Abstract
Objective:
To identify whether or not the same ultrasound features can be applied and should be considered to support the decision as to which subcentimeter nodules should be biopsied with fine needle aspiration (FNAB).
Methods:
Single-institution, IRB approved, retrospective study conducted from 2008 to 2016 that evaluated 1094 thyroid nodules smaller than 1.0 cm that were classified according to TIRADS and submitted for FNAB.
Results:
The value of FNAB of thyroid nodules smaller than 1.0 cm were assessed and correlated with the sonographic criteria by comparing the obtained results with the cytological findings in 1094 thyroid nodules. In the analysis considering all nodules, the proportion of malignancies among nodules with TIRADS 2 is 0.91% and for TIRADS 3 is 2.87%. Among those classified as 4A, 12.26%; with 4B classification, 34.43%; with 4C classification, 66.6%; and among those with 5 classifications, 85.7%.
Conclusion:
In conclusion, the TIRADS classification system, based on the sonographic features reported herein, may help detect which nodules should be investigated for potential malignancies.
Advances in knowledge:
Few reports compare the efficacy of ultrasound-FNAB for thyroid nodules smaller than 1.0 cm in diameter. The findings of malignancy in this subgroup of nodules may help in the clinical follow-up of which patients should be submitted to an early imaging evaluation or intervention.
Introduction
Over the last decade, the incidence of thyroid nodules has reached 70% of the general adult population and thyroid cancer incidence increased more than 100%.1 Almost 50% of these cancers consist of carcinomas smaller than 1.0 cm in diameter (microcarcinomas).1–11
Increased detection of subcentimeter nodules is most likely due to the use of higher resolution ultrasound, whereas detection of microcarcinomas was augmented mainly because of pathological confirmation by fine needle aspiration biopsy (FNAB).1–3,9
On the basis of ultrasound features, thyroid nodules may be categorized into three groups: low, intermediate and high malignancy risk. FNAB should be considered for nodules ≤10 mm diameter only when suspicious signs are present, while nodules ≤5 mm should be monitored rather than biopsied.
In 2016, the American Association of Clinical Endocrinologists recommend ultrasound-FNAB of nodules >0.5 cm with suspicious sonographic features and of nodules > 1.0 cm with other specific conditions, while nodules <5 mm should be monitored.12 In the Ultrasound Consensus Statement, the Society of Radiologists stated that ultrasound features that are associated with thyroid cancer include microcalcifications and a solid component.3, 11,13
Several ultrasound characteristics have been studied as potential predictors of thyroid malignancy. Recently, some reports have proposed a malignancy risk classification of thyroid nodules based on ultrasonographic features—Thyroid Imaging Reporting and Data System (TIRADS) showing the risk of malignancy in each category.14–16 These classification systems are based on the already established Breast Imaging Reporting and Data System (BI-RADS) which was designed to help improve and standardize breast imaging reports. It also helped to simplify the management of patients with breast lesions.17 It was recently reported that as the number of suspicious ultrasound features increases, the probability and risk of malignancy also increases. These studies also demonstrated that the US features associated with increased risk of malignancy were: presence of solid component, hypoechogenicity, marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape.18
A practical TIRADS classification to categorize thyroid nodules and stratify their malignancy risk19 was expressed with scores ranging from 1 to 5: TIRADS 1 corresponds to a normal gland, TIRADS 2 to a benign nodule (both with 0% malignancy), and TIRADS 3 to a highly probable benign nodule (<5% malignancy), TIRADS 4 (5 to 80% malignancy) and 5 (>80% malignancy) to suspicious nodule (TIRADS 4A, 4B and 4C corresponding to low, intermediate and moderate suspicion for malignancy, respectively and TIRADS 5 to a high suspicion for malignancy) with risk ranging from 5 to 80% of malignancy. Colour Doppler data were not used because of its poor reproducibility, as well as its interoperator and inter device variability.20, 21
The few reports that compared the efficacy of ultrasound-FNAB for thyroid nodules smaller than 0.5 cm in diameter have reached differing conclusions.9, 10 Moreover, none of these studies offered a correlation between the biopsied samples and their sonographic parameters and cytological findings. In a previous study including nodules larger and smaller than 1 cm, a correlation was demonstrated between the ultrasonographic findings of irregular margins, hypoechogenicity, marked hypoechogenicity, morphology taller than wide, microcalcifications and the risk of malignancy in the cytopathological analysis.22 The purpose of this study is to identify whether the same ultrasound features can be applied and should be considered to support the decision as to which subcentimeter nodules should be biopsied with FNAB. We also hope to validate the accuracy of biopsying small thyroid nodules.
