Abstract
Objective:
This study assessed the use of implanted hydrogel rectal spacers for stereotactic ablative radiotherapy-volumetric modulated arc therapy (SABR-VMAT) patients, investigating practicality, dosimetric impact, normal tissue complication probability (NTCP) and early toxicity.
Methods:
Data from the first 6 patients treated within a prostate SABR and rectal spacer trial were examined to determine spacer insertion tolerability, resultant changes in treatment planning and dosimetry and early toxicity effects. CT scans acquired prior to spacer insertion were used to generate SABR plans which were compared to post-insertion plans. Plans were evaluated for target coverage, conformity, and organs at risk doses with NTCPs also determined from resultant dose fluences. Early toxicity data were also collected.
Results:
All patients had successful spacer insertion under local anaesthetic with maximal Grade 1 toxicity. All plans were highly conformal, with no significant differences in clinical target volume dose coverage between pre- and post-spacer plans. Substantial improvements in rectal dose metrics were observed in post-spacer plans, e.g. rectal volume receiving 36 Gy reduced by ≥42% for all patients. Median NTCP for Grade 2 + rectal bleeding significantly decreased from 4.9 to 0.8% with the use of a rectal spacer (p = 0.031). To date, two episodes of acute Grade 1 proctitis have been reported following treatment.
Conclusion:
The spacer resulted in clinically and statistically significant reduction in rectal doses for all patients.
Advancesin knowledge:
This is one of the first studies to investigate the efficacy of a hydrogel spacer in prostate SABR treatments. Observed dose sparing of the rectum is predicted to result in meaningful clinical benefit.
Introduction
In light of the potentially low α/β ratio,1 stereotactic ablative radiotherapy (SABR) is increasingly being studied as a treatment option for prostate cancer, generating highly conformal dose distributions around the prostate, with steep dose gradients to spare neighbouring normal tissue.2–4 However, despite technological advances, a major limitation to dose escalation is the increased risk of normal tissue damage, particularly to the rectum and bladder. While prostate SABR appears to be well-tolerated, there are reports of increasing rectal toxicity in SABR trials that have dose-escalated ≥40 Gy in 5 fractions.5, 6 A number of solutions have recently been developed to address this rectal toxicity limitation, by modifying the patient’s anatomy to increase the separation between the anterior rectal wall and the prostate gland.7 Several of these spacer solutions are now commercially available and have been incorporated into a number of conventionally fractionated clinical trials which have demonstrated a reduction in acute and late rectal toxicity.7, 8
At the Northern Ireland Cancer Centre we are currently recruiting to a randomized feasibility study, evaluating SABR treatments in high-risk localized prostate cancer with or without elective nodal irradiation (ENI), the SPORT High-Risk Trial.9 All trial participants are implanted with a hydrogel rectal spacer system (SpaceOAR®, Augmenix, Waltham, MA) prior to SABR treatment. In this report, we present our initial experience of using a hydrogel rectal spacer system with prostate SABR. We evaluate the practicality and tolerability of the hydrogel spacer and quantify its rectal dosimetry benefits for prostate SABR treatments on our initial cohort of patients, including normal tissue complication probability (NTCP) for Grade 2 + rectal bleeding and acute toxicity.
methods and materials
Ethical approval and patient selection
Ethical approval for the clinical trial was granted by the Health and Social Care Research Ethics Committee (REC) A (REC reference 15/NI/0192). The study is open to male patients ≥18 years old with at least one of the following criteria: histologically confirmed prostate adenocarcinoma presented with clinical stage T3a N0 M0, Gleason score 7 (4 + 3) or above and/or PSA >20 and who were planned to receive 1–3 years ADT as part of their standard treatment. Patients with overt T3b stage cancer were excluded from the trial. Six patients have currently been recruited to the SPORT trial and their data were used for analysis in this investigation.
Rectum spacer and fiducial marker implantation
All procedures were performed transperineally with patients in the dorsal lithotomy position, with local anaesthetic only, under transrectal ultrasound (TR ultrasound) guidance. 10 mls 1% lidocaine was infiltrated into the sub-cutaneous tissues, with a further 10 mls infiltrated into the prostatic neurovascular bundles bilaterally. Six targeted intraprostatic prostate biopsies were then taken for translational biomarker research and three intraprostatic fiducial markers were inserted. Finally, an 18-gauge needle was inserted between the prostate and the rectum using sagittal TR ultrasound image guidance. Normal saline was used to hydrodissect the space between the rectoprostatic fascia and anterior rectal wall, and 10 mls of hydrogel liquid was injected into this space, where the hydrogel polymerized within seconds of injection to form a gel.
