were performed. (B) The tumor has sheets (left) and nests (right) of tumor cells. Tumor cells are seen adjacent to hepatocytes (asterisk) under the capsule (C) and surrounding bile ductules (D, center and right). Tumor is highlighted by a CD56 immunostain (E). Adjacent liver parenchyma and bile ductules are positive for a cytokeratin immunostain while tumor is negative (F). (G) There are occasional Homer-Wright rosettes scattered in the tumor (arrows). The rosetting, the presence of a low mitotic-karyorrhectic index and the patient’s young age (3 weeks) were consistent with a favorable histology of this poorly differentiated neuroblastoma (Original magnifications: A - 12.5; B - 40x; C–F - 200x; E - 400x).
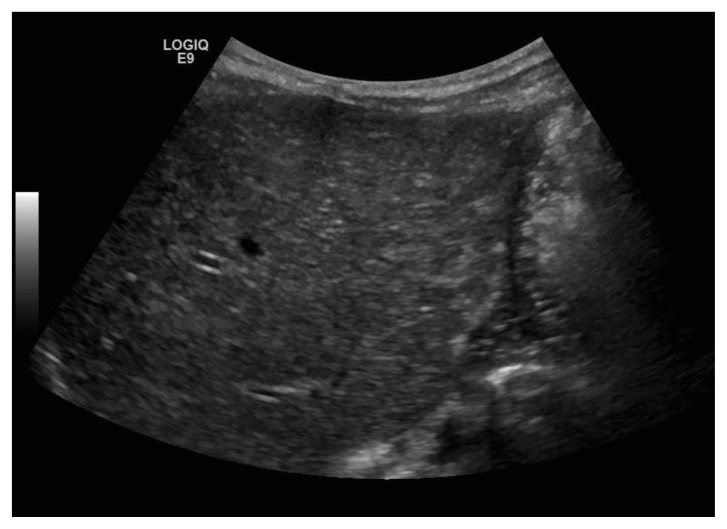
Initial hospital course was complicated by increase in abdominal girth, inability to tolerate oral intake requiring a J-Tube, tachypnea and coagulopathy. Given the progressive increase in abdominal girth and poor feeding, the decision was made to proceed with chemotherapy. Three weeks after initial presentation she was started on chemotherapy, which she received Carboplatin (18.6 mg/kg/dose × 1 dose) and etoposide (4mg/kg/dose × 3 doses). Neupogen was administered for expected neutropenia. She did well with chemotherapy. Tumor markers remained low and follow-up sonography of the liver revealed definite improvement [figure 6]. Eventually her Broviac line, which was placed for chemotherapy and her J – tube were removed. Currently, she is off chemotherapy and meeting appropriate developmental milestones.
Figure 6.
Same patient at six-month of age; status post treatment for neuroblastoma stage 4s. Sonogram findings: Gray scale sonogram of the liver (performed with a convex transducer, 3.5 MHz) in transverse view shows normal echotexture of the liver with no evidence of nodularity.
DISCUSSION
Etiology & Demographics
The etiology of neuroblastoma is not completely known. It is thought to be a failure of the neuroblasts to mature. The disease is sporadic, probably results from unknown gene changes. There are genetic markers that can predict prognosis and guide treatment.
Neuroblastoma is the most common extracranial solid tumor of childhood. It accounts for approximately 7.8% of childhood cancer. Over one-half of patients present with metastatic disease at diagnosis. The International (INSS/INRC) staging of neuroblastoma includes 5 stages according to the localization of the lesions at the time of admission [1, 2]:
Stage 1: Localized tumor with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph node negative for tumor microscopically (lymph nodes attached to and removed with the primary tumor may be positive)
Stage 2A: Localized tumor with incomplete gross excision; representative ipsilateral nonadherent lymph nodes negative for tumor microscopically
Stage 2B: Localized tumor with or without complete gross excision, with ipsilateral nonadherent lymph nodes positive for tumor. Enlarged contralateral lymph nodes must be negative microscopically
Stage 3: Unresectable unilateral tumor infiltrating across the midline, with or without regional lymph node involvement; or localized unilateral tumor with contralateral region lymph node involvement; or midline tumor with bilateral extension by infiltration (unresectable) or by lymph node involvement
Stage 4: Any primary tumor with dissemination to distant lymph nodes, bone, bone marrow, liver, skin and/or other organs (except as defined in stage 4S)
Stage 4S: Localized primary tumor (as defined in Stage 1, 2A, or 2B) with dissemination limited to skin, liver, and/or bone marrow (limited to infants age <1 year). In previous staging of neuroblastoma it was stated that stage 4S manifests without radiographic evidence of bone metastases on complete skeletal survey [3].