Methods and materials
Patients
Through a review of our medical database from 2008 to 2016, we retrospectively evaluated 1116 thyroid nodules obtained from 951 patients (age range, 15–87 years; mean, 47) that were referred to the interventional radiology department of our hospital by the attending physician to perform ultrasound-FNAB of nodules smaller than 1.0 cm, regardless of the nodule characteristics. Ultrasonographic reports and images of the biopsy thyroid nodules stored in the database of our hospital were used for the analysis. Cases where the nodules were documented with at least two incidents were considered (there was no minimum number of images per case).
The ultrasound report structure of the nodules that was analysed included the characteristics of echogenicity, presence of calcification, margin delimitation and 3-dimension nodule measurements. Although the Doppler pattern was present in the ultrasound reports of our hospital, this characteristic was not considered in the analysis because its large-scale reproducibility is compromised due to inter-examiner variability and differences seen between devices.23
We excluded from the analysis cases in which the nodules imaging were recorded in only one incidence or that the quality of the image was not adequate (patient with extreme obesity, causing attenuation of the acoustic beam). Patients’ thyroid ultrasound were evaluated and classified according to TIRADS22 (Tables 1 and 2) without prior knowledge of the cytological results (Bethesda) by 10 experienced radiologists. The following characteristics considered benign related to TIRADS 2 were cystic lesion or spongiform lesion. The probably benign characteristics related TIRADS 3 were hyperechogenic or isoechoic lesions with regular margins and without characteristics of malignancy. Features considered suspicious for malignancy were irregular margins, hypoechogenicity, marked hypoechogenicity, morphology taller than wide and microcalcifications. Of these, 22 nodules (in 20 patients) were excluded from the analysis due to the imaging or report exclusion criteria. The results were then paired with cytopathological analysis.
Table 1.
Sonographic features considered suspicious for malignancy
| Ultrasound features | Points |
| Irregular margins | 1 |
| Hypoechoic nodule | 1 |
| Markedly hypoechoic nodulea | 2 |
| Taller than wide | 1 |
| Microcalcifications | 1 |
aDefined as solid nodules, without further reinforcement or spots, with areas of greatest hypoechogenicity within the own node or in relation to other hypoechoic areas of the thyroid gland.
Table 2.
TIRADS classification
| Tirads | Definition | Ultrasound features |
| 1 | Negative | Normal thyroid |
| 2 | Benign | Benign features |
| 3 | Probably benign | Without suspicious features |
| 4 A | Low suspicion | One suspicion feature |
| 4 B | Intermediate suspicion | Two suspicion feature |
| 4C | Moderate suspicion | Three or four suspicion feature |
| 5 | High suspicion | Five suspicion feature |
| 6 | Known proved malignancy | Confirmed malignancy |
Sonographic analyses were performed using the Philips HDI 5000 device, iU22 Philips (Royal Philips Electronics, Amsterdam, Netherlands), Aplio 500 Platinum (Toshiba American Medical Systems, Tustin, CA) and My Lab 75 (Esaote, Genova, IT) all with high frequency transducers (up to 11 MHz).
Nodules were divided into two groups: smaller than or equal to 0.5 cm (ranging from 0.2 to 0.5 cm—Group A) and between 0.6 and 1.0 cm (Group B). The sonographic parameters considered to be associated to higher malignancy risks were: margins (irregular), echogenicity (hypoechogenicity or marked hypoechogenicity), morphology (taller than wide) and presence of microcalcifications.
All FNAB were performed by 1 of 10 experienced interventional radiologists (>5 years of experience). Nodule samples were obtained by fine needle aspiration (23G) under ultrasound guidance and local anaesthesia using Xylestesin® (lidocaine hydrochloride 2.0%). FNAB was performed by freehand technique: a 23-gauge needle attached to a 20-cc syringe. During nodule aspiration, a negative pressure was continuously maintained until blood appeared in the hub of the syringe. Transversal of thyroid vessels was avoided to prevent local bleeding. In mixed nodules, solid areas were punctured. A pathologist was present during all biopsy procedures to assess the quality of cytological specimens.