Two consultant clinical oncologists were trained to perform the implantation procedure, both of whom were experienced in prostate brachytherapy. The first three cases per clinician were supervized (i.e. all patients in this study). Antibiotic cover consisting of oral ciprofloxacin for 5 days was prescribed to start immediately prior to insertion. All patients were contacted on day 4 to assess the tolerability of the procedure, with any reported adverse effects scored against common terminology criteria for adverse events criteria.
Image acquisition and organ delineation
Prior to spacer implant, each patient had pre-spacer CT images acquired using a helical CT-simulator (512 × 512 field of view, 1 mm axial pixel resolution, 2.5 mm slice width). Patients were advised to follow a bowel and bladder preparation procedure before CT acquisition and each treatment session. One hour before the imaging or treatment session a micro-enema (Micralax®) was administered and the patients were also asked to empty their bladder. The patients were then advised to wait 10 min before drinking 500 mls of water within a 10 min period. Post-spacer CT and T2 weighted MR images were acquired on the same day, 1 week after spacer insertion. To assist with spacer delineation, the MR images were fused with the post-spacer CT images using the Eclipse™ treatment planning system v. 13.6 (Varian Medical Systems, Palo Alto, CA). Structures of interest were contoured manually in Eclipse by one of two consultant clinical oncologists, where the treating oncologist contoured the same structures on both CT image sets to allow comparison between pre- and post-spacer plans. Pelvic nodes clinical target volumes (CTVs) were defined for patients randomized to the ENI arm, however these were not utilized in this dosimetric investigation. In this study, only the prostate and proximal 10 mm of the seminal vesicles (PSV) CTV was used, reflecting the prostate only trial arm.
Treatment planning
A 5 mm margin was expanded isotropically around the CTV to generate the planning target volume (PTV). For this study, the contoured organs at risk (OARs) included the bladder, rectum, femoral heads and penile bulb. Contouring of the rectum was limited to the same number of corresponding CT slices on both image sets, to facilitate comparison between the plans generated. All planning was performed in Eclipse using the Progressive Resolution Optimization (v. 13.6) and Acuros XB dose calculation (v. 13.6.23) algorithms for a Varian TrueBeam Linac. The dose calculation grid size used was 2.5 mm and the heterogeneity correction and jaw tracking settings were enabled.
Plans were generated following our previously reported class solution,10 and employed a single 300° partial volumetric modulated arc therapy (VMAT) arc (210–150°), delivered using a 10 MV flattening filter free photon beam with a maximum dose-rate of 2400 MU min–1. For one patient (Patient 3) the class solution could not be applied due to the presence of a right hip prosthesis, therefore two 215° partial arcs (335–180°) were used instead. Planning objectives and constraints for targets and OARs are provided in Table 1. The prescribed dose was 40 Gy for the CTV and 36.25 Gy for the PTV, delivered simultaneously, in 5 fractions.
Plan evaluation
The dose metrics described in Table 1 were used to evaluate the plans generated on the two CT image sets. In addition to these metrics, the dose conformity index 11 was also determined, together with the medium-dose spillage outside the PTV ,12 where Vol95% and Vol50% are the tissue volumes receiving at least 95 and 50% of the PTV prescription dose respectively. Also, included in Table 1 are definitions for minor and major variations from the dose objectives considered in this investigation. To allow for comparison between optimized plans, priority was given to achieving target structure objectives and only variations from the OAR objectives were permitted. For both datasets every effort was taken to achieve the OAR objectives without compromising dose coverage of the target structures.
Table 1. .
Planning objectives and constraints for targets and OARs
| Target | Objective | Dose variation | |
| Minor | Major | ||
| PTV/CTV | VPrescription ≥ 95% | 90% ≤ VPrescription < 95% | VPrescription < 90% |
| PTV | D98% ≥ 34.4 Gy | ||
| Dmax < 48 Gy | |||
| D2% ≤ 42.8 Gy | |||
| OAR | Dose constraints | ||
| Rectum | V18.1 Gy < 50% V29 Gy < 20% V36 Gy < 1cc |
1 cc ≤ V36 Gy < 2 cc | V36 Gy≥ 2 cc |
| Bladder | V18.1 Gy < 40% V37 Gy < 10 cc |
10 cc ≤ V37 Gy < 20 cc | V37 Gy ≥ 20 cc |
| Penile bulb | V29.5 Gy < 50% | ||
| Femoral head | V14.5 Gy < 5% | ||
CTV, clinical target volume; OAR, organ at risk; PTV, planning target volume.
TCP and NTCP
Tumour control probabilities (TCP) and NTCPs were calculated using the Lyman–Kutcher–Burman model.13 Further information on these calculations, model parameters used and different endpoints are provided in (Supplementary Material 1, Supplementary material available online).
