Abstract
Acute ischaemic stroke is the second largest cause of death worldwide and a cause of major physical and psychological morbidity. Current evidence based treatment includes intravenous thrombolysis (IVT) and mechanical thrombectomy (MT), both requiring careful patient selection and to be administered as quickly as possible within a limited time window from symptom onset. Imaging plays a crucial role identifying patients who may benefit from MT or IVT whilst excluding those that may be harmed. For IVT, imaging must as a minimum exclude haemorrhage, stroke mimics and provide an estimate of non-viable brain. For MT, imaging must in addition detect and characterize intra-arterial thrombus and assess the intra and extracranial arterial architecture. More advanced imaging techniques may be used to assess more accurately the volume of non-viable and potentially salvageable brain tissue. It is highly likely that further research will identify patients who would benefit from treatment beyond currently accepted time windows for IVT (4.5 h) and MT (6 h) and patients with an unknown time of symptom onset. Current evidence indicates that best outcomes are achieved when treatment is instituted as soon as possible after symptom onset. A rapid, efficient imaging pathway including interpretation is fundamental to achieving the best outcomes. This review summarizes current techniques for imaging assessment of acute stroke, highlighting strengths and limitations of each. The optimum pathway is a balance between diagnostic information, local resources, specialization and the time taken to acquire, process and interpret the data. As new evidence emerges, it is likely that the minimum required imaging data will change.
INTRODUCTION
Ischaemic stroke is an acute injury to the brain parenchyma that results in physical and psychological morbidity affecting both the patient and their family. It is the fourth largest cause of death in the UK and is the second largest cause of death globally.1,2 Stroke causes 20% mortality within the first year with 50% of survivors suffering significant morbidity. The incidence in the UK ranges from 115 to 150 per 100,000 population and is estimated to cost the National Health Service approximately £9 billion per annum.1
Approximately 85% of strokes result from occlusion of the artery supplying the cerebral parenchyma, with the remainder haemorrhagic.3 The larger and more proximal the occlusive thrombus, the more likely it will result in significant functional and psychological deficit.4
The rationale behind intervention in hyperacute stroke is based on a three-compartment model of brain parenchyma following vascular occlusion. The infarct core represents non-viable brain that cannot be salvaged even with very prompt treatment. The surrounding ischaemic penumbra represents brain with reduced blood flow that has potential for survival if blood flow is restored sufficiently quickly. This zone is the target for treatment by intravenous thrombolysis (IVT) and mechanical thrombectomy (MT). The outer zone represents brain that is likely to survive without such treatment (Figure 1).
Figure 1.
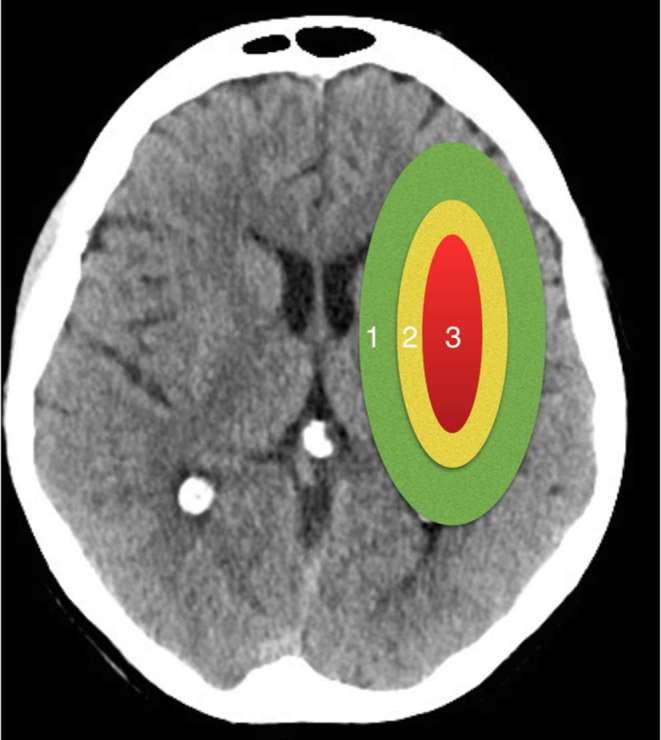
The three-compartment model of cerebral ischaemia. 1 = oligaemia, parenchyma that is likely to survive, 2 = ischaemic penumbra, parenchyma that is at risk of infarction without intervention, 3 = ischaemic core, parenchyma that is non-viable.