Cytological examination
FNAB samples were dispersed on slides and fixed by drying. These slides were stained using haematoxylin and eosin and evaluated by doctors from the pathology department of our hospital. The cytological material was classified according to Bethesda classification,20 in categories from I to VI. Only those nodules classified in category II (benign), IV, V and VI (suspicious of malignancy or malignancy) were considered. Nodules classified in category III (atypia/follicular lesion of undetermined significance) were not defined as benign or malignant, but were considered in the analysis. The nodules classified in categories I (non-diagnostic) were excluded from our statistical analysis. The hormonal profile was not considered in data analysis.
Statistical analysis
Correlation between cytological behaviour samples and ultrasonographic characteristics of individual nodules was performed using statistical program SPSS v. 20.0 (SPSS Inc., Chicago, IL) with Spearman correlation or χ2. A value of p < 0.05 was considered statistically significant.
The value of FNAB of thyroid nodules smaller than 1.0 cm were assessed and correlated with the sonographic criteria by comparing the obtained results with the cytological findings.
Ethical statement
The research ethics committee of our institution approved this study and waived the requirement of informed consent for the retrospective assessment of this work.
Results
A total of 1116 nodes were evaluated, 22 were excluded (due to imaging and reporting criteria) and 1094 were analysed. The 1094 FNAB thyroid nodules were divided into two groups: 272 nodules smaller than or equal to 0.5 cm (Group A) and 822 nodules between 0.6 and 1.0 cm (Group B). (Table 3) Cytological analysis categorized each nodule according to the Bethesda classification system. 35 nodules were excluded because Bethesda I cytological classification system: 16 from group A and 19 from group B.
Table 3.
Distribution of nodules according to size
| Group A | N (%) |
| 0.2 cm | 4 (1.5%) |
| 0.3 cm | 29 (10.7%) |
| 0.4 cm | 87 (32%) |
| 0.5 cm | 152 (55.8%) |
| Group B | |
| 0.6 cm | 161 (19.6%) |
| 0.7 cm | 176 (21.4%) |
| 0.8 cm | 177 (21.6%) |
| 0.9 cm | 151 (18.3%) |
| 1.0 cm | 157 (19.1%) |
The average number of FNAB passes was 3 (range, from 1 to 5). The cytological results revealed 35 nodules (3.2%) Bethesda I, 847 nodules (77.4%) Bethesda II, 68 nodules (6.2%) Bethesda III, 20 nodules (1.8%) Bethesda IV, 27 nodules (2.5%) Bethesda V and 97 nodules (8.9%) Bethesda VI. Only 13.2% of the nodules were categorized as suspicious or malignant (categories IV, and V, and VI, respectively).
The number of malignant results was slightly larger in males than in females (13.7 and 12.4%, respectively) but without statistical significance (p = 0.58). Malignancy rates were similar between group A and group B (n = 35; 13.7% and n = 109; 13.6%, respectively, p = 0.968).
The distribution of the TIRADS classification was: TIRADS 2 (n = 109, 10%), TIRADS 3 (n = 418, 38.5%), TIRADS 4A (n = 367, 33.2%), TIRADS 4B (n = 151, 13.8%), TIRADS 4C (n = 42, 3.8%) and TIRADS 5 (n = 7, 0.7%). In the analysis considering all nodules the proportion of malignancies among nodules with TIRADS 2 is 0.91% (n = 1); TIRADS 3, 2.87% (n = 12); TIRADS 4A, 12.26% (n = 45); TIRADS 4B, 34.43% (n = 52); TIRADS 4C, 66.6% (n = 28) and TIRADS 5, 85.7% (n = 6).
TIRADS and Bethesda criteria were correlated by Spearman analysis yielding a positive statically significant correlation (Table 4) demonstrating that with the increase of suspicious features in the nodule, the malignancy rate associated with it also increases.
Table 4.
