Abstract
Iatrogenic injury to the oesophagus is a serious complication which is increasingly seen in clinical practice secondary to expansion and greater acceptability of surgical and endoscopic oesophageal procedures. Morbidity and mortality following such injury is high. This is mostly due to an inflammatory response to gastric contents in the mediastinum, and the negative intrathoracic pressures that may further draw out oesophageal contents into the mediastinum leading to mediastinitis. Subsequently, pulmonary complications such as pneumonia or abscess may ensue leading to rapid clinical deterioration. Optimized and timely cross-sectional imaging evaluation is necessary for early and aggressive management of these complications. The goal of this review is to make the radiologist aware of the importance of early and accurate identification of postoperative oesophageal injury using optimized CT imaging protocols and use of oral contrast. Specifically, it is critical to differentiate benign post-operative findings, such as herniated viscus or redundant anastomosis, from clinically significant postoperative complications as this helps guide appropriate management. Advantages and drawbacks of other diagnostic methods, such as contrast oesophagogram, are also discussed.
INTRODUCTION AND EXTENT OF PROBLEM
A multitude of surgical and endoscopic oesophageal procedures are performed in current clinical practice and the indications are ever increasing (Table 1), with iatrogenic oesophageal injury becoming one of the most common causes of injury to the oesophagus.
Table 1.
Expanding spectrum of oesophageal surgery and endoscopic procedures
| Endoscopic procedures | Surgical procedures |
| Endoscopic dilation and stenting | Oesophagectomy |
| Endoscopic ultrasound and biopsy of peri-oesophageal masses | Treatment of hiatal hernia—Nissen fundoplication |
| Endoscopic removal of foreign bodies | Resection of benign oesophageal masses |
| Peroral endoscopic Myotomy (POEM) for treatment of achalasia | Oesophageal myotomy for treatment of achalasia |
| Endoscopic radiofrequency ablation for cardiac diseases | Surgical Procedures close to the oesophagus (discectomy, aortic stent–graft placement) |
It is estimated that 7.2 to 14% of patients develop post-surgical anastomotic leaks.1,2 Leak-related mortality after thoracic anastomotic dehiscence has been reported to be as high as 35%.1,3 The wide range in reported incidence as well as the morbidity and mortality depends on a few factors. This includes coexistent patient comorbidities, location of the anastomosis (lower mortality is associated with a cervical anastomosis as compared to an intrathoracic anastomosis) as well as in part due to varying clinical and radiological definitions of leaks used by different groups.4
The true incidence of anastomotic leaks also depends on the sensitivity of the test used to assess it. A chest CT performed without oral contrast has a much lower sensitivity for detection of leaks compared to a contrast oesophagogram and chest CT obtained with oral contrast (Figure 1).
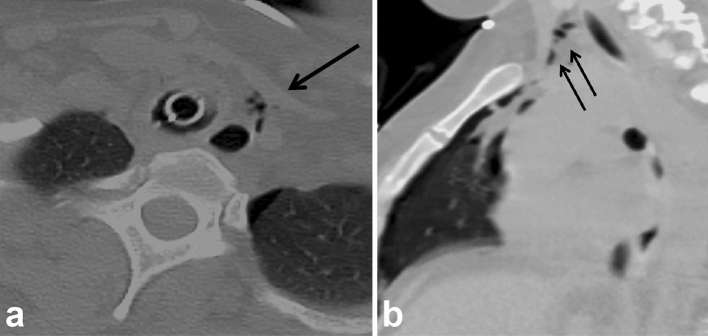
Figure 1.
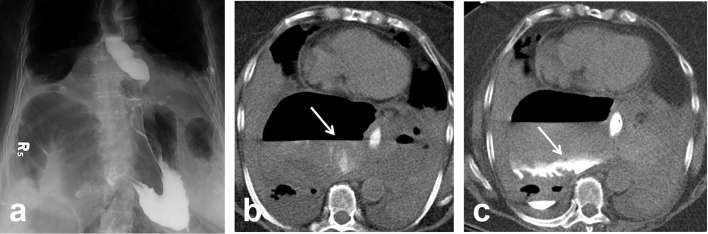
Post-oesophagectomy with fever. Chest CT done on POD 17 (a) Axial non-contrast CT chest reveals tiny pockets of gas (arrow) close to proximal anastomosis which was initially attributed to normal postoperative appearance. (b) Right-sided multiloculated pleural effusion was suspicious for empyema vs chylous leak. (c) Subsequent UGI study on POD 18 identified presence of an anastomotic leak, and hence, the effusion was likely an empyema secondary to underlying leak (arrow). Teaching point: chest CT without oral contrast is inadequate for evaluation of anastomotic leak. UGI, upper gastrointestinal. POD, post-operative day.
