Abstract
Objective:
The hypodense sign (HyS) on CT imaging is highly suggestive of pulmonary invasive mould disease (IMD) in patients with haematological malignancies, but its diagnostic utility has not been systematically evaluated on contrast-enhanced CT. The objective of this study was to compare the diagnostic performance of the HyS to other common CT findings in a cohort of haematology patients with proven, probable or possible IMD based on European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria.
Methods:
We analysed the diagnostic performance of the HyS to other common CT signs among 127 neutropenic patients with haematological malignancies submitted to both non-contrast-enhanced and contrast-enhanced CT scans of the lungs, including CT pulmonary angiography.
Results:
The HyS was detected in 15.7% of patients imaged without contrast, and 44.1% after contrast administration. A contrast-aided HyS was detected in 86.6, 78.0 and 15.5% of patients with European Organization for Research and Treatment of Cancer/Mycoses Study Group proven, probable and possible IMD, respectively. When analysed per clinical diagnosis (proven, probable and highly possible IMD—i.e. no alternative diagnosis to mould disease reached), the contrast-enhanced HyS was as sensitive as the halo sign but significantly more specific [halo sign 0.56, 95% CI (0.39–0.71) vs HyS 0.98, 95% CI (0.87–1.00)]. Only the vessel occlusion sign was more sensitive [0.97, 95% CI (0.91–0.99)] and specific [0.97, 95% CI (0.86–0.99)] than the HyS for IMD diagnosis.
Conclusion:
The high specificity of the HyS strongly supports the diagnosis of pulmonary IMD in neutropenic patients, and is highly suggestive breakthrough fungal disease in patients on mould-active antifungal prophylaxis.
Advances in knowledge:
This is the first systematic analysis of the hypodense sign on contrast-enhanced CT; the sign can support the diagnosis of IMD when other CT signs are uncertain.
INTRODUCTION
Opportunistic filamentous fungi such as Aspergillus spp. cause invasive mould disease (IMD) in immunosuppressed individuals as the consequence of inhalation of conidia. Histological characteristics of invasive pulmonary aspergillosis are filamentous growth within the pulmonary parenchyma and angioinvasion of small- to medium-sized pulmonary arteries by fungal hyphae.1, 2 Fungal hyphae are internalized by pneumocytes and endothelial cells, most of them are destroyed and causing endothelial injury and intravascular thrombosis leading to tissue infarction with coagulative necrosis, and occasionally haematogenous dissemination.3–5 Thus, pulmonary infarction with coagulative necrosis is the typical pulmonary lesion of IMD in patients with neutropenia and it consist of a central zone of necrotic parenchymal tissue infected by fungal hyphae and a peripheral zone of alveolar haemorrhage.6 This corresponds to pulmonary nodule with a surrounding haemorrhagic area of ground-glass opacity (the “halosign”), or to pleural-based wedge-shaped haemorrhagic pulmonary infarcts on CT imaging.1, 7,8
In 2008, the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) revised the definitions for IMD for clinical and epidemiological research.9 The definitions proposed three levels of diagnostic probability. A diagnosis of proven IMD is fulfilled if patients have histological evidence of tissue invasion by a fungus. However, this level of diagnostic certainty is infrequently reached in patients with haematological malignancies, as invasive procedures required to collect tissue (bronchoscopy and/or lung biopsy) are often unfeasible in the setting of severe thrombocytopenia. A diagnosis of probable IMD is defined by lung CT exam compatible with IMD in presence of host risk factors for fungal infections (e.g. prolonged neutropenia) and microbiological evidence infection, either a positive respiratory culture or presence of the Aspergillus cell wall antigen galactomannan in serum or bronchoalveolar lavage fluid. Cultures, however, have a relatively poor sensitivity and the galactomannan antigen test is limited in patients receiving certain antifungal prophylaxis (e.g. azoles reduce sensitivity) or antibiotic therapy (e.g. false-positive results in patients receiving piperacillin–tazobactam, a common antipseudomonal antibiotic for febrile neutropenia).10, 11 Patients with host risk factors and suggestive CT findings only, but no microbiological evidence of infection, are considered to have possible IMD. Chest radiography often demonstrates normal or non-specific findings during early phases of infection in up to 50% of patients with invasive aspergillosis, while non-contrast high-resolution CT (HRCT) can, at earlier stages of disease, detect pulmonary infiltrates suggestive of IMD and is frequently used to monitor the response to therapy.12, 13 The radiological criteria of possible pulmonary IMD include HRCT findings of dense, well-circumscribed lesion(s) with or without a halo sign (ground-glass opacity surrounding a pulmonary nodule or mass), air-crescent sign (air in a crescent shape in a nodule or mass) and cavity.9 However, these CT signs are not specific for mould disease and may be associated with other infectious and non-infectious causes.7, 14,15
Consequently, improvements in the sensitivity and specificity of CT imaging are a major unmet need in the management of invasive fungal diseases.
In 2005, Sonnet first proposed the use of contrast-enhanced CT with pulmonary angiography (CTPA) for the diagnosis of pulmonary IMD. The interruption (occlusion) of a vessel at the border of a pulmonary dense lesion, without depiction of the vessel inside the lesion or peripheral to the lesion, was defined as a positive vessel occlusion sign (VOS) and considered indicative of angioinvasion.16 Stanzani and colleagues later demonstrated that VOS documented by CTPA was a more sensitive and specific sign of IMD than other common CT findings in patients with haematological malignancies.17,18
However, CTPA has some technical limitations including breath or motion artefacts, and a low resolution for small (<10–12 mm in diameter) or peripherally-located pulmonary lesions. CTPA also requires additional radiation and administration of iodinated contrast-media, which is only recommended in current treatment guidelines for aspergillosis when lesions surround large vessels.19
The hypodense sign (HyS) is a pulmonary CT findings firstly described by Horger et al in 43 immunocompromised patients submitted to chest HRCT.20 It is a central hypodensity in lung consolidation or nodule imaged by HRCT, corresponding to a central area of necrosis caused by vascular obstruction with secondary lung infarction and sequestration in angioinvasive pulmonary aspergillosis (IPA). The authors found that the HyS was highly specific (100%) for proven or probable IPA, but had a relatively low sensitivity (30%). They also proposed that the HyS may be more readily detected with contrast-enhanced CT scan, and could possibly discriminate fungal from bacterial pneumonia. However, no study to date has systematically evaluated the sensitivity of the HyS for IMD on contrast-enhanced CT, even though hypodense areas in pulmonary lesions are occasionally reported in studies describing CT findings of patients with invasive pulmonary aspergillosis.21,22
The aim of this study was to determine if contrast-enhanced CT examination increases the sensitivity of the HyS for the detection of pulmonary IMD. We also compared the sensitivity and specificity of contrast-enhanced HyS vs other common CT findings of pulmonary IMD.