Statistical analysis
Statistical analysis was conducted to compare the dose volume histogram (DVH) metrics analysed for pre-spacer and post-spacer plans. This analysis was conducted in MATLAB (v. 8.2-R2013b) using the non-parametric two-sided paired-sample Wilcoxon signed rank test, the significance level was set at p ≤ 0.05.
Results
The intraprostatic biopsy procedure with fiducial marker and hydrogel spacer insertion was well tolerated under local anaesthetic. The total procedure time was 20–25 min per patient and all patients were discharged 1 h after completion. Telephone follow up on day 4 identified two patients who experienced Grade 1 rectal bleeding, two patients who had Grade 1 haematuria and one patient with Grade 1 urgency post-procedure. All toxicities resolved within 24–48 h.
Dosimetric comparison of optimized plans
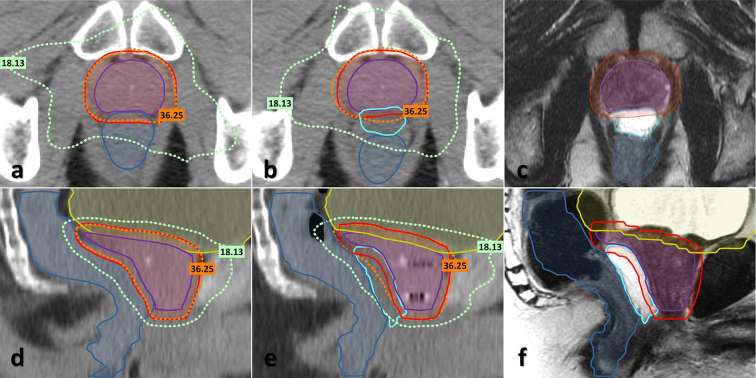
Figure 1 displays examples of axial and sagittal CT views, including target and OAR contours acquired before (a, d) and after (b, e) spacer and FM insertion. It is evident from these figures that the spacer is not easily distinguished from neighbouring soft tissue. Therefore registered MR images, acquired on the same day as the post-spacer CT, are also included in Figure 1 panels (c, f) which allow the spacer to be easily delineated. Overlaid on the CT images are the 100% (36.25 Gy) and 50% (18.13 Gy) isodose lines of the PTV prescription dose. The images demonstrate how the spacer has displaced the rectum away from the prostate and as a result has reduced the volume of the rectum that overlaps with the high radiation dose region.
Figure 1.
Axial (a–c) and sagittal (d–f) CT and MRI images acquired from patient 6 prior to and after hydrogel spacer insertion. (a, d) CT images acquired prior to spacer insertion. (b, e) CT images acquired following spacer and fiducial marker insertion. (c, f) Corresponding post-spacer MR images. Images include contours for the prostate and seminal vesicles CTV (purple) and PTV (red), bladder (yellow), rectum (blue) and spacer (cyan) structures and dashed lines corresponding to the 100 and 50% isodoses of the PTV prescription dose. CTV, clinical target volume; PTV, planning target volume. (Colour available online only)
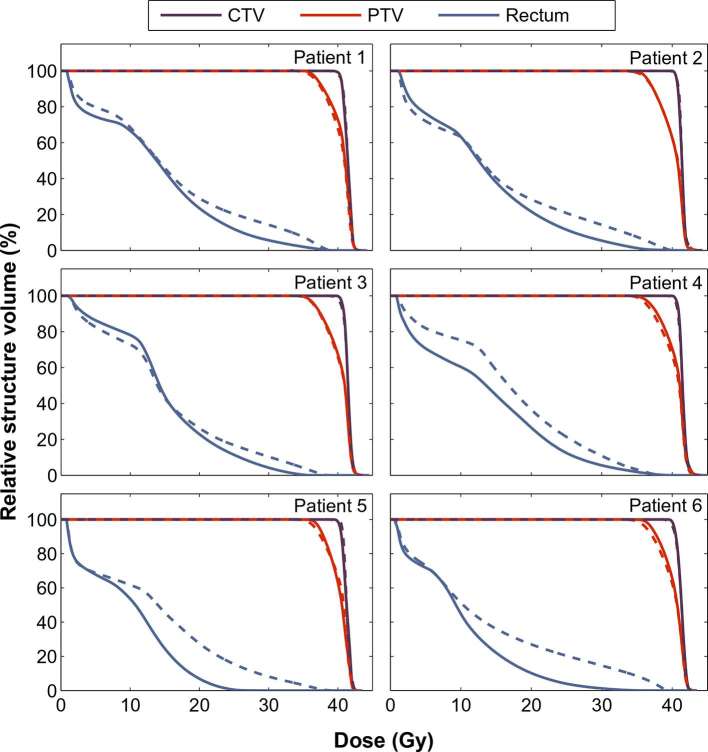
Figure 2 displays individual DVHs for target and rectum structures for the 6 patients included in this study. When generating the optimized plans, priority was given to achieving the target volume constraints. As a result, DVHs generated for the CTVs using the two CT image sets are very similar. However, even with this approach, the pre-spacer PTV dose coverage was reduced compared to the post-spacer plans for the majority of patients, as a result of trying to achieve the rectum constraints. This is also reflected in the target dose metrics extracted from the DVHs reported in Table 2 (full details provided in Supplementary Table 1). While there were no significant differences in the CTV metrics, the median pre-spacer PTV volume receiving the prescription dose was significantly reduced by 2.1% (98.1 vs 96.0 for post-spacer and pre-spacer CTs respectively, p = 0.031).