The management of patients with post-operative oesophageal leaks depends on several patient-related factors such as age, clinical picture, comorbidities and time since injury. However, as expected, further clinical decision-making regarding surgical or endoscopic management requires accurate information regarding the site and size of the perforation. Here, imaging plays a crucial role in identifying an anastomotic leak, detecting drainable collections and delineating the extent of mediastinal, pleural or peritoneal contamination. Asymptomatic patients with an occult leak can be managed conservatively, while patients with large fulminant leaks and extensive mediastinal and pleural contamination can rapidly progress to sepsis and multiorgan failure, including organ necrosis, and hence require prompt surgical and endoscopic management with stent placement.5
Imaging modalities
A contrast oesophagogram or upper gastrointestinal series remains the initial imaging modality for diagnosing anastomotic leaks, often performed on a routine basis prior to commencement of enteral feeding. Besides evaluating for leaks, it can also confirm absence of post-surgical downstream obstruction or spasm prior to initiating oral feeds. In most cases, approximately 20–100 ml of undiluted non-ionic water soluble contrast such as iohexol (Omnipaque 240 mg iodine ml–1, GE Healthcare, Princeton, NJ, USA) is administered with the patient in a standing position. Subsequently, images are acquired using a frame rate of 2–3 fps. Overall, the study is inexpensive and involves smaller radiation dose to the patient. In select patients with higher risk of aspiration, thin dilute barium can be given as it less toxic to the lungs. Due to its increased density, it provides improved visualization of the contrast column in larger patients and is helpful to exclude post-operative oedema or dysmotility. Therefore, some have proposed the use of high-density barium as a second-line investigation when no leak is detected by contrast swallow performed with water soluble contrast, and this increased the sensitivity of the swallow by 60% in a prospective study.6 However, it has been shown that overall barium oesophagogram is an ineffective screening modality in symptomatic patients due to its low sensitivity, as it misses anastomotic leak in 40–50% of the cases.7
Compared to the oesophagogram, there are fewer practical obstacles to transferring a clinically unstable patient to a CT scanner than to a fluoroscopy suite. CT with oral contrast has a higher sensitivity for delineating small leaks. In a study by Upponi et al8, 67% of the patients found CT more tolerable compared to fluoroscopy. Importantly the sensitivity, specificity, positive- and negative-predictive values were 100, 80, 40 and 100%, respectively, for CT and 67, 100, 100 and 96%, respectively, for fluoroscopy.8 In a febrile symptomatic patient, a chest CT with oral contrast is the preferred imaging modality as it can detect and characterize peri-anastomotic collections and assess pulmonary complications such as pneumonia and abscess which need to be tackled promptly (Figure 2). Furthermore, CT helps in planning image-guided drainage of collections, stent placement or surgical debridement.5,9 This is of a particular benefit in clinically unstable patients, where delay in treatment significantly increases the risk of death. It is worth noting that while oral contrast is extremely helpful in delineating a leak, the risk of aspiration exists and may further precipitate clinical deterioration due to development of pulmonary complications, such as pulmonary edema or pneumonitis in critically sick patients.9 Hence, the route of oral contrast administration (oral or via feeding tube) and the volume of oral contrast is selected after close consultation with the surgical team, and the amount is judiciously tailoured to whatever is tolerated by the patient.
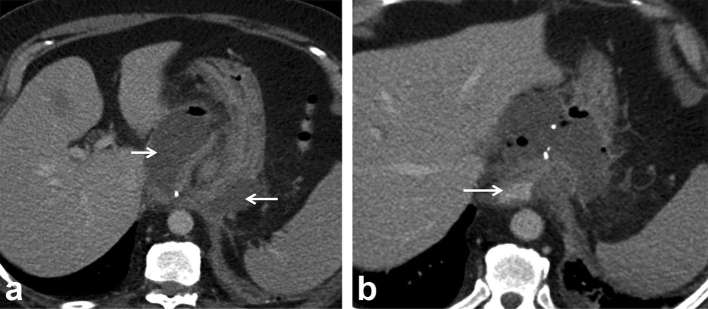
Figure 2.