METHODS AND MATERIALS
Patients
We performed a single-centre, retrospective non-randomized study to compare the sensitivity of non-contrast-enhanced vs contrast-enhanced HyS for the diagnosis of IMD. The study design was approved by the Institutional Research Committees in accordance with principles outlined in the Declaration of Helsinki.
Between May 2008 and April 2015, we identified 804 consecutive patients with haematological malignancies, who had been submitted to unenhanced chest HRCT within 72–120 h from the beginning of the empiric antibiotic therapy for a febrile episode. Most patients had an initial assessment with standard chest radiography, but because of its known limitations in neutropenic patients (normal or nonspecific findings during early phase of infection in up to 50% of patients with IMD),21 all patients subsequently underwent further evaluation with HRCT.
The standard management for febrile episode in neutropenic patient at our institution consists in starting an empirical antibiotic therapy (generally piperacillin/tazobactam ± amikacin) on the first day of fever previous to collection of blood cultures. Test result of serum Aspergillus cell wall antigen galactomannan and biomarkers (reactive C protein, procalcitonin) are taken three times weekly. Mould-active antifungal therapy, typically liposomal amphotericin-B or voriconazole, are started empirically in any patients with radiological suspicion of fungal infection (based on contrast-enhanced HRCT) irrespective of culture or galactomannan results.
Among the 804 consecutive patients undergoing HRCT examination, 127 (15.8%) were submitted to contrast-enhanced CT with CTPA because they met the radiological criteria described below and were included in the study. 100 patients were reported in previous studies on CTPA for diagnosis of pulmonary IMD18 but the diagnostic value of the HyS was not analysed.
Chest CT
All of the 804 patients who underwent an initial HRCT scan were immediately evaluated by a radiologist who proceed to contrast-aided studies (CTPA) if one or more dense well-circumscribed pulmonary lesion(s) >12 mm in diameter, with or without halo sign, were identified (lesion with diameter ≤12 mm or localized in peripheral lung are not exhaustively assessable).17, 18 Patients with lesions having the air-crescent sign or cavitation were excluded, as they develop days to weeks after the early radiographic abnormalities, with recovery from neutropenia.23, 24 Therefore, these signs are absent in the early phase of IMD and are not useful for early diagnosis.7, 23,25
127 patients met the inclusion criteria for CTPA (patients <18 years old and/or with previous reaction to contrast-media and/or at high risk for contrast-media exacerbated acute renal injury were excluded). The CT examinations were performed with a multidetector CT scanner (Lightspeed 16 or VCT 64, GE Milwaukee, WI; Brilliance iCT 128 Philips Healthcare, Cleveland, OH). The CTPA acquisition was performed according the procedure previously described.17, 18 In a subgroup of 37 patients (29.1%), the CTPA (arterial phase scan) was followed by a venous phase scan including the pulmonary lesion for different diagnostic purpose (characterization of mediastinal, pleural, pericardial, systemic vessels and/or liver incidental findings).
The CT images were transferred to a dedicated workstation (Advantage 4.3, GE, Milwaukee, WI) and evaluated with multiplanar reformatting software. All images were reviewed separately by two expert radiologists blinded to the clinical course and patient diagnosis. If disagreement occurred, the radiologists discussed the findings and reached a consensus interpretation. CT images were analysed for the presence of dense nodule or well-circumscribed consolidation, halo sign, VOS, and HyS. The VOS was defined, according to criteria proposed by Sonnet, as an interruption of the vessel at the border of a focal lesion without depiction of the vessel itself inside the lesion.16
The HyS was defined using criteria described by Horger as the presence of a central area of hypodensity assessed using a dedicated narrow window setting [width: 110–140 Hounsfield Units (HU); level: 15–40 HU].20 If at visual evaluation, a certain finding was present at least once in a patient, the patient was considered positive for this finding. We also performed a densitometric analysis in HU. Densitometric values for pulmonary lesions were calculated using CT image analysis workstation software, drawing multiple regions of interest (ROIs) of at least 10 mm2 within the central and the peripheral areas of the lesions. Densitometric evaluation was performed in 98/127 patients (77.2%), excluding lesions too small to place a ROI in the central and/or peripheral zone. The visual evaluation of lesions and densitometric measurement analysis were made on both the unenhanced and contrast-enhanced CT scans as well as adjunctive venous phase scans.
Diagnostic assessment
All patients met EORTC/MSG criteria of possible IMD at the time of the radiological examination. We defined a third category of patients as “highly possible” IMD, who did not meet mycological criteria for probable or proven infection, but had no other diagnoses established according to criteria proposed by Nivoix et al.26
In addition to the radiological evaluation, an extensive diagnostic and microbiologic workup was performed to establish the cause of infection. The majority (96.8%) of patients were screened for serum galactomannan (Platelia, TM Bio-Rad Laboratories, Hercules, CA and Bio-Rad, Marnes-la-Coquette, France) two to three times weekly during fever, with an optical density index ≥0.5 on two consecutive tests considered positive. When bronchoscopy was performed (29.9%), lavage fluid was tested for galactomannan in conjunction with cultures and/or histology using the same positive index cut-off for a single test.
The baseline risk for IMD prior to CT imaging was also estimated individually for each patient using an institutionally, validated mold infection risk score.27 Briefly, the score accurately discriminates whether haematology patients have a cumulative probability of IMD greater than 5%, or less than 1% within 90 days on the basis of four weighted risk factors: (1) malignancy status (controlled or uncontrolled); (2) neutropenia last longer than 10 days; (3) lymphocyte dysfunction defined as CD4+ <50 mm3 or post-allogeneic haematopoietic stem cell transplant acute graft vs host diseases receiving calcineurin inhibitors and/or steroids; and (4) prior history of IMD. Data related to microbiological or histological diagnosis, underlying malignancy status and treatment, and infection outcome were prospectively collected for up to 90 days or until discharge. Alternative diagnosis for lung infiltrates was established based on cultures from the bloodstream or respiratory tract (bronchoalveolar lavage), and/or histology from fine-needle biopsy or autopsy. To ensure a consistency in case assessment, the diagnosis was reviewed by a haematologist, two radiologists, and an infectious diseases specialist based on EORTC/MSG criteria, and the final clinical diagnosis was recorded based on the microbiological and clinical findings.