A recent meta-analysis of nine trials5–13 investigating IVT has demonstrated that improved patient outcomes are achieved if treatment is delivered within 4.5 h of stroke onset, despite risk of fatal intracranial haemorrhage. The efficacy of such treatment can be limited, with functional independence gained in 19.1% patients (modified Rankin Score 0–2) compared with 32.6% in MT.14
Since 2015, there have been five major randomized control trials14–18 that provide Level 1 evidence that MT is superior to IVT for treatment of anterior circulation large vessel occlusion stroke when performed within 6 h of symptom onset. The HERMES (Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials) collaboration meta-analysis of the data from the five trials determined that the number needed to treat to achieve a one-point improvement in the modified Rankin Score at 90 days is 2.6 [adjusted cOR (crude odds ratio) 2.49, 95% CI (1.76–3.53); p < 0.0001].19 This is irrespective of patient demographic and geographical location. At 2 years post-treatment, the patients of the MR CLEAN (Multicenter Randomised Clinical Trial of Endovascular Treatment for Acute Ischaemic Stroke in the Netherlands) trial who received MT continue to benefit.20 Current guidelines recommend that in eligible patients MT, in addition to IVT if within 4.5 h, may be performed for large arterial occlusion in the anterior circulation up to 6 h after symptom onset.21
“Time is brain” is an apt phrase when dealing with an acute stroke. In 1 min, 1.9 million neurons die with the brain ageing approximately 3.6 years each hour without treatment.22 This emphasizes the importance of a rapid and efficient workflow to ensure patients receive timely treatment. Analysis of SWIFT PRIME and STAR trial data showed that treatment provided in 2.5 h of symptom onset resulted in independent function in 91% of patients,23 with a 1 h delay in treatment decreasing a positive clinical outcome by 38%.24 Every 60 min delay 3.5 h after symptom onset results in a 20% lower chance of regaining function independence.23
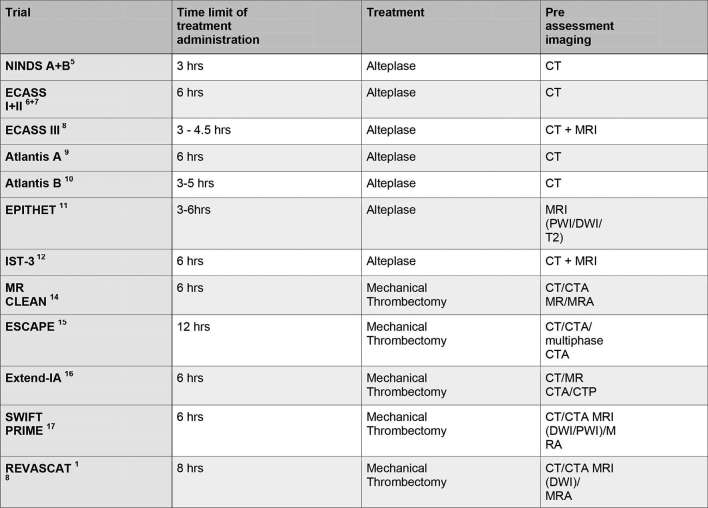
Rapid imaging plays an essential part in assessing and selecting patients and determining their treatment course. Hyperacute stroke trials have utilized a variety of imaging methods (Figure 2).
Figure 2.
Hyperacute stroke trials listing treatment and imaging protocols used. CTA, CT angiography; DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging.
For IVT, imaging must as a minimum exclude haemorrhage and stroke mimics and provide an estimate of the volume of non-viable brain tissue in order to identify patients who will not benefit or risk being harmed. For MT, vascular imaging is also essential to identify the presence, location and morphology of occlusive thrombus, to identify tandem lesions and to assess the collateral circulation.