Figure 2.
Dose volume histograms for target structures and rectum for individual patients, for optimized plans generated using the post-spacer (solid lines) or pre-spacer (dashed lines) CT images.
Table 2.
Summary of key plan evaluation and target structure DVH metrics for patient cohort, median (min–max)
| Metric | Objective | Target volume | Pre-spacer plans | Post-spacer plans |
| VPrescription (%) | >95% | CTV | 98.8 (96.3–99.7) | 99.5 (98.6–100.0) |
| PTV | 96.0 (95.4–97.1) | 98.1a (96.5–99.4) | ||
| D98% (Gy) | PTV> 34.4 Gy | 35.6 (35.4–35.9) | 36.2a (35.8–36.7) | |
| D2% (Gy) | PTV < 42.8 Gy | 42.3 (42.2–42.4) | 42.3 (42.0–42.7) | |
| MU | 2272 (2248–2475) | 2240 (2143–2423) | ||
| CI | <1.2 | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) |
CTV, clinical target volume; DVH, dose volume histogram; PTV, planning target volume.
ap ≤ 0.05 considered statistical significant.
Figure 2 also indicates that all six patients benefited from a large reduction in the volume of rectum exposed to high radiation doses (>18 Gy), as a result of the spacer insertion. Key metrics for the OAR structures are reported in Table 3, with full metrics reported in Supplementary Table 2. Of the pre-spacer plans, only one plan (Patient 4) met all target and OAR objectives and constraints. For three patients (1, 2 and 6) it was not possible to generate treatment plans on the pre-spacer anatomy without a major variation from the V36 Gy <1 cc rectum objective, i.e. V36 Gy >2 cc, due to substantial overlap of the PTV and rectum volumes. In contrast, no major or minor violations of the rectum dose objectives were observed in any of the post-spacer plans and the rectum volume receiving 36 Gy was significantly reduced (median 2.6 cc pre-spacer vs 0.3 cc post-spacer, p = 0.031). Further analysis of individual patient data (Supplementary Table 2) revealed that all patients had a reduction in their rectal V36 Gy volume of more than 42% for plans generated on the post-spacer CT image sets. Table 3 also indicates a median reduction of 5.2 Gy in the maximum dose absorbed by 2 cc of the rectum for plans generated using the spacer-modified anatomy, from 36.3 Gy in the pre-spacer plans to 31.1 Gy in the post-spacer plans. No significant difference was observed in the dose metrics recorded for the bladder, femoral heads or penile bulb when pre-spacer and post-spacer plans were compared.
Table 3.
Key DVH metrics of patient cohort for rectum and bladder OARs, median (min–max)
| OAR | Metric | Constraint | Pre-spacer plans | Post-spacer plans |
| Rectum | DMean (Gy) | 15.0 (13.8–16.9) | 13.4a (9.9–14.9) | |
| D2cc (Gy) | 36.3 (34.1–37.8) | 31.1a (21.5–33.6) | ||
| V18.1 Gy (%) | <50% | 33.0 (30.0–44.2) | 28.0a (11.9–33.4) | |
| V29 Gy (%) | <20% | 14.1 (9.4–15.7) | 5.9a (0.0–6.6) | |
| V36 Gy (cc) | <1 cc | 2.6 (1.0–4.1) | 0.3a (0.0–1.0) | |
| V38 Gy (cc) | 0.5 (0.2–1.7) | 0.1 (0.0–0.3) | ||
| Bladder | DMean (Gy) | 8.5 (5.8–11.1) | 9.2 (5.8–15.3) | |
| D2 cc (Gy) | 39.9 (38.7–41.1) | 39.6 (38.1–41.6) | ||
| V18.1 Gy (%) | <40% | 20.6 (11.0–25.4) | 21.7 (11.9–38.5) | |
| V37 Gy (cc) | <10cc | 6.9 (4.4–13.9) | 7.0 (3.4–12.5) |
DVH, dose volume histogram; OAR, organ at risk.
ap ≤ 0.05 considered statistical significant.