Statistical analysis
Baseline demographic characteristics were analysed as absolute numbers and their relative frequencies and compared by the Fisher’s exact test. Continuous variables were expressed as mean ± standard deviation if normally distributed, or as median and interquartile range if non-normally distributed and compared using Student’s t-test or Mann–Whitney U test according to their distribution.
The diagnostic accuracy of contrast-enhanced HyS was compared to other HRCT findings and CTPA on a per patient basis using 2 × 2 tables (i.e. patient classified as positive if they ever had a positive test). The sensitivity, specificity, positive and negative likelihood ratio and diagnostic odds ratio and corresponding 95% confidence intervals were calculated for each sign analysed as a binary variable. Optimal cut-off values for densitometric analysis in the HyS that differentiate mould vs non-mould causes of the HyS were examined using area under the receiver operator curve analysis (aROC). All analysis was performed with STATA v. 13 (Stata Corp, College Station, TX).
RESULTS
Patient population
Patient, clinical and demographic characteristics are presented in Table 1. An unenhanced HRCT and a contrast-enhanced CTPA technique were both performed in 127 patients with clinical suspicion of IMD during the time period of the study. More than one-half of patients were affected by acute myeloid leukaemia (56.7%). Most patients had an active haematological malignancy (67.7%). 73 (57.4%) patients were receiving chemotherapy and 37 patients (29.1%) had received an allogeneic haematopoietic stem cell transplant.
Table 1.
Patient characteristics
| Variable | All patients | IMD diagnosis | No IMD diagnosis | p-valuea |
| n = 127 | n = 86 | n = 41 | ||
| Age years, median (IQR) | 54 (21–78) | 53 (21–78) | 55 (21–71) | 0.48 |
| Sex no. (%) | 0.05 | |||
| Male | 69 (54.3) | 42 (49) | 27 (65.8 ) | |
| Female | 58 (45.7) | 44 (51) | 14 (34.1) | |
| Malignancy no. (%) | ||||
| Acute myeloid leukaemia/myelodysplastic | 72 (56.7) | 52 (60.5) | 20 (48.8) | 0.25 |
| Acute lymphocytic leukaemia | 22 (17.3) | 15 (17.4) | 7 (17.1) | 0.99 |
| Lymphoma | 21 (16.5) | 9 (10.5) | 12 (29.3) | 0.01 |
| Chronic myelodysplastic syndrome | 7 (5.5) | 7 (8.1) | 0 (0) | 0.10 |
| Chronic lymphocytic leukaemia | 2 (1.6) | 1 (1.2) | 1 (2.4) | 0.54 |
| Myeloma | 2 (1.6) | 2 (2.3) | 0 (0) | 0.32 |
| Other | 1 (1) | 0 (0) | 1 (2.4) | 0.99 |
| Treatment phase no. (%) | ||||
| Induction chemotherapy | 20 (15.7) | 13 (15.1) | 7 (17.1) | 0.80 |
| Other chemotherapy | 53 (41.7) | 33 (38.4) | 20 (48.8) | 0.34 |
| Allogeneic HSCT | 37 (29.1) | 31 (36.0) | 6 (14.6) | 0.01 |
| Autologous HSCT | 2 (1.6) | 0 (0) | 2 (4.9) | 0.56 |
| No chemotherapy | 15 (11.8) | 9 (10.5) | 6 (14.6) | 0.10 |
| Disease phase no. (%) | ||||
| Initial diagnosis | 24 (18.9) | 14 (16.3) | 10 (24.4) | 0.84 |
| Complete or partial remission | 41 (32.2) | 27 (31.4) | 14 (34.1) | 0.33 |
| Relapse, resistance or progression | 62 (48.8) | 45 (52.3) | 17 (41.5) | 0.26 |
| Mold infection risk scoreb | ||||
| Neutropeniac | 105 (82.7) | 77 (89.5) | 28 (68.3) | 0.01 |
| Lymphopenia or lymphocyte dysfunctiond | 53 (41.7) | 44 (51.2) | 9 (22.0) | 0.18 |
| Uncontrolled malignancye | 42 (33.1) | 59 (68.6) | 23 (56.1) | 0.99 |
| History of previous IMD | 8 (6.3) | 7 (8.1) | 1 (2.4) | 0.05 |
| Median score (IQR)f | 5 (4–7) | 6 (4–7) | 4 (3.7–6) | 0.02 |
| Antifungal prophylaxis | ||||
| Non-systemic | 13 (10.2) | 6 (7.0) | 7 (17.1) | 0.11 |
| Fluconazole | 53 (41.7) | 41 (47.7) | 6 (14.6) | 0.08 |
| Itraconazole | 15 (11.8) | 7 (8.1) | 8 (19.5) | 0.06 |
| Posaconazole | 27 (21.3) | 20 (23.3) | 6 (14.6) | 0.49 |
| Liposomal amphotericin B | 2 (1.6) | 2 (2.3) | 0 (0) | 0.99 |
| Caspofungin | 1 (0.8) | 1 (1.2) | 0 (0) | 0.39 |
| None | 16 (12.6) | 9 (10.5) | 5 (12.2) | 0.99 |
| Serum galactomannan | ||||
| Positive | 52 (41.0) | 48 (55.8) | 4 (9.8) | <0.001 |
| Negative | 71 (55.9) | 38 (44.2) | 33 (80.4) | |
| Not available | 4 (3.1) | 0 (0) | 4 (9.8) | 0.01 |
| Bronchoalveolar lavage | ||||
| Negative for IMD | 29 (22.8) | 22 (25.6) | 7 (17.0) | 0.37 |
| Positive for IMD | 3 (2.4) | 3 (3.5) | 0 (0) | |
| Galactomannan positive | 13 (10.2) | 13 (15.1) | 0 (0) | 0.02 |
| Positive for other pathogen | 6 (4.7) | 2 (2.3) | 4 (9.8) | 0.09 |
| Not available | 89 (70.0) | 59 (68.6) | 30 (73.2) | 0.68 |
HSCT, haematopoietic stem cell transplantation; IMD, invasive mould disease; IQR, interquartile range.
ap values determined by Mann–Whitney test or Pearson Χ2 test.
bFrom Stanzani et al.27
cAbsolute neutrophil count <500 cells mm–3 >10 days within 30 days prior to admission or after chemotherapy.
dAbsolute lymphocyte count <50 cells mm–3, or allogeneic HSCT patient receiving calcineurin inhibitor, corticosteroids, or antithymocyte globulin for acute graft vs host disease.
eNewly diagnosed or relapsed/uncontrolled malignancy.
fRisk scores of ≥6 identify higher risk populations (baseline incidence of invasive mold disease independent of CT findings >5%).