Cross-tablation of TIRADS classification and the Bethesda classification for the cytological material
| TIRADS | Bethesda | Total | r (p) | ||||||||||
| 2 | 3 | 4 | 5 | 6 | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| 2 | 104 | 9.8 | 1 | 0.1 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 109 | 10.0 | 0,413 (<0,001) |
| 3 | 376 | 35.5 | 20 | 1.9 | 5 | 0.5 | 1 | 0.1 | 6 | 0.6 | 418 | 38.5 | |
| 4A | 274 | 25.9 | 33 | 3.1 | 3 | 0.3 | 9 | 0.8 | 33 | 3.1 | 367 | 33.2 | |
| 4B | 83 | 7.8 | 11 | 1.0 | 8 | 0.8 | 11 | 1.0 | 33 | 3.1 | 151 | 13.8 | |
| 4C | 9 | 0.8 | 3 | 0.3 | 3 | 0.3 | 5 | 0.5 | 20 | 1.9 | 42 | 3.8 | |
| 5 | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | 5 | 0.5 | 7 | 0.7 | |
| Total | 847 | 80.0 | 68 | 6.4 | 20 | 1.9 | 27 | 2.5 | 97 | 9.2 | 1094 | 100 | |
TIRADS, ThyroidImaging Reporting and Data System. Spearman correlation.
In the analysis of malignancy and benignity, the nodules that presented with cytological result Bethesda III were excluded (n = 68, group A = 11 nodules group B = 57 nodules), since this result is considered indeterminate (limitation of the method). These patients underwent a follow-up puncture between 3 and 6 months, but the results of the new puncture were not included in the present analysis.
Discussion
Cytological results revealed a rate of 13.2% of malignancy (Bethesda V and VI) or suspicious (Bethesda IV) for the subcentimeter nodules analysed (those categorized as Bethesda IV, V and VI), with similar results when compared with nodules smaller than 0.5 cm to nodules between 0.5–1 cm in diameter. These data suggest that, in the case of subcentimeter nodules, size alone is not a good predictor of malignancy. This observation is consistent with findings reported by Sharma et al24, which states that subcentimeter nodules are significantly associated with the risk of malignancy and certain ultrasonographic features may be used in risk stratification.
Some sonographic criteria such as presence of microcalcification, solid composition, and margins are important to define nodules <1.0 cm as potentially malignant and indicate for biopsy. Our results reveal that these characteristics as well as echotexture should be considered when analysing subcentimeter thyroid nodules, a finding that contradicts some opinions.7
Mazzaferri et al25 suggest that nodules smaller than 5 mm, even in the presence of ultrasonographic findings suspected of malignancy, should not be submitted to FNAB, since it increases the patient's anxiety and there is a high rate of inconclusive cytology associated with these nodules. Our experience was similar from that reported by Kwak et al with the malignancy of 66.6% in TIRADS 4c nodules and 85.7% in TIRADS 5. The low rate of malignancy in TIRADS 3 nodules (2.87%), 4a (12.26%) and 4b (34.43%) is also similar to that which is already reported.13 The effect of the size on the accuracy of TIRADS has not been investigated in depth. Moon et al26 suggest that despite increasing the proportion of inadequate FNAB samples with reduced nodule size, FNAB demonstrated good diagnostic accuracy in nodules smaller than 1 cm.
Despite the retrospective nature of this study our findings are still relevant because they reveal that subcentimeter thyroid nodules should be evaluated according to the sonographic criteria used to analyse larger lesions.
This study had several limitations including, retrospective design, sonographic assessment performed by different operators, use of cytology data instead of pathological data despite high sensitivity and specificity of cytology, and the lack of uniformity of criteria establishing the indication for biopsy of thyroid nodules.
Conclusion
In conclusion, the TIRADS classification system, based on the sonographic features reported herein, may help detect which nodules should be investigated for potential malignancies. Despite the over diagnosis of thyroid cancer due to FNAB of nodules smaller than 10 mm, the findings of malignancy in this subgroup of nodules may help in the clinical follow-up of which patients should be submitted to an early imaging evaluation or intervention.
Figure 1.
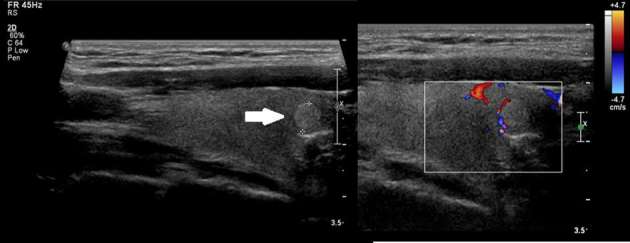
Hyperechoic nodule in the right thyroid lobe, with well-defined margins (arrow) and peripheral Doppler flow. TIRADS: 3. Cytology: Colloid goiter (Classification II Bethesda).