Age distribution of neuroblastoma is as follows: 40% of patients are younger than 1 year when diagnosed, 35% are aged 1–2 years, and 25% are older than 2 years when diagnosed [4]. According to Surveillance, Epidemiology, and End Report (SEER), incidence decreases every consecutive year up to age 10 years, after which the disease is rare. The male-to female ratio is 1.2:1 and the incidence of neuroblastoma is slightly higher in white children than in black children.
Stage 4s neuroblastoma represents approximately 7–10% of all neuroblastoma cases [5]. Its hallmark is the possibility of spontaneous regression despite a large tumor burden, and an overall good prognosis [6].
Shimada classification of histopathologic features was developed for patients with neuroblastoma [7]. Important features of the classification include (1) the degree of neuroblast differentiation, (2) the presence or absence of Schwannian stromal development (stroma-rich, stroma-poor), (3) the index of cellular proliferation, (4) nodular pattern, and (5) age. Using these components, patients can be classified into favorable and unfavorable histology groups.
Clinical & Imaging findings
Clinical manifestations can vary according to the organ involved. At presentation, the infants can be tachypneic, pale, and lethargic. The abdomen is usually distended and hepatomegaly may be the sole clinical finding. Associated skin findings of stage 4S disease include non-tender, bluish ‘blueberry muffin’ subcutaneous nodules [8]. Urinary catecholamines vanillylmandelic acid (VMA) and homovanillic acid (HVA) are usually elevated. If the liver is involved, serum lactate dehydrogenase (LDH) and liver enzymes may be elevated.
I-123-metaiodobenzylguanidine (MIBG) was developed in the early 1980s to visualize tumors of the adrenal medulla.
MIBG enters the neuroendocrine cells by an active uptake mechanism via the epinephrine transporter and is stored in the neurosecretory granules, resulting in a specific concentration in contrast to cells of other tissues. MIBG scintigraphy is used to image tumors of neuroendocrine origin, particularly pheochromocytomas, paragangliomas and neuroblastomas, although other neuroendocrine tumors such as carcinoids and medullary thyroid carcinoma can be visualized.
I-123-metaiodobenzylguanidine (MIBG) is an indispensable tool in evaluating neuroblastoma, both at initial staging and in assessment of subsequent tumor response to therapy [9]. There is a physiologic MIBG uptake by the liver. Here, we present a case in which lack of a clearly identifiable adrenal mass on imaging led to misinterpretation of diffuse MIBG accumulation within the liver. Also, the suspicion for neuroblastoma on her ultrasound and MR was low given the normal size and appearance of her adrenals. Majority of reported cases of the 4S subtype of neuroblastoma are newborns with an associated small, localized primary adrenal mass, mostly solid, and rarely (<2% cases) cystic [10]. In this context, our patient presented an interesting dilemma. Diffuse hepatic uptake of MIBG in context of absence of an associated adrenal lesion led to this being interpreted as a false-negative finding related to normal tracer biodistribution, since liver is a site of catecholamine degradation. However, the subsequent biopsy did confirm metastatic liver involvement by neuroblastic cells. Hepatic metastatic involvement by neuroblastoma on MR presents either as a diffusely infiltrative pattern (seen in infants with stage 4S disease), or focal masses [11]. Sonographic involvement of the liver present as coarse diffuse increased echogenicity or nodular echogenic appearance. This case presented these typical findings.