Most of the patients (82.7%) were neutropenic at the time of the CT examination. Approximately one-third (35.4%) of patients were receiving mould-active prophylaxis. The baseline risk for IMD was estimated at 3% for the study population (risk score of 5; IQR 4–7), but was significantly higher in patients who developed IMD vs those who did not (median 6 vs 4, p = 0.02).
Diagnostic outcome
Among the 127 patients submitted to HRCT (without contrast-enhancement) and CTPA (with arterial contrast-enhancement), the HyS was detected visually in only 20 (15.7%) patients without contrast enhancement (HRCT scan) but in 56 (44.1%, p < 0.0001) patients after contrast administration (arterial CTPA scan) (Table 2) (Figure 1). The CTPA was technically adequate to assess the VOS in 116 patients (readable cases) of which 77 (66.4%) were positive for the VOS (Figure 2). In the remaining 11 patients (9%), the VOS was indeterminate because of technical limitations. A contrast-aided HyS was appreciable in 3 of those 11 (27.3%) patients with indeterminate VOS on CTPA and was concurrent with the final diagnosis of IMD.
Table 2.
Frequency of the hypodense sign in proven, probable and possible IMD (127 patients) by EORTC diagnostic criteria
| EORTC diagnostic category | Frequency in all patients | Hypodense sign without contrast (HRCT) | Hypodense sign with contrast CT arterial phase (CTPA) | Hypodense sign with contrast CT venous phase |
| n = 127 (%) | n = 20 (15.7%) | n = 56 (44.1%) | n = 19 (51.3%)a | |
| Proven IMD | 15 (11.8) | 6 (40.0) | 13 (86.7) | 5 (100) |
| Probable IMD | 41 (32.3) | 12 (29.3) | 32 (78.0) | 10 (71.4) |
| Possible IMD | 71 (55.9) | 2 (2.8) | 11 (15.5) | 4 (22.2) |
| Highly possible IMDb | 30 (23.6) | 2 (6.7) | 10 (33.3) | 4 (40.0) |
| No IMDc | 41 (32.3) | 0 (0) | 1 (2.4) | 0 (0) |
CTPA, CT pulmonary angiography; EORTC, European Organization for Research and Treatment of Cancer; HRCT, high-resolution CT; IMD, invasive mould disease.
aValues related to 37/127 (29.1%) patients who carried out CT venous phase (5 proven IMD; 14 probable IMD; 18 possible IMD; 10 highly possible IMD).
bNo evidence of an alternative diagnosis and patient responded to antifungal therapy.
cIncluding 27 bacterial pneumonia.
Figure 1.
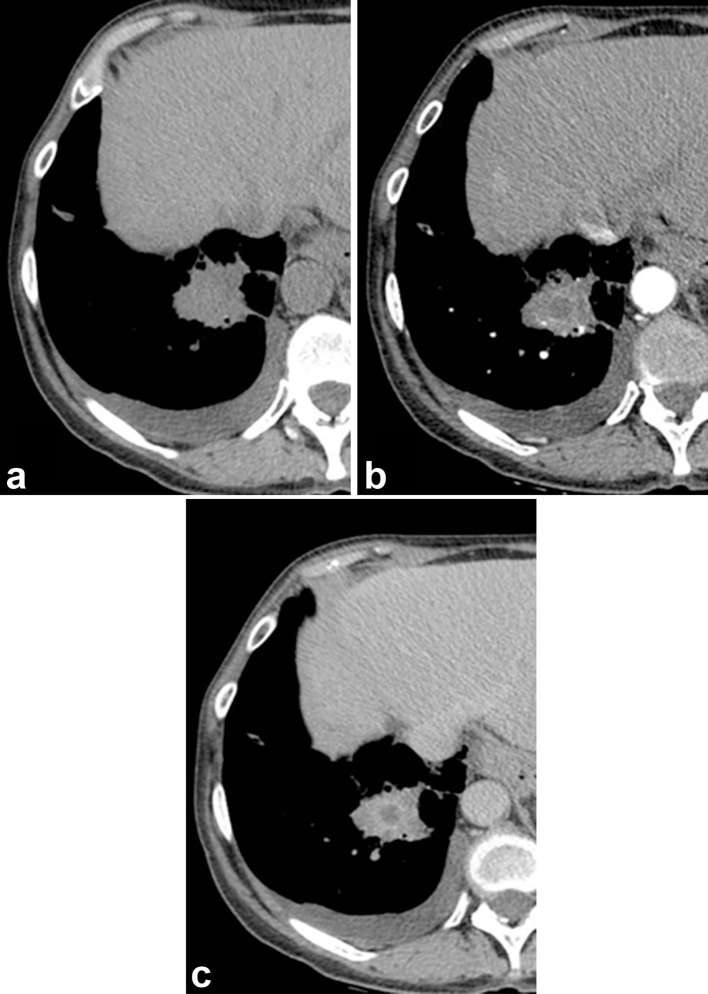
Visual assessment of the hypodense sign. The HyS is absent on a non-contrast-enhanced CT (a) but it appears on contrast-enhanced CT, both in arterial phase (CTPA), (b) and in venous phase (c) The VOS was indeterminate because of CTPA limits. Other findings include small pleural effusion to the right and left (not shown), and a hepatic atypical haemangioma that was previously diagnosed. CTPA, CT pulmonary angiography; HyS, hypodense sign; VOS, vessel occlusion sign.
Figure 2.