CT perfusion (CTP) and MRI techniques can provide additional information that, although not essential, may improve patient selection in some circumstances.
CT
Unenhanced CT
Unenhanced CT is a widely available technique producing images rapidly in the acute stroke setting. It has a proven track record for assessing acute ischaemic stroke and ruling out haemorrhage and has been used in almost all major trials.
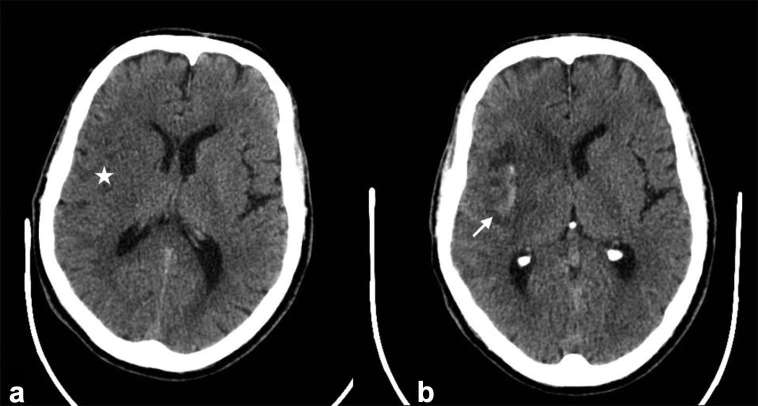
Acute infarction results in a relative reduction in the attenuation of grey matter owing due to cytotoxic oedema and an overall increase in water content. Associated swelling of the infarcted parenchyma produces focal sulcal effacement. Brain swelling without cytotoxic oedema may represent early ischaemic change (ischaemic penumbra or oligaemic tissue) rather than severe ischaemia.25 Characteristic signs are loss of definition of the basal ganglia from the adjacent capsules and loss of distinction of cortex from underlying white matter typified by the insular ribbon sign26 (Figure 3). Unenhanced CT has been shown to have a sensitivity of approximately 52% in assessment of abnormal parenchymal changes indicative of ischaemia.27 One of the greatest benefits from unenhanced CT is the ability to rule out acute haemorrhage, an absolute contraindication to IVT. The high sensitivity has been demonstrated in early major trials.6,7,27–29
Figure 3.
Unenhanced CT. (a) Demonstrates a right MCA territory infarct with loss of differentiation of the caudate and lentiform nuclei and the insula ribbon (Star). There is swelling with overlying sulcal effacement. (b) Demonstrates haemorrhagic transformation of the right MCA territory infarct in the same patient (Arrow), a complication of large territory infarction.
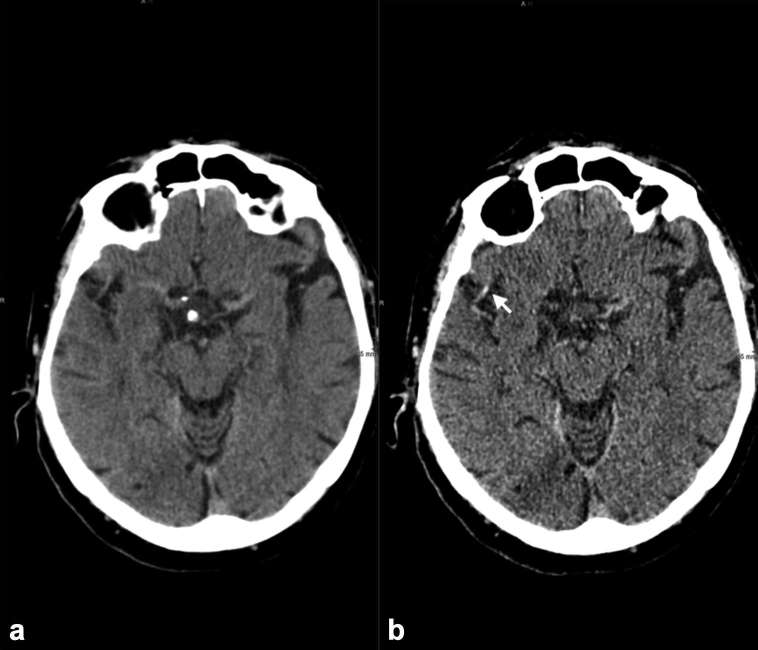
Unenhanced CT is best viewed as series comprising thick and thin slices, typically 5 and 0.5 mm. If sub-millimetre slices are considered too noisy, an intermediate thickness such as 2.5 mm may be preferred. The thicker slices provide good appreciation of the brain parenchyma, detection of haemorrhage and assessment of grey white matter differentiation. Thin slice imaging offers increased spatial resolution for resolving partial volume averaging and detecting very small infarcts. It is particularly useful at identifying small thrombi, increasing sensitivity from 67 to 97%28 (Figure 4). As well as the standard axial plane, reviewing images in reformatted coronal and sagittal planes may be useful in accurate localization of infarction and confirming suspicion of abnormality in the axial plane. This becomes particularly useful when assessing beam hardening artefact at the skull base and localizing small peripheral cortical infarcts.