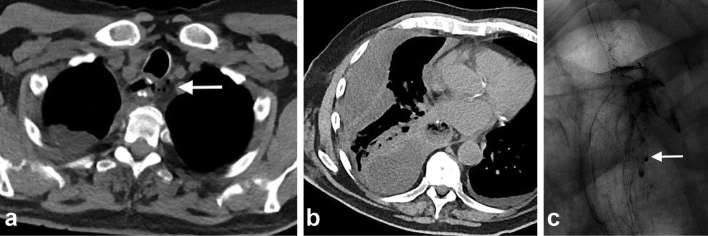
Status postrepair of paraoesophageal hernia, concern for leak. (a) Frontal radiograph from an UGI series with water soluble oral contrast demonstrates smooth passage of contrast into the stomach with no extraluminal contrast leak into the mediastinum or pleural space. Axial images of chest CT with oral contrast (b, c) repeated due to continued concern for leak showed extravasation of oral contrast into the hernia sac (arrows). Note the presence of large volume air and fluid in the hernia sac. Teaching point. This case emphasizes the importance of proceeding with CT in patients with initial negative UGI study, especially when there is continued concern for leak. UGI, upper gastrointestinal.
Institutional imaging protocol for evaluation of post-operative esophageal leaks
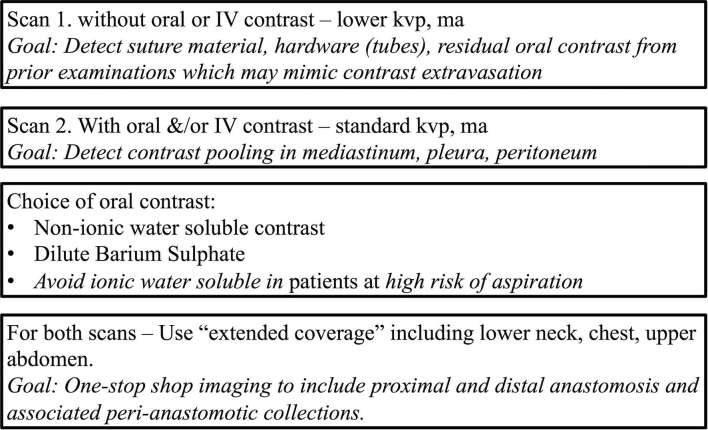
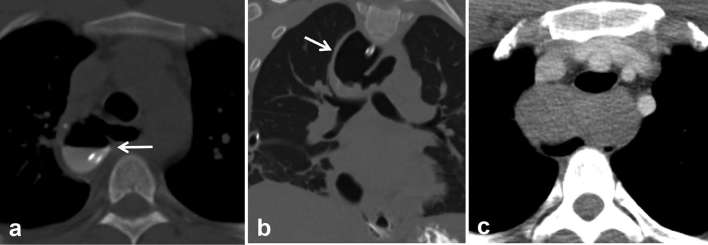
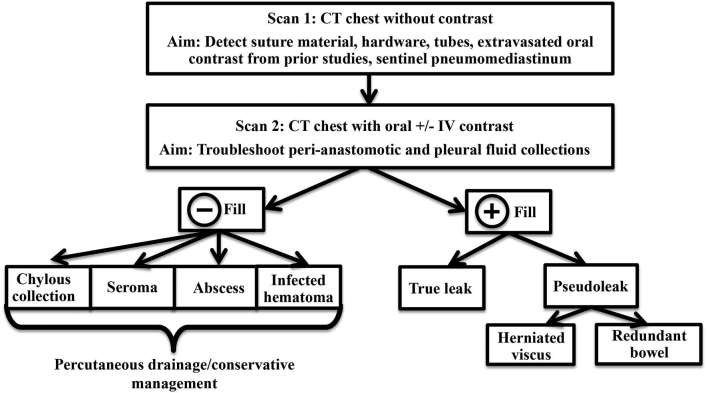
At our institution at Brigham and Women's hospital, patients with concern for oesophageal injury or anastomotic leak undergo a dual phase extended coverage chest CT with and without oral contrast (CT oesophageal leak protocol. Figures 3 and 4).10 The initial non-contrast study is performed at a lower radiation dose without administration of oral or intravenous contrast, and the purpose is to detect suture material, hardware, extravasated oral contrast from prior studies (these entities may be hard to distinguish from a new leak and can confound the diagnosis if only a single phase scan is done). Baseline non-contrast study may also help delineate sentinel pneumomediastinum, a finding which may be obscured following administration of a large bolus of dense oral contrast (Figure 5). Following the baseline scan, the second phase is repeated using standard radiation dose through the same coverage area using dilute non-ionic