EUD and NTCP comparison
Reported in Table 4 are the TCP values estimated for the prostate CTV and NTCP values for rectum and bladder toxicity endpoints. While there was no significant difference in the TCPs for the two datasets, the median NTCP for Grade 2 + rectal bleeding complications15 was significantly reduced from 4.9% in the pre-spacer dataset to 0.8% in the post-spacer dataset. NTCPs for Grade 2 + rectal bleeding toxicity calculated for each patient are displayed in Figure 3. The maximum complication probability was 9.9%, calculated for the pre-spacer image set of patient 6, this was subsequently reduced to 0.0% for the plan generated using the patient’s rectal spacer image set. There was no significant difference in the bladder cystitis NTCP between the two datasets.
Table 4.
TCP/NTCP analysis performed on the CTV, bladder and rectum structures, median (min–max)
| Structure | TCP/NTCP | Pre-spacer plans | Post-spacer plans |
| CTV | Tumour control14 | 98.8 (98.6–98.8) | 98.7 (98.6–98.9) |
| Rectum | Bleeding 2+15 | 4.9 (2.5–9.9) | 0.8a (0.0–1.7) |
| Incontinence16 | 3.4 (3.0–3.9) | 2.8a (1.9–3.0) | |
| Severe frequency17 | 0.9 (0.5–1.0) | 0.3a (0.0–0.3) | |
| Bladder | Cystitis/loss of volume18 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
CTV, clinical target volume; NTCP, normal tissue complication probability; TCP, Tumour control probabilities.
ap ≤ 0.05 considered statistical significant.
Figure 3.
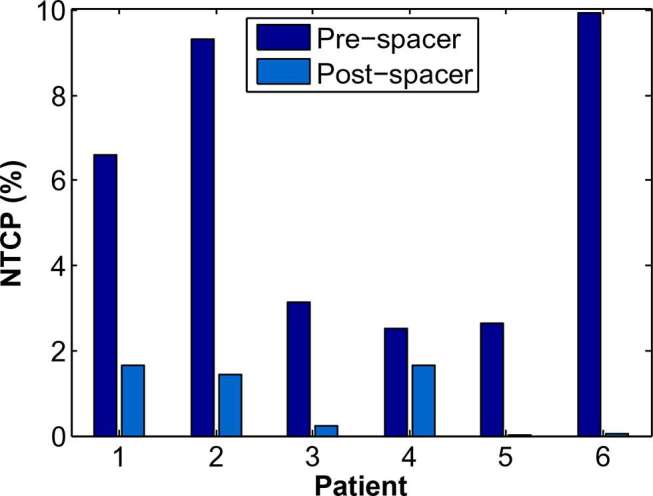
Change in Grade 2 + rectal bleeding NTCP, calculated using QUANTEC LKB model parameters,15 for individual patients as a result of using a hydrogel rectal spacer. LKB, Lyman–Kutcher–Burman; NTCP, normal tissue complication probability.
Early Toxicity
For the 6 patients (3 prostate only, 3 ENI), acute rectal and gastrointestinal toxicity from treatment has been satisfactory to date. With five patients beyond three months' follow up and the sixth patient beyond 6 weeks, only 2 episodes of Grade 1 proctitis have been observed, both of which lasted less than 5 days. Clearly, longer follow-up in a larger cohort of patients is required to assess treatment-related toxicity.
Discussion
In this report we have evaluated the potential dose sparing benefits from use of a hydrogel rectal spacer system in the first six patients recruited to a prostate SABR clinical trial. The spacer has been well-tolerated and allowed more patients to achieve both target volume objectives and OAR constraints, with significant improvements in rectal DVHs and corresponding NTCPs observed in the post-spacer radiotherapy plans. To date, prostate SABR has been well-tolerated by the patient cohort, although further recruitment and follow-up is required. As reported in other studies,7, 18 spacer delineation using CT images alone was difficult, therefore a multi-imaging modality approach (including MR imaging) was necessary for accurate contouring.
There is increasing evidence that biochemical control of localized prostate cancer is improved by dose-escalated radiotherapy.18 Despite recent technological advancements, further dose-escalation is limited by the proximity of the prostate to the anterior wall of the rectum, with the rectum volume receiving ≥60 Gy associated with a greater risk of Grade 2 + rectal toxicity or bleeding in conventionally fractionated radiotherapy.15 To reduce the dose to the rectum many clinical protocols have used an anisotropic CTV-PTV margin, limiting posterior expansion to reduce rectal radiation doses; however, a rectal spacer system provides an alternative option without having to potentially compromise target coverage.