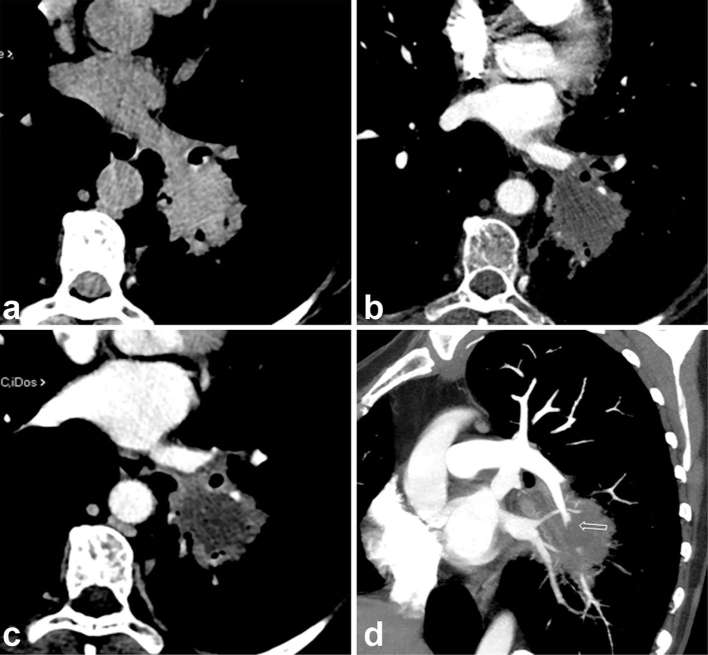
CT shows a nodular dense lesion in left inferior lobe nodular lesion without evidence of the hypodense sign (a) After contrast administration, on arterial phase scan (CTPA), the nodular lesion shows a slight ring enhancement (b) that becomes clearly evident in venous phase (c) revealing the hypodense sign. The oblique reformatted image (d) displays the vessel occlusion sign (abruption of the arterial vessel entering the pulmonary lesion). CTPA, CT pulmonary angiography.
By analysing data according to EORTC/MSG criteria for IMD diagnosis, a proven IMD was reached in 15/127 patients (11.8%). Among these patients, a HyS was documented in only 6/15 (40.0%) by HRCT (without contrast enhancement) but in 13/15 (86.7%) by CTPA (with contrast enhancement) (p = 0.02). Among patients with EORTC/MSG probable IMD (n = 41/127; 32.3%), the HyS was detected in only 12/41 (29.3%) by HRCT but in 32/41 (78.0%) by CTPA (p = 0.001). Among patients with EORTC/MSG possible IMD (n = 71/127, 55.9%), the HyS was detected in only 2/71 patients (2.8%) by HRCT but in 11/71 (15.5%) by CTPA (p = 0.02) (Table 2).
Among the 37 patients who underwent an adjunctive venous contrast-enhanced CT scan, the hypodense sign was detected in 5/37 (100%), 10/37 (71.4%) and 4/37 (22.2%) patients with proven, probable and possible IMD, respectively (Table 2) (Figures 1 and 2).
A “final diagnosis” was reached after multidisciplinary (clinical, microbiological, radiological) evaluation of all the 127 patients. The 56 patients classified as having a proven and probable IMD according to EORTC/MSG criteria, fulfilled a final diagnosis of IMD. Among the remaining 71 patients (71/127; 56%) classified as having a possible IMD according to EORTC/MSG criteria, 41 (41/71; 57.7%) reached a final diagnosis different from IMD (no-IMD). These diagnoses included, 27 bacterial pneumonias, 2 viral pneumonias, 1 polymicrobial pneumonia, 1 possible case of tuberculosis, 6 cases of lymphoma, 1 graft vs host disease, 1 bronchiolitis obliterans with organizing pneumonia, 1 drug-related lung reaction, and 1 case where no diagnosis could be established.
Among the 41 patients with no-IMD diagnosis, a contrast-enhanced HyS was visible in only one (1/41; 2%) patient who had a diagnosis of previous pulmonary tuberculosis who developed disseminated fusariosis (diagnosed 2 months after CT imaging). In the remaining 30 of 71 (42.2%) patients with no-IMD diagnosis, no alternative diagnosis to IMD was reached and all patients responded to empiric mould-active antifungal therapy. These 30 cases fulfilled the final diagnosis of “highly possible” IMD.
If patients with proven, probable, and highly possible diagnosis are grouped as a “final diagnosis” of IMD (86 patients; 67.7%), the hypodense sign was appreciable in only 20/86 (23.2%) by HRCT (without contrast-enhancement) but in 55/86 (63.9%) cases by CTPA (arterial contrast-enhancement).
Among the 37 patients with the adjunctive venous contrast-enhanced CT scan and a “final diagnosis” of IMD, the hypodense sign was detected in 19/37 (51.3%) cases (Table 2).
Among the 41 patients with a “final diagnosis” of no-IMD, only 1 patient (2.4%) had a contrast-enhanced positive HyS that likely represented a pre-existent cavity (exitus of previous tuberculosis) filled with mucus.
Among the 86 patients with a “final diagnosis” of IMD, the CTPA was technically adequate in 78 patients of which 76/78 (97%) showed the VOS (Figure 2).
The overall diagnostic performance of the HySvs other common CT signs is presented in Table 3. The HyS demonstrated a specificity of 1.00 and a sensitivity of 0.23 on unenhanced HRCT. After contrast administration, with arterial enhancement (CTPA), the specificity of the HyS was similar (0.98), while the sensitivity increased to 0.64. A CTPA-detected VOS was associated with the highest specificity and diagnostic odds ratio of any CT sign.
Table 3.
Diagnostic performance of the hypodense sign for proven, probable and highly-possible IMD (86 patients) relative to other CT findings
| CT finding | Frequency of sign in 86 patients | Sensitivity(95% CI) | Specificity(95% CI) | Positive LR(95% CI) | Negative LR(95% CI) | Diagnostic odds ratio (95% CI) |
| Halo sign (on HRCT and CTPA) | 67.7% | 0.68 (0.57–0.78) | 0.56 (0.39–0.71) | 1.58 (1.13–2.38) | 0.53 (0.36–0.83) | 2.94 (1.27–6.84) |
| Hypodense sign on HRCT | 23.2% | 0.23 (0.13–0.36) | 1.00 (0.95–1.00) | ∞ (4.33–∞) | 0.77 (0.65–0.87) | ∞ (4.50–∞) |
| Hypodense sign on CTPA | 63.9% | 0.64 (0.52–0.74) | 0.98 (0.87–1.00) | 26.22 (5.00–148.82) | 0.37 (0.27–0.48) | 68.90 (10.11–299) |
| Vessel occlusion sign (on CTPA)a | 97.4% | 0.97 (0.91–0.99) | 0.97 (0.86–0.99) | 37.02 (5.35–256.19) | 0.02 (0.007–0.10) | 1406.00 (123–16009) |
| Vessel occlusion sign (on CTPA)b | 88.4% | 0.88 (0.79–0.94) | 0.97 (0.87–0.99) | 36.23 (5.22–251.50) | 0.12 (0.07–0.21) | 304.00 (37–2460) |
CI, confidence interval; CTPA, CT pulmonary angiography; HRCT, high resolution CT; LR, likelihood ratio; VOS, vessel occlusion sign. ∞, infinity sign, not calculable.