water-soluble oral contrast such as iohexol (Omnipaque 240 mg iodine ml–1, GE Healthcare, Princeton, NJ, USA). A 50 ml vial is diluted with approximately 500 ml of water and the contrast is given just before the CT examination. In addition, if there is concern for infectious complications such as abscess formation, 50 cc of non-ionic iodinated intravenous contrast such as iohexol (Omnipaque 350 mg iodine ml–1, GE Healthcare, Princeton, NJ, USA) is administered at 3 ml s–1 and images are acquired after a 30 s delay. Oral contrast helps to troubleshoot and characterize postoperative peri-anastomotic collections as identification of oral contrast extravasation helps make the diagnosis of an anastomotic leak and distinguishes this from other peri-anastomotic collections such as an abscess, haematoma, seroma or a chylous collection which do not fill-in with oral contrast (Figures 6 and 7).
Figure 3.
Specifics of dual phase extended field of view CT oesophageal leak protocol for evaluation of patients with suspected oesophageal injury.
Figure 4.
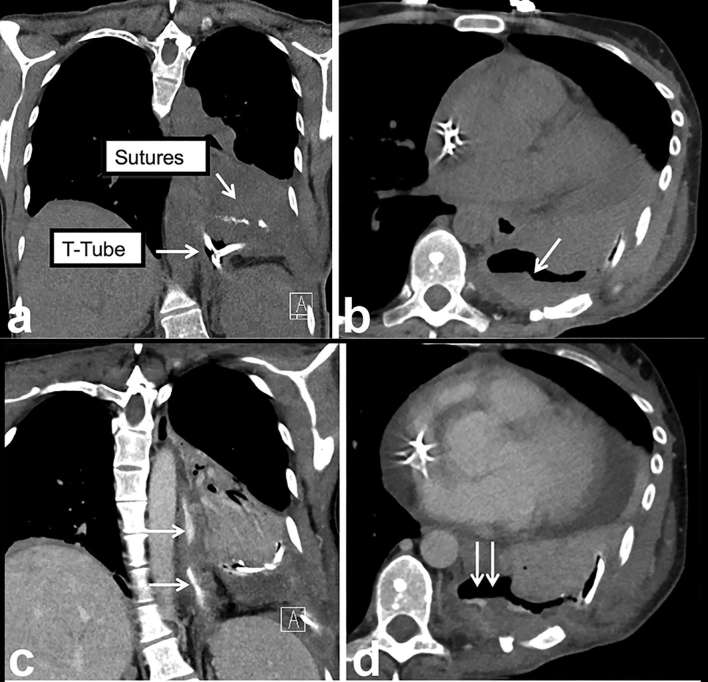
Nuts and bolts of CT oesophageal leak protocol highlighted using an example. (a) Coronal non-contrast CT image (without oral or intravenous contrast) demonstrates metallic suture material and a T-tube (arrows). (b) Persistent thick-walled basilar loculated hydropneumothorax (arrow) raises the possibility of a leak. (c, d) Coronal and axial images from second phase of the study following administration of oral and intravenous contrast demonstrate extravasation of contrast into the thick-walled collection (double arrows) confirming the leak (oesophagus—single arrows). Teaching point: since true leaks can be small and subtle, the presence of hardware, suture and residual oral contrast from prior imaging studies can confound and mimic a leak on a single phase study.