Our patient presented with a variant of Stage 4s subtype of neuroblastoma, in whom manifestations of the disease included a diffusely involved liver (Pepper syndrome), with associated respiratory compromise and coagulopathy [12]. Also, there was no identifiable adrenal mass, either on prenatal or postnatal evaluation. She had favorable histology by the Shimada classification, and no evidence of bone marrow infiltration by neuroblastic cells. 4S neuroblastoma tumors are generally considered low risk and have an excellent prognosis. These may spontaneously regress.
Differential Diagnoses
In case of an adrenal mass the differential diagnosis may include neuroblastoma and other masses that originate from the adrenal gland or the surroundings, such as Mesoblastic nephroma, Wilms’ tumor, Rhabdomyosarcoma, Rhabdoid tumor, adrenal cortical carcinoma, adrenal hemorrhage, and adrenal infection such as an abscess. In our case of neuroblastoma 4s with an absence of an adrenal mass, the differential diagnosis is more intriguing as one needs to consider the disease and evaluate the case with I-123-MIBG scintigraphy and urinary VMA and HVA. The differential diagnosis of our case with liver involvement includes any infiltrative or focal primary or metastatic liver disease, such as hepatoblastoma, leukemia, lymphoma, hemangioblastomatosis, teratoma, and infections.
Neuroblastoma can be differentiated from other tumors with I-123-MIBG scintigraphy and urinary VMA and HVA. Cross sectional images are nonspecific and the differential diagnosis based on imaging is difficult. However, epicenter of a tumor based on imaging and different tumor markers may help in narrowing the differential. Tissue samples are essential in establishing the final diagnosis.
Treatment & Prognosis
Treatment for neuroblastoma varies and depends on the stage of the disease. It may include surgery, radiation therapy, chemotherapy or any combination of these. The outcome observed with the different successive treatment approaches suggests that if infants with stage 4s neuroblastoma do require therapy, a more intensive chemotherapy may be more beneficial [5]. In one study a more intensive treatment by CE regimen (carboplatin and etoposide), only two chemotherapy courses were required. Results of studies suggest that a prompt initiation of a more intensive treatment may be necessary in order to push the neuroblastic cells towards the regression pathway [5]. The CE regimen proved to be associated with a high response rate and good clinical tolerance when used as second-line or as first-line therapy, a schedule which has also shown good response rates and clinical tolerance in infants with localized, unresectable neuroblastoma [13]. The CE regimen is now being proposed as first-line therapy in the NB99 Infant SIOP study for patients with stage 4s neuroblastoma who do require medical intervention. Stage 4s neuroblastoma is associated with an excellent survival rate and with overall survival of 88% [5, 14].
The presented case is unique in a sense that a neuroblastoma 4s was diagnosed and confirmed pathologically without any evidence for associated adrenal tumor by imaging.
TEACHING POINT
A case of pathologically proven, neonatal neuroblastoma 4s with diffuse hepatic metastatic involvement at presentation and without adrenal mass is extremely rare. The initial evaluation may lead to erroneous interpretation. Diffuse hepatic uptake of MIBG in context of absence of an associated adrenal lesion may be interpreted as a false-negative finding related to normal tracer biodistribution, since liver is a site of catecholamine degradation.
Table 1.
Summary table for neuroblastoma stage 4s.
| Etiology | Unknown. Thought to be a failure of neuroblasts to mature. Neuroblasts continue to grow and divide. |
| Incidence | Neuroblastoma – 7.8% of childhood cancers. For neuroblastoma 4s – 7 to 10% of all neuroblastomas, |
| Gender Ratio | Male to female ratio of 1.2:1 |
| Age Predilection | Neuroblastoma – below 5 years; For Neuroblastoma 4s – below 1 year. |
| Risk factors | Sporadic. Probably unknown gene changes. There are genetic markers that can predict prognosis and guide treatment. |
| Treatment | Vary according to stage and presentation. Most preferably for stage 4s is chemotherapy with CE regimen. |
| Prognosis | Excellent. Overall survival rate of 88% |
Table 2.