Figure 4.
(a) (5-mm slices) Fails to demonstrate the proximal right sylvian M2 thrombus observed on (b) (Arrow) (0.5-mm slices) showing the importance of thin slice review.
Sensitivity to parenchymal changes in acute ischaemic stroke has been shown to improve by utilizing “stroke windows”. The window centre is set at white matter attenuation with a narrower width to increase grey white matter differentiation, increasing sensitivity from 57 to 71% in some studies.26,29 Typical values are window width 40, centre 40. In the authors’ experience optimum windowing can vary from scanner to scanner, protocol used and individual preference.
The extent of early ischaemic changes on CT correlates with stroke severity scores such as the National Institute of Health Stroke Score (NIHSS) and is a predictor of clinical outcome.29–31 Accurate and consistent assessment of the extent of these changes by simple visual inspection is difficult.
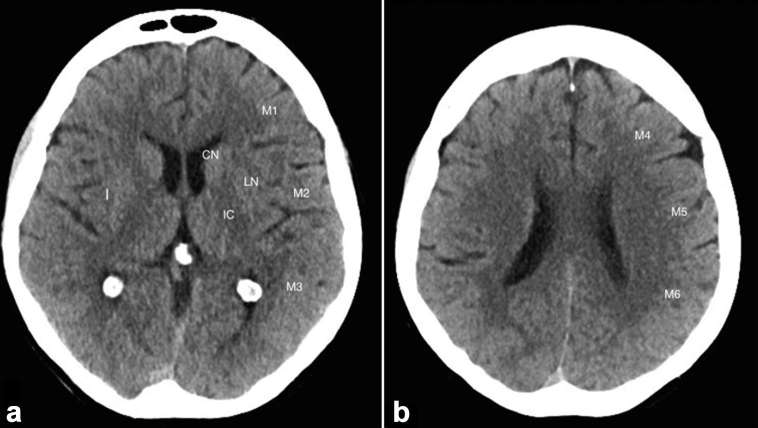
The Alberta Stroke Program Early CT Score (ASPECTS) was originally devised as a simple topographic scoring system for assessment of the extent of early ischaemic changes in middle cerebral artery territory on unenhanced CT. The MCA territory is divided into 10 regions (Figure 5). Each region scores 1 point if normal and 0 if abnormal, thus a normal scan scores 10 and a total MCA territory infarct scores 0. This validated technique is useful for predicting radiological and neurological outcome.32 A score of less than eight is associated with a poorer neurological outcome.32 e-ASPECTS (Brainomix™) is medical imaging software that provides a rapid, automated ASPECTS from plain CT data. It has been shown to perform as well as stroke experts and neuroradiologists [specificity, 95% CI (80.9–95.2%)].33
Figure 5.
The 10 Territories identified in ASPECTS. Ischaemia in one of these regions leads to a one point reduction in the score. ASPECTS, Alberta Stroke Program Early CT Score.