Early investigations identified Polyethylene-glycol (PEG)-based19 and Hyaluronic acid-based20 hydrogels as potential prostate-rectum spacers. Commercial versions of these hydrogels, e.g. SpaceOAR (Augmenix, Waltham, MA), NASHA Spacer gel (Q-Med AB, Uppsala, Sweden)21 and Hylaform (Genzyme Corporation, Cambridge, MA),22 have now been incorporated into a number of clinical trials including this SABR study.9 Human collagen has also been investigated as a potential spacer option,23 while Gez et al24 have evaluated the safety and efficacy of ProSpace™ (BioProtect Ltd, Kfar-Saba, Israel), a transperineal implantable biodegradable balloon system.24
The dosimetric benefit of hydrogel rectal spacers has been reported in a number of conventionally fractionated IMRT studies.25–27 In keeping with our results, these studies have reported >95% of spacer patients having a ≥25% reduction in rectal volume receiving high doses (V70 Gy).26, 27 Similar to the results reported by Pinkawa et al, we also observed a learning curve to the hydrogel spacer placement.28 While the hydrogel placement in the first two patients exhibited a degree of asymmetry, it became increasing symmetric with each successive patient. Fischer-Valuck et al have reported that significant reduction of rectal dose can still be achieved even with asymmetric hydrogel spacer placement29 and this was also observed in our study.
Clinical results from a recent phase III trial, that employed the SpaceOAR hydrogel rectal spacer and recruited 222 patients, have described the acute and late toxicity benefits to a spacer system.8, 27,30 In agreement with the NTCPs predicted from our SABR study, long-term (3 year) follow-up found that the reduction in rectal dose, as a result of using the spacer, correlated with a significant reduction in both Grade 1+ (9.2 vs 2.0%) and Grade 2+ (5.7 vs 0%) rectal toxicity. Given the life expectancy of these patients, these results are both important and clinically meaningful. Quality of life, scored using the Expanded Prostate Cancer Index Composite questionnaire, demonstrated statistically significant improvements in bowel quality of life scores in the spacer patient group.30 Interestingly, the authors have also reported a significant increase in the percentage of patients where the penile bulb mean dose constraint (<23 Gy) was achieved using the rectal spacer system, which correlated with an improvement in sexual function.30
Increased occurrences of high grade rectal toxicities have been reported in a number of recent SABR clinical trials. For instance, in a phase I/II study of prostate SABR with doses of 45–50 Gy in 5 fractions, 6 out of 91 patients developed Grade 3 or 4 rectal toxicity, with 5 patients requiring diverting colostomy.5 In a separate prostate SABR study, rates of late rectal bleeding were 19.4% in 258 patients treated to 35–40 Gy in 5 fractions.31 In their study, the volume of rectum receiving 38 Gy was found to be a predictor for rectal bleeding (odds ratio = 4.7 if >2.0 cm3). Both studies demonstrated a direct dose response for rectal toxicity with prostate SABR. The dose-sparing observed in our investigation suggests that the use of a rectal spacer may reduce the incidence of high grade rectal toxicities, although this needs tested in large prospective clinical trials.
Some groups have attempted to quantify the cost-effectiveness of rectal spacers. Vanneste et al performed a cost-benefit analysis, comparing treatment follow-up and toxicity costs with quality-adjusted life years.32 They calculated a cost of €55,880 per quality-adjusted life years gained and, assuming the €80,000 ceiling ratio used in the Netherlands, determined a 77% probability of spacer use being cost-effective, but acknowledged that this probability could be considerably lower using the £30,000 threshold set by the UK National Institute for Health and Clinical Excellence. The group subsequently developed a set of decision rules, based on clinical risk factors, to identify the patients expected to benefit the most from a rectal spacer.33 Hutchinson et al evaluated the 10-year costs associated with rectal toxicity complications across different RT modalities and found that spacer use was immediately cost-effective for high dose (50 Gy) SABR.34 This recommendation will be of particular relevance to future dose-painting trials where radiation boosts to the dominant intraprostatic lesions (DILs) may be limited by rectum dose constraints,35, 36 with UK radiotherapy centres keenly awaiting NICE guidance for rectal spacer use in prostate radiotherapy.37
Conclusion
Early clinical results from this UK cohort indicate that the insertion of a perirectal spacer was well tolerated by all patients. To our knowledge this is the first reported UK experience of a hydrogel rectal spacer system. Use of the spacer resulted in significant sparing of the rectum, with improvements in clinically significant rectal dose metrics and corresponding reductions in NTCPs for different rectal toxicity endpoints. This promising treatment approach merits further investigation in future prostate SABR studies.
ACKNOWLEDGEMENTS
This work was supported through grants from Prostate Cancer UK, CRUK and the Movember foundation (grant number CEO13_2-004 (FASTMAN Centre)), the R&D division of the Public Health Agency (grant number: COM/4965/14) and Friends of the Cancer Centre, registered with The Charity Commission of Northern Ireland (NIC101345). The planning study was carried out using equipment kindly donated by the Friends of the Cancer Centre.
Contributor Information
Raymond B King, Email: raymond.king@belfasttrust.hscni.net.