Figure 2.
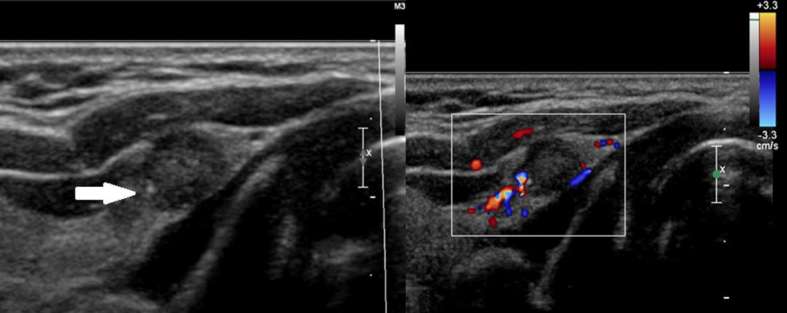
Hypoechoic nodule of 0.8 cm in the transition between the isthmus and the right thyroid lobe, with few microcalcifications (arrow) and without flow Doppler. TIRADS: 4B. Result: Suspicious for follicular neoplasm (Classification IV of Bethesda). TIRADS,Thyroid Imaging Reporting and Data System.
Figure 3.
(a) Longitudinal image shows anodule measuring 0.4 cm, markedly hypoechoic with irregular contours. TIRADS 4C. (b) Result: papillary carcinoma (Classification VI Bethesda).
Contributor Information
Guilherme F Mendes, Email: guilfmendes@gmail.com.
Marcio RT Garcia, Email: mrtgarcia@gmail.com.
Priscila M Falsarella, Email: primina@gmail.com.
Antonio Rahal, Email: antoniorahal@yahoo.com.br.
Francisco A Cavalcante Junior, Email: facj2014@gmail.com.
Daniela R Nery, Email: daniela_nery@hotmail.com.
Rodrigo G Garcia, Email: rodrigo.gobbo@einstein.br.
REFERENCES
- 1.Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg 2003; 237: 399–407. doi: https://doi.org/10.1097/01.SLA.0000055273.58908.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomimori EK, Bisi H, Medeiros-Neto G, Camargo RYAde. Avaliação ultra-sonográfica dos nódulos tireóideos: comparação com exame citológico e histopatológico. Arquivos Brasileiros de Endocrinologia & Metabologia 2004; 48: 105–13. doi: https://doi.org/10.1590/S0004-27302004000100012 [DOI] [PubMed] [Google Scholar]
- 3.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002; 178: 687–91. doi: https://doi.org/10.2214/ajr.178.3.1780687 [DOI] [PubMed] [Google Scholar]
- 4.Shimura H, Haraguchi K, Hiejima Y, Fukunari N, Fujimoto Y, Katagiri M, et al. Distinct diagnostic criteria for ultrasonographic examination of papillary thyroid carcinoma: a multicenter study. Thyroid 2005; 15: 251–8. doi: https://doi.org/10.1089/thy.2005.15.251 [DOI] [PubMed] [Google Scholar]
- 5.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol 2004; 60: 21–8. doi: https://doi.org/10.1046/j.1365-2265.2003.01912.x [DOI] [PubMed] [Google Scholar]
- 6.Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid 2009; 19: 27–31. doi: https://doi.org/10.1089/thy.2008.0106 [DOI] [PubMed] [Google Scholar]
- 7.Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab 1999; 84: 24–8. doi: https://doi.org/10.1210/jcem.84.1.5418 [DOI] [PubMed] [Google Scholar]
- 8.Berker D, Aydin Y, Ustun I, Gul K, Tutuncu Y, Işik S, et al. The value of fine-needle aspiration biopsy in subcentimeter thyroid nodules. Thyroid 2008; 18: 603–8. doi: https://doi.org/10.1089/thy.2007.0313 [DOI] [PubMed] [Google Scholar]
- 9.Lee NS, Bae JS, Jeong S-R, Jung CK, Lim DJ, Park WC, et al. Risk factors of lymph node metastasis in papillary thyroid microcarcinoma. J Korean Surg Soc 2010; 78: 82–6. doi: https://doi.org/10.4174/jkss.2010.78.2.82 [Google Scholar]
- 10.Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg 2008; 32: 747–53. doi: https://doi.org/10.1007/s00268-007-9453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SJ, Kim EK, Park CS, Chung WY, Oh KK, Yoo HS. Ultrasound-guided fine-needle aspiration biopsy in nonpalpable thyroid nodules: is it useful in infracentimetric nodules? Yonsei Med J 2003; 44: 635–40. doi: https://doi.org/10.3349/ymj.2003.44.4.635 [DOI] [PubMed] [Google Scholar]