The diagnostic performance of the halosign is the same on unenhanced HRCT and CTPA; the vessel occlusion sign is visible only on enhanced CTPA scan, and the CTPA increases the sensitivity of the hypodense sign respect to HRCT.
aExcluding indeterminate VOS (8/86 of patients who underwent CTPA had not-evaluable results because of coughing, breathing, technical noise or insufficient nodule volume).
bIncluding indeterminate VOS (8/86 of patients who underwent CTPA had not-evaluable results because of coughing, breathing, technical noise or insufficient nodule volume).
Densitometric analysis of the hypodense sign
A densitometric analysis of the lesions was performed in 98/127 patients (77.2%) (Table 4). Of them, 65/98 (66.3%) had a final diagnosis of IMD and 33/98 (33.7%) reached an alternative diagnosis. On unenhanced HRCT images, among patients with final diagnosis of IMD and positive HyS, the densitometric analysis of pulmonary lesions revealed a slight difference of 16.33 HU between the peripheral (average: 36.55 HU) and central area (average: 20.22 HU) of the lesion. After contrast media injection, the difference between peripheral and central area increased to 25.06 HU on arterial phase scan (CTPA), and even more in venous phase scan (difference of 38.93 HU). In patients with “final diagnosis” of IMD but negative HyS, pulmonary lesions showed a very low (only 7.04 HU) difference between peripheral and central area on unenhanced HRCT scan, that decreased after contrast media administration (2.91 HU and 2.28 HU on arterial and venous scan images, respectively). Only one patient with no-IMD diagnosis had a positive HyS on contrast-enhanced CTPA images, with a difference greater than 20 HU between the periphery and centre of the pulmonary lesion.
Table 4.
Densitometric analysis of lesions in IMD vs non-IMD cases (98/127 patients)
| Patients | Area of analysis | Positive HyS | Negative HyS | ||||
| HRCT(n = 18) | CTPA(n = 45) | VP(n = 15) | HRCT(n = 47) | CTPA(n = 20) | VP(n = 5) | ||
| IMD 65/98 | Peripheral | 36.55 | 46.84 | 64.13 | 27.72 | 44.35 | 53.88 |
| Centre | 20.22 | 21.78 | 25.20 | 20.68 | 41.44 | 51.60 | |
| Difference | 16.33 | 25.06 | 38.93 | 7.04 | 2.91 | 2.28 | |
| HRCT(n = 0) | CTPA(n = 1) | VP(n = 0) | HRCT(n = 33) | CTPA(n = 32) | VP(n = 13) | ||
| No-IMD 33/98 | Peripheral | – | 37.03 | – | 24.85 | 54.82 | 60.92 |
| Centre | – | 13.25 | – | 23.67 | 52.73 | 58.77 | |
| Difference | – | 23.78 | – | 1.18 | 2.09 | 2.15 | |
CTPA, CT pulmonary angiography; HRCT,high-resolution CT; HyS,hypodense sign; IMD, invasive mould disease; VP, venous phase.
The average HU values of the pulmonary lesions in peripheral zone, central zone, and the difference between peripheral and central zone, respectively on unenhanced HRCT, arterial phase (CTPA) and VP, are compared with evaluation of HyS, in both the two groups of patients, with IMD and without IMD.
Among the 33 cases with no-IMD diagnosis and with negative HyS, the densitometric analysis showed a negligible difference of 1.18 HU between the peripheral and central area of the pulmonary lesion on HRCT. After contrast injection, this difference was still negligible (2.09 HU and 2.15 HU on the CTPA and the venous scan, respectively) because both the peripheral and central area enhanced similarly. The greatest difference between peripheral and central densities of the pulmonary lesions in patients with IMD was observed in the subset of patients who had contrast-enhanced venous CT scans (Table 4).
A difference >15 HU between the peripheral and central area of the pulmonary lesions reliably differentiated the HyS associated to mould-related pulmonary lesions vs non mould-related pulmonary lesions [aROC 0.86; 95% CI (0.69–0.96)] (Figure 3).
Figure 3.
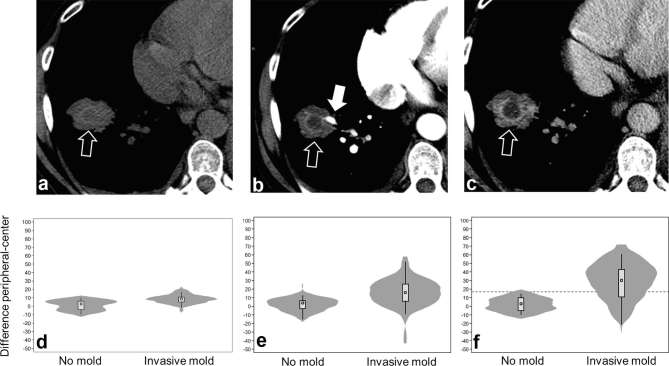
Hypodense sign visualized in a single patient on (a) non-contrast-enhanced CT, (b) contrast-enhanced CT performed in CTPA arterial phase, and (c) contrast-enhanced CT performed in venous phase. Open arrows point to hypodense sign, the filled arrow points to an occluded vessel. Panels below show violin plots of difference in the software calculated density between the peripheral vs central density of the lesion in patients with a hypodense sign following (d) non-contrast-enhanced CT; (e) contrast-enhanced CT visualized arteries; and (f) contrast-enhanced CT in venous phase. The plots show the median (circle), interquartile range (box), upper and lower adjacent values (spikes) and kernel density (shaded area). All difference in the peripheral–central density were statistically significant between patients with invasive mould diseases vs those without invasive mould disease, two-tailed t-test p < 0.0001. A provisional difference of >15 for venous scans differentiated mould vs non-mould associated HyS [aROC aROC 0.86; 95% CI (0.69–0.96)].