Differential diagnosis table for neuroblastoma.
| Diagnosis | Ultrasound | CT | MRI | Scintigraphy |
|---|---|---|---|---|
| Neuroblastoma | Heterogeneous solid lesion, mostly echogenic. | Large, heterogeneous, lobulated soft- tissue masses. Encasing vessels. 90% calcified. Heterogeneous or little enhancement. |
T1-Hypointense. T2-Hyperintense. DWI-restricted diffusion. |
I-123-MIBG avid. I-123-MIBG is superior to Tc99m-MDP. |
| Wilms’ Tumor | Heterogeneous soft tissue echotexture. | Heterogeneous soft-tissue density masses with infrequent areas of calcification. Displacing vessels. Patchy enhancement and allows for better delineation of the relationship between the mass and kidney. |
T1-Hypointense. T2-Hyperintense. DWI-restricted diffusion. |
Tc-99m DMSA to assess the volume of functioning renal tissue. |
| Mesoblastic Nephroma | Well-defined mass with low-level homogeneous echoes. | Solid hypoattenuating renal lesion. Variable enhancement. |
T1-Hypointense. T2-Variable. DWI-Restricted diffusion. |
Does not play a role in diagnosis. |
| Rhabdoid tumor | Heterogeneous echotexture. | Lobulated with individual lobules separated by intervening areas of decreased attenuation, relating to either previous hemorrhage or necrosis. Calcifications may be seen. Heterogeneous enhancement. |
T1-Variable. T2-Variable. |
Does not play a role in diagnosis. |
| Adrenal Hemorrhage | At early stage -diffuse or inhomogeneous echogenicity. Later liquefies and becomes heterogeneously anechoic. | Round or oval, often with surrounding stranding of the periadrenal fat. Early stage – hyperattenuating. Later liquefies with variable attenuation. Calcifications may be seen. Thin and somewhat uniform or heterogeneous and irregular enhancement. |
Acute stage: T1-isointense or slightly hypointense. T2- markedly hypointense. Subacute stage: Hyperintense on T1 and T2. Chronic stage: hypointense rim is present on T1 and T2. |
Photon deficient. |
| Rhabdomyosarcoma | Heterogeneous well-defined irregular mass of low to medium echogenicity. | Soft tissue density. Some enhancement. |
T1-Low to intermediate intensity. T2-Hyperintensity DWI-restricted diffusion. |
Tc99m-MDP and Gallium-67 may be useful for diagnosis. |
| Hepatoblastoma | Variable echogenicity is common. | Heterogeneous mass, which is usually hypoattenuating compared to surrounding liver. Heterogeneous enhancement. |
T1-Hypointense. T2-Hyperintense. |
Photopenic regions following either Tc-99m SC or IDA agents. |
| Leukemia | Heterogeneous echogenicity. | Hepatosplenomegaly. Lymphadenopathy. Heterogeneous enhancement. |
T1-Hypointense. T2-Hyperintense. |
Does not play a role in diagnosis. |
| Lymphoma | Heterogeneous soft tissue masses. | Lymphadenopathy in different sites. Heterogeneous enhancement. |
T1-Hypointense. T2-Hyperintense. |
Does not play a role in diagnosis. |
| Hemangioma | Increased echogenicity. | Variable attenuation. Centripetal enhancement. |
T1-Hypointense T2-Heterogeneous. |
Does not play a role in diagnosis. |
| Teratoma | Echogenicity due to fat and calcifications. | Mass that contains fat and calcifications. Heterogeneous enhancement. |
Fat and/or calcifications in different sequences. | Does not play a role in diagnosis. |
| Abscess | Heterogeneous mass. | Heterogeneous mass containing air. Heterogeneous rim enhancement. |
T1-Hypointense. T2-Hyperintense. |
Scintigraphy with labeled leukocytes. |
ABBREVIATIONS
- CE
Carboplatin and etoposide
- DMSA
Dimercaptosuccinic acid
- DTPA
Diethylene-triamine-pentacetic acid
- FDG
Fluorodeoxyglucose
- FIESTA
Fast Imaging Employing Steady-state Acquisition
- INSS
International Neuroblastoma Staging System
- INRC
International Neuroblastoma Response Criteria
- MDP
Methylene diphosphonate.