- 12.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. American Association of Clinical Endocrimologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocrine Practice 2016; 22(Suppl 1): 1–60. doi: https://doi.org/10.4158/EP161208.GL [DOI] [PubMed] [Google Scholar]
- 13.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: society of radiologists in ultrasound consensus conference statement. Radiology 2005; 237: 794–800. doi: https://doi.org/10.1148/radiol.2373050220 [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid 2009; 19: 1257–64. doi: https://doi.org/10.1089/thy.2008.0021 [DOI] [PubMed] [Google Scholar]
- 15.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab 2009; 94: 1748–51. doi: https://doi.org/10.1210/jc.2008-1724 [DOI] [PubMed] [Google Scholar]
- 16.Hambly NM, Gonen M, Gerst SR, Li D, Jia X, Mironov S, et al. Implementation of evidence-based guidelines for thyroid nodule biopsy: a model for establishment of practice standards. AJR Am J Roentgenol 2011; 196: 655–60. doi: https://doi.org/10.2214/AJR.10.4577 [DOI] [PubMed] [Google Scholar]
- 17. American College of Radiology. Breast imaging reporting and data system, breast imaging atlas. 4th ed Reston, VA: The British Institute of Radiology.; 2003. [Google Scholar]
- 18.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 2011; 260: 892–9. doi: https://doi.org/10.1148/radiol.11110206 [DOI] [PubMed] [Google Scholar]
- 19.Rago T, Vitti P, Chiovato L, Mazzeo S, De Liperi A, Miccoli P, et al. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in 'cold' thyroid nodules. Eur J Endocrinol 1998; 138: 41–6. doi: https://doi.org/10.1530/eje.0.1380041 [DOI] [PubMed] [Google Scholar]
- 20.Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, et al. Thyroid nodule shape suggests malignancy. Eur J Endocrinol 2006; 155: 27–31. doi: https://doi.org/10.1530/eje.1.02177 [DOI] [PubMed] [Google Scholar]
- 21.Cibas ES, Ali SZ. The bethesda system for reporting thyroid cytopathology. Thyroid 2009; 19: 1159–65. doi: https://doi.org/10.1089/thy.2009.0274 [DOI] [PubMed] [Google Scholar]
- 22.Rahal A, Falsarella PM, Rocha RD, Lima JP, Iani MJ, Vieira FA, et al. Correlation of thyroid imaging reporting and data system [TI-RADS] and fine needle aspiration: experience in 1,000 nodules. Einstein 2016; 14: 119–23. doi: https://doi.org/10.1590/S1679-45082016AO3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faria MASde, Casulari LA. Comparação das classificações dos nódulos de tireoide ao Doppler colorido descritas por Lagalla e Chammas. Arquivos Brasileiros de Endocrinologia & Metabologia 2009; 53: 811–7. doi: https://doi.org/10.1590/S0004-27302009000700004 [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Gabriel H, Nemcek AA, Nayar R, Du H, Nikolaidis P. Subcentimeter thyroid nodules: utility of sonographic characterization and ultrasound-guided needle biopsy. AJR Am J Roentgenol 2011; 197: W1123–W1128. doi: https://doi.org/10.2214/AJR.10.5684 [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferri EL, Sipos J. Should all patients with subcentimeter thyroid nodules undergo fine-needle aspiration biopsy and preoperative neck ultrasonography to define the extent of tumor invasion? Thyroid 2008; 18: 597–602. doi: https://doi.org/10.1089/thy.2008.0100 [DOI] [PubMed] [Google Scholar]
- 26.Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol 2012; 19: 52–9. doi: https://doi.org/10.1245/s10434-011-1813-1 [DOI] [PubMed] [Google Scholar]