DISCUSSION
The main finding of this study was that the HyS was a highly specific sign for IMD on CT scan with or without contrast-media. However, the sensitivity of the HyS was low on unenhanced CT, but improved in patients who received iodinated contrast-media. These findings are consistent with the expectation that the necrotic central area of infarcted lesions will not take up contrast-media to an appreciable degree compared to peripheral regions, resulting in 50% lower densitometric values.20, 22 We observed that the peripheral ring enhances mostly in venous phase than in arterial phase, while necrotic vascular (infarcted) parenchyma does not enhance in both contrast phases. Obviously, the diagnostic value of a “whole-chest” venous phase scan is the same as a selected venous phase scan limited to the pulmonary lesion (the “ROI”), but the latter has a lower radiologic burden than a whole-chest CT scan.
Our results are consistent with those reported by Horger et al who found that non-contrast-enhanced HyS was a relatively insensitive but highly-specific finding of IPA.20 Our results are also in agreement with the data of Schulze et al that suggested that the HyS would be more readily detected at earlier stages in patients undergoing contrast-enhanced vs noncontrast-enhanced studies.22
We found no significant differences in the detection of the HyS by arterial CTPA imaging vs venous imaging, but the venous phase imaging make it easier to detect the HyS thanks to higher densitometric differences of central vs peripheral zones of the lesion. However, the limited number of patients who underwent venous phase scans in our series analysis may limit interpretations of the diagnostic performance.
All but one of the patients in our series with positive visual HyS had a final diagnosis of IMD. The single “false-positive” patient had a possible diagnosis of tuberculosis, therefore, the hypodensity could be the result of a colliquative necrosis or a pre-existent cavity filled with mucus. Hence, the HyS may not be able to differentiate fungal vs mycobacterial pneumonia, although that latter is a relatively uncommon cause of infection in neutropenic patients.
Our results supports those of Horger et al who did not detect the HyS in patients with bacterial or viral pneumonias.20 Therefore, as suggested by Horger, we think that in neutropenic patients, differentiation from bacterial abscess seems highly reliable. Indeed, the HyS can be found also in bacterial infections with lung abscess and cavitation, but these complications are rarely encountered in neutropenic patients because abscess formation is related to leukocytosis and neutrophilia.
In the group of patients with IMD and positive visual HyS, the densitometric analysis showed a difference between central and peripheral area always >16 UH, which increased after contrast injection. Similarly, excluding the patient with tuberculosis, no other cases who reached an alternative diagnosis to IMD and with HyS with a peripheral–central density zone difference >20 UH. aROC confirmed that a cut-off of <15 UH [aROC 0.86; 95% CI (0.69–0.96)] could provisionally discriminate patients with lower-density HyS into a category of lower probability of IMD.
As compared to other CT findings of IMD, the contrast-enhanced HyS was as sensitive as the halo sign but significantly more specific [halo sign: 0.56, 95% CI (0.39–0.71) vs HyS: 0.98, 95% CI (0.87–1.00)]. Only the VOS was more sensitive [0.97, 95% CI (0.91–0.99)] and specific [0.97, 95% CI (0.86–0.99)] than the HyS for IMD diagnosis (Table 3). Those results are in keeping with those by Sonnet et al16 and Stanzani et al17, 18 that showed the utility of the CTPA-detected VOS sign for IMD diagnosis.
We also observed that the HyS was appreciable in three patients who had indeterminate VOS on CTPA scans due to technical reasons, thus the HyS could support the diagnosis of IMD when the VOS cannot be assessed.
Our analysis of the diagnostic performance of CT signs was based on the EORTC/MSG definitions for proven, probable and possible IMD, but one-third (35.4%) of patients who had a final clinical diagnosis of IMD were receiving mould-active triazoles, liposomal amphotericin B or a echinocandin at the time of CT scan. The rate of serum galactomannan positivity was significantly lower among patients receiving mould-active prophylaxis vs patients not receiving mould-active agents (27.6 vs 70.2%, p < 0.0001). A similar reduction in sensitivity was evident in testing bronchoalveolar lavage galactomannan (16.7 vs 80.0%, p = 0.007). In contrast, the rate of HyS (68.4 vs 55.2%, p = 0.23) or VOS positivity (98.0 vs 96.2%, p = 0.64) was not significantly affected by mould-active prophylaxis. Therefore, HyS and VOS are particularly useful signs in patient who develop breakthrough IMD given the poorer sensitivity of serum biomarkers in patients receiving mould-active antifungal therapy.
Finally, the evaluation of HyS does not require an additional irradiation as the assessment is performed on CTPA scan. A possible additional dose to perform the venous CT scan would be limited, since the scan would be narrowed to the area of interest.
CONCLUSION
In neutropenic patients with suspected IMD, the HyS is a highly specific sign for IMD with poor sensitivity on unenhanced CT that is improved with contrast-enhanced scan. The high specificity of the HyS strongly supports the diagnosis of IMD, and is a highly suggestive sign of breakthrough mould disease in patients who develop pulmonary lesions on mould-active antifungal therapy or in patients with infiltrates in which the VOS cannot be technically assessed by CTPA. Further studies are needed to confirm the added utility of densitometric analysis for improving the specificity of HyS cause by IMD.
Contributor Information
Claudia Sassi, Email: claudia.sassi3@unibo.it.
Marta Stanzani, Email: marta.stanzani2@unibo.it.
Russell E Lewis, Email: russeledward.lewis@unibo.it.
Giancarlo Facchini, Email: giancarlo.facchini2@unibo.it.
Alberto Bazzocchi, Email: abazzocchi@gmail.it.
Michele Cavo, Email: michele.cavo@unibo.it.
Giuseppe Battista, Email: g.battista@unibo.it.