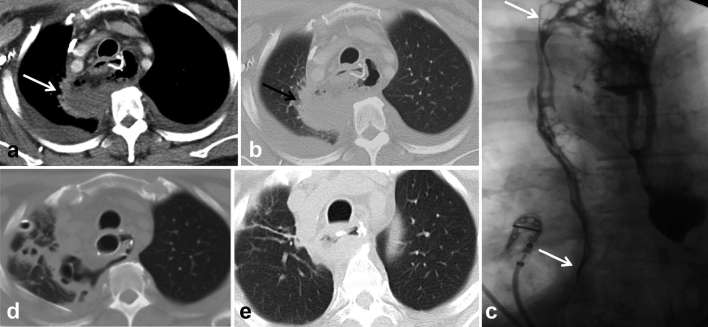
Figure 5.
Suspected injury of the upper thoracic oesophagus following traumatic intubation. Patient intubated and unable to swallow contrast. (a, b) Axial and sagittal chest images from a chest CT done without oral or i.v. contrast in lung windows shows sentinel pneumomediastinum (arrows) and left pneumothorax. Since this could emanate from either a tracheal or oesophageal injury, endoscopy was done and confirmed injury to the upper thoracic oesophagus. Teaching point: this case highlights the value of doing a non-contrast CT for detection of subtle pneumomediastinum, confirming suspicion of oesophageal injury. Often the patients’ clinical condition may preclude transfer to the fluoroscopic suite and administration of oral contrast.
Figure 6.
Nissen fundoplication surgery, fever and peri-anastomotic collection. (a) Axial contrast-enhanced CT image through the upper abdomen shows thick-walled perianastomotic collection (arrows) with fluid and air pockets; suspicious for abscess or infected haematoma vs an underlying leak. (b) Study repeated with oral contrast (arrow) reveals no extravasation, ruling out an oesophageal leak and confirming abscess complicating underlying wrap ischaemia. The patient defervesced with antibiotics and percutaneous drainage. Teaching point: oral contrast helps troubleshoot and characterize the aetiology of peri-anastomotic collections.
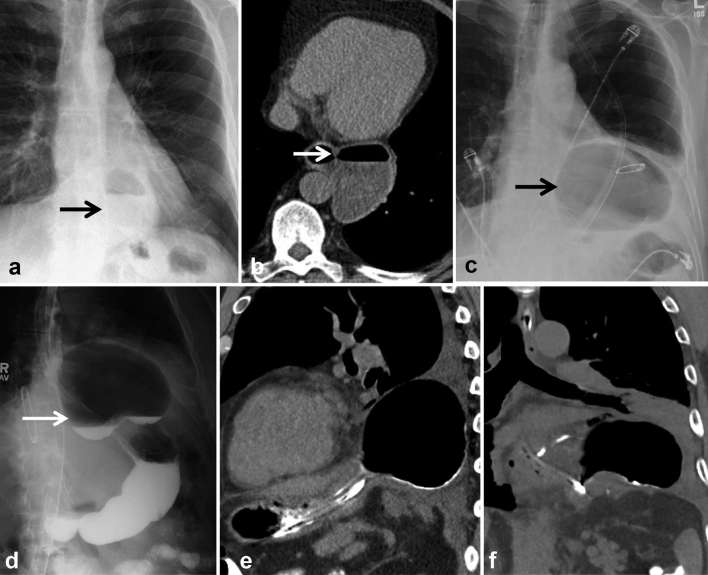
Figure 7.
Post-oesophagectomy, fever and peri-anastomotic collection.(a, b) Axial CT shows thick-walled air and fluid collection adjacent to proximal anastomosis (arrows), extending posterior to the conduit into the right upper lobe. (c) Contrast oesophagogram confirms the presence of a leak extending into the upper lobe (upper arrow) and pleural space (lower arrow). (d, e) Covered oesophageal stent was used to treat leak and subsequent CT shows decreasing size of the collection. Teaching point: mediastinal air/fluid collection may be due to abscess, infected haematoma or ongoing leak. CT with oral contrast or contrast oesophagogram is required to identify underlying anastomotic leak to guide management.
Our institutional CT protocol also uses extended coverage to include the neck from the level of the hyoid bone, the entire chest and the upper abdomen. This allows comprehensive one stop-shop imaging evaluation for patients with oesophagectomy, covering any proximal cervical, mediastinal and distal intra-abdominal anastomosis and any associated collections.