Sarah OS Osman, Email: s.osman@qub.ac.uk.
Ciaran Fairmichael, Email: c.fairmichael@qub.ac.uk.
Denise M Irvine, Email: denise.irvine@belfasttrust.hscni.net.
Ciara A Lyons, Email: ciaralyons@gmail.com.
Ananth Ravi, Email: ananth.ravi@sunnybrook.ca.
Joe M O'Sullivan, Email: joe.osullivan@belfasttrust.hscni.net.
Alan R Hounsell, Email: alan.hounsell@belfasttrust.hscni.net.
Darren M Mitchell, Email: darren.mitchell@belfasttrust.hscni.net.
Conor K McGarry, Email: conor.mcgarry@belfasttrust.hscni.net.
Suneil Jain, Email: s.jain@qub.ac.uk.
Declaration of interest
The Belfast Health and Social Care Trust has a research agreement with oncology systems limited (OSL, Shrewsbury, UK), UK distributor of SpaceOAR®,for the use of SpaceOAR in the SPORT clinical trial.
REFERENCES
- 1.Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys 2013; 85: 89–94. doi: https://doi.org/10.1016/j.ijrobp.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol 2011; 6: 3. doi: https://doi.org/10.1186/1748-717X-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loblaw A, Cheung P, D'Alimonte L, Deabreu A, Mamedov A, Zhang L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol 2013; 107: 153–8. doi: https://doi.org/10.1016/j.radonc.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 4.Alongi F, Cozzi L, Arcangeli S, Iftode C, Comito T, Villa E, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 2013; 8: 171–8. doi: https://doi.org/10.1186/1748-717X-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DW, Cho LC, Straka C, Christie A, Lotan Y, Pistenmaa D, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89: 509–17. doi: https://doi.org/10.1016/j.ijrobp.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Bauman G, Ferguson M, Lock M, Chen J, Ahmad B, Venkatesan VM, et al. A phase 1/2 trial of brief androgen suppression and stereotactic radiation therapy (FASTR) for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2015; 92: 856–62. doi: https://doi.org/10.1016/j.ijrobp.2015.02.046 [DOI] [PubMed] [Google Scholar]
- 7.Mok G, Benz E, Vallee JP, Miralbell R, Zilli T. Optimization of radiation therapy techniques for prostate cancer with prostate-rectum spacers: a systematic review. Int J Radiat Oncol Biol Phys 2014; 90: 278–88. doi: https://doi.org/10.1016/j.ijrobp.2014.06.044 [DOI] [PubMed] [Google Scholar]
- 8.Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys 2017; 97: 976–85. doi: https://doi.org/10.1016/j.ijrobp.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 9.ClinicalTrials.gov. SPORT High-Risk Trial Evaluating SABR in Prostate Cancer (SPORT). 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03253978 [accessed 19.12.17]
- 10.Osman SO, Jeevanandam P, Kanakavelu N, Irvine DM, Lyons CA, Jain S, et al. Class solutions for SABR-VMAT for high-risk prostate cancer with and without elective nodal irradiation. Radiat Oncol 2016; 11: 155. doi: https://doi.org/10.1186/s13014-016-0730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006; 64: 333–42. doi: https://doi.org/10.1016/j.ijrobp.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 12.Stanley DN, Popp T, Ha CS, Swanson GP, Eng TY, Papanikolaou N, et al. Dosimetric effect of photon beam energy on volumetric modulated arc therapy treatment plan quality due to body habitus in advanced prostate cancer. Pract Radiat Oncol 2015; 5: e625–e633. doi: https://doi.org/10.1016/j.prro.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 13.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35. doi: https://doi.org/10.1016/0360-3016(91)90172-Z [DOI] [PubMed] [Google Scholar]
- 14.Park JY, Lee JW, Chung JB, Choi KS, Kim YL, Park BM, et al. Radiobiological model-based bio-anatomical quality assurance in intensity-modulated radiation therapy for prostate cancer. J Radiat Res 2012; 53: 978–88. doi: https://doi.org/10.1093/jrr/rrs049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl): S123–S129. doi: https://doi.org/10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters ST, Hoogeman MS, Heemsbergen WD, Hart AA, Koper PC, Lebesque JV. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006; 66: 11–19. doi: https://doi.org/10.1016/j.ijrobp.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 17.Murray LJ, Lilley J, Thompson CM, Cosgrove V, Mason J, Sykes J, et al. Prostate stereotactic ablative radiation therapy using volumetric modulated arc therapy to dominant intraprostatic lesions. Int J Radiat Oncol Biol Phys 2014; 89: 406–15. doi: https://doi.org/10.1016/j.ijrobp.2014.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009; 74: 1405–18. doi: https://doi.org/10.1016/j.ijrobp.2008.10.091 [DOI] [PubMed] [Google Scholar]