REFERENCES
- 1.Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. Br J Haematol 2010; 150: 406–17. doi: https://doi.org/10.1111/j.1365-2141.2010.08283.x [DOI] [PubMed] [Google Scholar]
- 2.Stergiopoulou T, Meletiadis J, Roilides E, Kleiner DE, Schaufele R, Roden M, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007; 127: 349–55. doi: https://doi.org/10.1309/UJRV9DLC11RM3G8R [DOI] [PubMed] [Google Scholar]
- 3.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2006; 2: e129. doi: https://doi.org/10.1371/journal.ppat.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes Bezerra LM, Filler SG. Interactions of aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood 2004; 103: 2143–9. doi: https://doi.org/10.1182/blood-2003-06-2186 [DOI] [PubMed] [Google Scholar]
- 5.Kamai Y, Chiang LY, Lopes Bezerra LM, Doedt T, Lossinsky AS, Sheppard DC, et al. Interactions of Aspergillus fumigatus with vascular endothelial cells. Med Mycol 2006; 44(Suppl 1): 115–7. doi: https://doi.org/10.1080/13693780600897989 [DOI] [PubMed] [Google Scholar]
- 6.Shibuya K, Ando T, Hasegawa C, Wakayama M, Hamatani S, Hatori T, et al. Pathophysiology of pulmonary aspergillosis. J Infect Chemother 2004; 10: 138–45. doi: https://doi.org/10.1007/s10156-004-0315-5 [DOI] [PubMed] [Google Scholar]
- 7.Franquet T, Müller NL, Giménez A, Guembe P, de La Torre J, Bagué S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 2001; 21: 825–37. doi: https://doi.org/10.1148/radiographics.21.4.g01jl03825 [DOI] [PubMed] [Google Scholar]
- 8.Denning DW. Invasive aspergillosis. Clin Infect Dis 1998; 26: 781–803. doi: https://doi.org/10.1086/513943 [DOI] [PubMed] [Google Scholar]
- 9.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–21. doi: https://doi.org/10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the aspergillus galactomannan enzyme immunoassay. Clin Infect Dis 2005; 40: 1762–9. doi: https://doi.org/10.1086/429921 [DOI] [PubMed] [Google Scholar]
- 11.Vergidis P, Razonable RR, Wheat LJ, Estes L, Caliendo AM, Baden LR, et al. Reduction in false-positive aspergillus serum galactomannan enzyme immunoassay results associated with use of piperacillin-tazobactam in the United States. J Clin Microbiol 2014; 52: 2199–201. doi: https://doi.org/10.1128/JCM.00285-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauggaard A, Ellis M, Ekelund L. Early chest radiography and CT in the diagnosis, management and outcome of invasive pulmonary aspergillosis. Acta Radiol 2002; 43: 292–8. doi: https://doi.org/10.1034/j.1600-0455.2002.430310.x [DOI] [PubMed] [Google Scholar]
- 13.De Marie S. New developments in the diagnosis and management of invasive fungal infections. Haematologica 2000; 85: 88–93. [PubMed] [Google Scholar]
- 14.Lee YR, Choi YW, Lee KJ, Jeon SC, Park CK, Heo JN. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol 2005; 78: 862–5. doi: https://doi.org/10.1259/bjr/77712845 [DOI] [PubMed] [Google Scholar]
- 15.Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis 2011; 52: 1144–55. doi: https://doi.org/10.1093/cid/cir122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnet S, Buitrago-Téllez CH, Tamm M, Christen S, Steinbrich W. Direct detection of angioinvasive pulmonary aspergillosis in immunosuppressed patients: preliminary results with high-resolution 16-MDCT angiography. AJR Am J Roentgenol 2005; 184: 746–51. doi: https://doi.org/10.2214/ajr.184.3.01840746 [DOI] [PubMed] [Google Scholar]
- 17.Stanzani M, Battista G, Sassi C, Lewis RE, Tolomelli G, Clissa C, et al. Computed tomographic pulmonary angiography for diagnosis of invasive mold diseases in patients with hematological malignancies. Clin Infect Dis 2012; 54: 610–6. doi: https://doi.org/10.1093/cid/cir861 [DOI] [PubMed] [Google Scholar]
- 18.Stanzani M, Sassi C, Lewis RE, Tolomelli G, Bazzocchi A, Cavo M, et al. High resolution computed tomography angiography improves the radiographic diagnosis of invasive mold disease in patients with hematological malignancies. Clin Infect Dis 2015; 60: 1603–10. doi: https://doi.org/10.1093/cid/civ154 [DOI] [PubMed] [Google Scholar]
- 19.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis 2016; 63: e1–e60. doi: https://doi.org/10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horger M, Einsele H, Schumacher U, Wehrmann M, Hebart H, Lengerke C, et al. Invasive pulmonary aspergillosis: frequency and meaning of the "hypodense sign" on unenhanced CT. Br J Radiol 2005; 78: 697–703. doi: https://doi.org/10.1259/bjr/49174919 [DOI] [PubMed] [Google Scholar]
- 21.Kang EY, Kim DH, Woo OH, Choi JA, Oh YW, Kim CH. Pulmonary aspergillosis in immunocompetent hosts without underlying lesions of the lung: radiologic and pathologic findings. AJR Am J Roentgenol 2002; 178: 1395–9. doi: https://doi.org/10.2214/ajr.178.6.1781395 [DOI] [PubMed] [Google Scholar]
- 22.Schulze M, Vogel W, Spira D, Sauter A, Hetzel J, Horger M. Reduced perfusion in pulmonary infiltrates of high-risk hematologic patients is a possible discriminator of pulmonary angioinvasive mycosis: a pilot volume perfusion computed tomography (VPCT) study. Acad Radiol 2012; 19: 842–50. doi: https://doi.org/10.1016/j.acra.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Gefter WB, Albelda SM, Talbot GH, Gerson SL, Cassileth PA, Miller WT. Invasive pulmonary aspergillosis and acute leukemia. Limitations in the diagnostic utility of the air crescent sign. Radiology 1985; 157: 605–10. doi: https://doi.org/10.1148/radiology.157.3.4059547 [DOI] [PubMed] [Google Scholar]
- 24.Caillot D, Latrabe V, Thiébaut A, Herbrecht R, De Botton S, Pigneux A, et al. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: an useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol 2010; 74: e172–e175. doi: https://doi.org/10.1016/j.ejrad.2009.05.058 [DOI] [PubMed] [Google Scholar]
- 25.Franquet T, Giménez A, Hidalgo A. Imaging of opportunistic fungal infections in immunocompromised patient. Eur J Radiol 2004; 51: 130–8. doi: https://doi.org/10.1016/j.ejrad.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 26.Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 2008; 47: 1176–84. doi: https://doi.org/10.1086/592255 [DOI] [PubMed] [Google Scholar]
- 27.Stanzani M, Lewis RE, Fiacchini M, Ricci P, Tumietto F, Viale P, et al. A risk prediction score for invasive mold disease in patients with hematological malignancies. PLoS One 2013; 8: e75531. doi: https://doi.org/10.1371/journal.pone.0075531 [DOI] [PMC free article] [PubMed] [Google Scholar]