CT can accurately characterize the size of leak, the extent of mediastinal and pleural contamination and provide a roadmap for the surgeon. Based on our institutional experience of oesophageal leaks, the size of leaks may be graded as follows: Grade 1: blind ending tract; Grade 2: contained localized mediastinal collection with air/fluid/contrast; Grade 3: Non-contained collection with air/fluid contrast in mediastinum; and Grade 4: free contrast extravasation and spillage into the pleural space.
Pitfalls and oesophageal leak mimics
False-positives (pseudoleaks) and false-negatives: a critical point to remember while imaging patients with recent oesophageal surgery is that not all peri-anastomotic collections which fill-in with oral contrast represent oesophageal perforations or leaks. For instance, oral contrast may enter a herniated viscus or redundant bowel adjacent to the anastomosis and can mimic a leak (Figures 8 and 9). Here, the relationship of the “collection” to adjacent bowel and diaphragm, the presence of a bowel signature on imaging and identification of a mucosal pattern on endoscopy can help differentiate this from a leak. The importance of making a clear distinction between true leaks and pseudoleaks due to herniating viscous or redundant anastomosis cannot be overemphasized since inadvertent placement of a percutaneous drain or tube into a hollow viscus may cause much harm to the patient. False negative interpretations can be minimized by means of using optimized CT protocols, including use of dual phase studies and oral contrast for CT examinations (Figure 1). Figure 10 summarizes the value of different components of the dual phase extended coverage chest CT protocol, and a decision tree which can help the radiologist troubleshoot cases with suspected postoperative oesophageal injury.
Figure 8.
New gas collection following diverticulectomy and fundoplication for epiphrenic diverticulum. (a, b) Pre-operative frontal chest radiograph and chest CT show large narrow necked diverticulum (arrows). (c) Post-operative chest radiograph shows large air-filled collection which does not decompress following NG tube placement raising concern for leak. (d) The collection communicates with the oesophagus filling with oral contrast (arrow). (e, f) Sagittal and coronal chest CT images reveal air-filled structure to be supradiaphragmatic, with mucosal folds. Findings are consistent with herniation and incarceration of the gastric fundus above the wrap mimicking a leak. This was confirmed on endoscopy. Teaching point: peri-anastomotic collections which fill with oral contrast may sometimes represent a herniating viscus rather than a true leak; hence, knowledge of post operative anatomy is key. Percutaneous drainage of perianastomotic collections should not be recommended unless these are accurately characterized as true leaks and a herniating viscous is excluded.
Figure 9.
Post-resection and myomectomy for large bilobed oesophageal leiomyoma. (a, b) Axial and coronal CT images done with oral contrast following surgery show an air-filled mediastinal collection communicating with the oesophagus and filling in with oral contrast (arrows). Since this patient was febrile, this was initially thought to represent a leak at the site of myomectomy. (c) Review of pre-operative CT revealed a large bilobed oesophageal mass distending the oesophagus. The outpouching was correctly interpreted to be redundant oesophagus. Teaching point: differentiating outpouching due to redundant bowel from true leak can be difficult since both fill with oral contrast. Knowledge of the surgical details and pre-operative imaging can help in making the correct diagnosis.
Figure 10.
Decision tree to characterize and manage peri-anastomotic collections in patients following oesophageal surgery.
Close communication and teamwork between radiologists and surgical team
Accurate assessment of post-operative oesophageal injuries requires detailed knowledge of the operative details and post-operative anatomy (i.e. surgical access, type of anastomosis and time since surgery). This requires close communication with the surgeon including face-to-face discussion to understand the post-operative anatomy, to ascertain the clinical question to be answered and use this information to tailor imaging protocols. In patients with oesophagectomy, the site of leak and nature of complications have direct correlation with the surgical access chosen by the surgeon (transthoracic, transhiatal or minimally invasive laparoscopic approach).
CONCLUSIONS
In asymptomatic patients with recent oesophageal surgery and cervical/intrathoracic anastomosis, contrast oesophagogram is obtained on a routine basis prior to initiating oral feeds. CT chest with oral contrast is not advocated on a systematic basis, and is instead reserved for patients with symptoms and probability of underlying leak/complication.