- MIBG
Metaiodobenzylguanidine
- MRI
Magnetic Resonance Imaging
- SFOP
French Society of Pediatric Oncology
- SIOP
International Collaboration for Neuroblastoma Research
- SSFSE
Single Shot Fast Spin Echo
REFERENCES
- 1.Brodeur GM, Pritchard J, Berthold F, Carlson NLT, Castel V, Castleberry RP, et al. for the INSS/INRC. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Onco. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 2.van Noesel MM, Hählen K, Hakvoort-Cammel FG, Egeler RM. Neuroblastoma 4S: a heterogeneous disease with variable risk factors and treatment strategies. Cancer. 1997 Sep 1;80(5):834–43. [PubMed] [Google Scholar]
- 3.Evans AE, D’Angio GJ, Randolph JA. A proposed staging system for children with neuroblastoma. Children’s Cancer Study Group. Cancer. 1971;27:374–8. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. SEER Pediatric Monograph. National Cancer Institute; [Accessed: February 25, 2002]. Available at http://www.seer.ims.nci.nih.gov/Publications/PedMono/sympathetic.pdf. [Google Scholar]
- 5.Schleiermacher G, Rubie H, Hartmann O, Bergeron C, Chastagner P, Mechinaud F, Michon J Neuroblastoma Study Group of the French Society of Paediatric Oncology. Treatment of stage 4s neuroblastomareport of 10 years’ experience of the French Society of Paediatric Oncology (SFOP) Br J Cancer. 2003 Aug 4;89(3):470–6. doi: 10.1038/sj.bjc.6601154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pritchard J, Hickman JA. Why does stage 4s neuroblastoma regress spontaneously. Lancet. 1994;344:869–870. doi: 10.1016/s0140-6736(94)92834-7. [DOI] [PubMed] [Google Scholar]
- 7.Shimada H, Chatten J, Newton WA, Jr, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug;73(2):405–16. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JP, Tweddle DA. Neonatal Neuroblastoma. Semin Fetal Neonatal Med. 2012 Aug;17(4):207–15. doi: 10.1016/j.siny.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008 Dec;52(4):388–402. [PubMed] [Google Scholar]
- 10.Avanzini S, Conte M, Granata C, Zamorani EM, Sementa AR, Garaventa A, Buffa P, Sorrentino S. Life-threatening bilateral adrenal cystic neuroblastoma in an infant. J Pediatr Hematol Oncol. 2009 Dec;31(12):963–4. doi: 10.1097/MPH.0b013e3181b79641. [DOI] [PubMed] [Google Scholar]
- 11.Nour-Eldin NE, Abdelmonem O, Tawfik AM, Naguib NN, Klingebiel T, Rolle U, Schwabe D, Harth M, Eltoukhy MM, Vogl TJ. Pediatric primary and metastatic neuroblastoma: MRI findings: pictorial review. Magn Reson Imaging. 2012 Sep;30(7):893–906. doi: 10.1016/j.mri.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Levitt GA, Platt KA, De Byrne R, Sebire N, Owens CM. 4S neuroblastoma: the long-term outcome. Pediatr Blood Cancer. 2004 Aug;43(2):120–5. doi: 10.1002/pbc.20067. [DOI] [PubMed] [Google Scholar]
- 13.Rubie H, Plantaz D, Coze C, Michon J, Frappaz D, Baranzelli MC, Chastagner P, Peyroulet MC, Hartmann O Neuroblastoma Study Group, Societe Francaise d’Oncologie Pediatrique Localised and unresectable neuroblastoma in infants: excellent outcome with primary chemotherapy. Neuroblastoma Study Group, Société Française d’Oncologie Pédiatrique. Med Pediatr Oncol. 2001;36:247–250. doi: 10.1002/1096-911X(20010101)36:1<247::AID-MPO1061>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Nickerson HJ, Matthay KK, Seeger RC, Brodeur GM, Shimada H, Perez C, Atkinson JB, Selch M, Gerbing RB, Stram DO, Lukens J. Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: a Children’s Cancer Group study. J Clin Oncol. 2000;18:477–486. doi: 10.1200/JCO.2000.18.3.477. [DOI] [PubMed] [Google Scholar]