- 19.Susil RC, McNutt TR, DeWeese TL, Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 1251–8. doi: https://doi.org/10.1016/j.ijrobp.2009.07.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prada PJ, Fernández J, Martinez AA, de la Rúa A, Gonzalez JM, Fernandez JM, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys 2007; 69: 95–102. doi: https://doi.org/10.1016/j.ijrobp.2007.02.034 [DOI] [PubMed] [Google Scholar]
- 21.Chapet O, Udrescu C, Devonec M, Tanguy R, Sotton MP, Enachescu C, et al. Prostate hypofractionated radiation therapy: injection of hyaluronic acid to better preserve the rectal wall. Int J Radiat Oncol Biol Phys 2013; 86: 72–6. doi: https://doi.org/10.1016/j.ijrobp.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 22.Wilder RB, Barme GA, Gilbert RF, Holevas RE, Kobashi LI, Reed RR, et al. Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010; 77: 824–30. doi: https://doi.org/10.1016/j.ijrobp.2009.05.069 [DOI] [PubMed] [Google Scholar]
- 23.Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 1918–22. doi: https://doi.org/10.1016/j.ijrobp.2011.02.034 [DOI] [PubMed] [Google Scholar]
- 24.Gez E, Cytron S, Ben Yosef R, London D, Corn BW, Alani S, et al. Application of an interstitial and biodegradable balloon system for prostate-rectum separation during prostate cancer radiotherapy: a prospective multi-center study. Radiat Oncol 2013; 8: 96. doi: https://doi.org/10.1186/1748-717X-8-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinkawa M, Corral NE, Caffaro M, Piroth MD, Holy R, Djukic V, et al. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol 2011; 100: 436–41. doi: https://doi.org/10.1016/j.radonc.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Song DY, Herfarth KK, Uhl M, Eble MJ, Pinkawa M, van Triest B, et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys 2013; 87: 81–7. doi: https://doi.org/10.1016/j.ijrobp.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy.Int J Radiat Oncol Biol Phys 2015; 92: 971–7. doi: https://doi.org/10.1016/j.ijrobp.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 28.Pinkawa M, Klotz J, Djukic V, Schubert C, Escobar-Corral N, Caffaro M, et al. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology 2013; 82: 963–8. doi: https://doi.org/10.1016/j.urology.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol 2017; 7: 195–202. doi: https://doi.org/10.1016/j.prro.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 30.Hamstra DA, Shah D, Kurtzman S, Sylvester J, Zimberg SH, Hudes RS, et al. Evaluation of sexual function on a randomized trial of a prostate rectal spacer. Journal of Clinical Oncology 2017; 35(6Suppl): 69. doi: https://doi.org/10.1200/JCO.2017.35.6_suppl.69 [Google Scholar]
- 31.Musunuru HB, Davidson M, Cheung P, Vesprini D, Liu S, Chung H, et al. Predictive parameters of symptomatic hematochezia following 5-fraction gantry-based SABR in prostate cancer. Int J Radiat Oncol Biol Phys 2016; 94: 1043–51. doi: https://doi.org/10.1016/j.ijrobp.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 32.Vanneste BG, Pijls-Johannesma M, Van De Voorde L, van Lin EN, van de Beek K, van Loon J, et al. Spacers in radiotherapy treatment of prostate cancer: is reduction of toxicity cost-effective? Radiother Oncol 2015; 114: 276–81. doi: https://doi.org/10.1016/j.radonc.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 33.Vanneste BG, Hoffmann AL, van Lin EN, Van De Voorde L, Pinkawa M, Lambin P. Who will benefit most from hydrogel rectum spacer implantation in prostate cancer radiotherapy? A model-based approach for patient selection. Radiother Oncol 2016; 121: 118–23. doi: https://doi.org/10.1016/j.radonc.2016.08.026 [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson RC, Sundaram V, Folkert M, Lotan Y. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol Oncol 2016; 34: 291.e19–291.e26. doi: https://doi.org/10.1016/j.urolonc.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 35.Murray LJ, Lilley J, Thompson CM, Cosgrove V, Mason J, Sykes J, et al. Prostate stereotactic ablative radiation therapy using volumetric modulated arc therapy to dominant intraprostatic lesions. Int J Radiat Oncol Biol Phys 2014; 89: 406–15. doi: https://doi.org/10.1016/j.ijrobp.2014.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onjukka E, Uzan J, Baker C, Howard L, Nahum A, Syndikus I. Twenty fraction prostate radiotherapy with intra-prostatic boost: results of a pilot study. Clin Oncol 2017; 29: 6–14. doi: https://doi.org/10.1016/j.clon.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence. Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer. 2017. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ipg10018 [Accessed 16/06/17]