Optimization of CT protocol is necessary for comprehensive one stop-shop imaging evaluation of oesophageal injury and leaks. This involves having extended coverage including lower neck, chest and upper abdomen as well as scanning the patient pre- and post-oral contrast administration. Imaging pitfalls-related to presence of hardware, suture material or oral contrast from prior examinations can be avoided by their detection on the initial non-contrast phase.
The differential diagnosis of peri-anastomotic and mediastinal/pleural collections in these post-operative patients is broad and further characterization is necessary before these can be appropriately managed. Non-filling collections usually represent abscesses, seromas, chylous collections and haematomas and can be drained percutaneously or conservatively managed.
Peri-anastomotic and mediastinal/pleural collections which fill with oral contrast usually represent leaks, and may require endoscopic stent placement or re-operation. Occasionally “collections” may represent herniating or redundant viscous. Close attention must be paid to surgical details to recognize this entity and avoid harm to the patient by erroneously placing percutaneous drains in these “collections”.
Thorough communication between radiologist and physician team is essential for appropriate management of patients with oesophageal anastomotic leaks.
ACKNOWLEDGMENTS
Dr Brett Carter receives royalty from Elsevier.
Contributor Information
Rachna Madan, Email: RMadan@partners.org.
Olga Laur, Email: olaur@partners.org.
Breland Crudup, Email: brudup@umc.edu.
Latia Peavy, Email: ldpeavy@gmail.com.
Brett W Carter, Email: BCarter2@mdanderson.org.
REFERENCES
- 1.Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004; 10: 71–5. [PubMed] [Google Scholar]
- 2.Jones CM, Clarke B, Heah R, Griffiths EA. Should routine assessment of anastomotic integrity be undertaken using radiological contrast swallow after oesophagectomy with intra-thoracic anastomosis? Best evidence topic (BET. Int J Surg 2015; 20: 158–62. doi: https://doi.org/10.1016/j.ijsu.2015.06.076 [DOI] [PubMed] [Google Scholar]
- 3.Whooley BP, Law S, Alexandrou A, Murthy SC, Wong J. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg 2001; 181: 198–203. doi: https://doi.org/10.1016/S0002-9610(01)00559-1 [DOI] [PubMed] [Google Scholar]
- 4.Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013; 96: 1919–26. doi: https://doi.org/10.1016/j.athoracsur.2013.07.119 [DOI] [PubMed] [Google Scholar]
- 5.Messager M, Warlaumont M, Renaud F, Marin H, Branche J, Piessen G, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017; 43: 258–69. doi: https://doi.org/10.1016/j.ejso.2016.06.394 [DOI] [PubMed] [Google Scholar]
- 6.Tanomkiat W, Galassi W. Barium sulfate as contrast medium for evaluation of postoperative anastomotic leaks. Acta Radiol 2000; 41: 482–5. doi: https://doi.org/10.1080/028418500127345730 [DOI] [PubMed] [Google Scholar]
- 7.Cools-Lartigue J, Andalib A, Abo-Alsaud A, Gowing S, Nguyen M, Mulder D, et al. Routine contrast esophagram has minimal impact on the postoperative management of patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol 2014; 21: 2573–9. doi: https://doi.org/10.1245/s10434-014-3654-1 [DOI] [PubMed] [Google Scholar]
- 8.Upponi S, Ganeshan A, D'Costa H, Betts M, Maynard N, Bungay H, et al. Radiological detection of post-oesophagectomy anastomotic leak - a comparison between multidetector CT and fluoroscopy. Br J Radiol 2008; 81: 545–8. doi: https://doi.org/10.1259/bjr/30515892 [DOI] [PubMed] [Google Scholar]
- 9.Fadoo F, Ruiz DE, Dawn SK, Webb WR, Gotway MB. Helical CT esophagography for the evaluation of suspected esophageal perforation or rupture. AJR Am J Roentgenol 2004; 182: 1177–9. doi: https://doi.org/10.2214/ajr.182.5.1821177 [DOI] [PubMed] [Google Scholar]
- 10.Madan R, Bair RJ, Chick JF. Complex iatrogenic esophageal injuries: an imaging spectrum. AJR Am J Roentgenol 2015; 204: W116–W125. doi: https://doi.org/10.2214/AJR.14.12476 [DOI] [PubMed] [Google Scholar]