Isolated linear hyperdensity on CT can represent organized thrombus in the context of acute ischaemic stroke (attenuation ≅ 80 HU).34 Linear hyperdensity in the terminal internal carotid artery has 100% specificity for distal internal carotid artery thrombosis with a high predictive value for distal internal carotid artery thrombus on CT angiography (CTA).35 Acute thrombus in the distal MCA can readily be detected on unenhanced CT as a linear hyperdensity or dot sign36 (Figure 6), but would not necessarily benefit from MT compared with revascularization of proximal occlusion.35 Hyperdensity in the basilar artery has a sensitivity of 71% and specificity of 98%. Visualization of the basilar artery can be difficult due to the presence of beam hardening artefact at the base of skull and lack of presence of other arteries at this level for comparison.35
Figure 6.
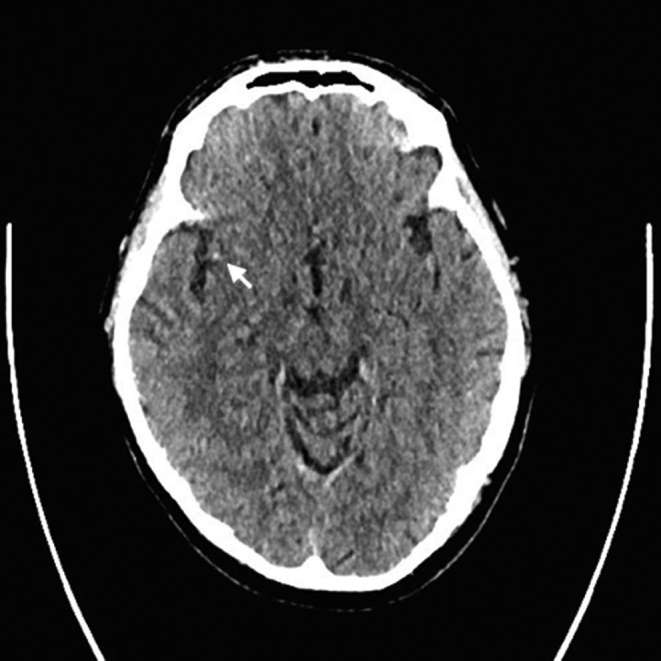
MCA dot sign—small focus of high density in the sylvian branch of the right MCA representing acute thrombus (arrow) (0.5 mm slices, unenchanced CT).
Limitations of unenhanced CT
Plain CT does not accurately indicate the infarct core or provide information about the ischaemic penumbra. The European Cooperative Acute Stroke Study showed that acute ischaemic involvement of more than one-third of the MCA territory is a contraindication to IV thrombolysis due to the risk of haemorrhagic transformation.6 ASPECTS provides a template to estimate the size of baseline infarct, but assessment of early change within 90 min of stroke onset is less reliable and small core infarction may not be picked up.37 Patient factors that reduce sensitivity to ischaemia include cerebral atrophy and leukoariosis. CT has poor sensitivity for detection of posterior fossa infarcts.
Other pitfalls when assessing acute thrombus include variability in slice thickness, vessel wall calcification and increased haematocrit.38
Summary unenhanced CT
Unenhanced CT can provide sufficient information to select patients for IVT according to current guidelines. It is highly sensitivity for acute haemorrhage. Detectable changes of early infarction correlate with outcome and the risk of treatment related haemorrhage. It is able to detect intraluminal thrombosis with reasonable sensitivity.
CT angiography (CTA)
CTA demonstrates the presence, location and size of occlusive thrombus and may provide information on collateral supply to the ischaemic territory. It is widely available, rapid and provides excellent spatial resolution in assessment of the intra and extracranial vasculature. The source data can be reformatted into two-dimensionla and three-dimensional multiplanar images.29 The sensitivity of CTA in the detection of significant stenosis or thrombotic occlusion of large intracranial and extracranial vessels is 95 to 99%.39 Those patients who have a low National Institute of Health Stroke Score score with distal occlusion or patent vasculature on CTA assessment have better chances of early improvement and early independence with lower haemorrhagic complications when treated with IVT.39,40 This modality was included in patient assessment in all five major MT trials.
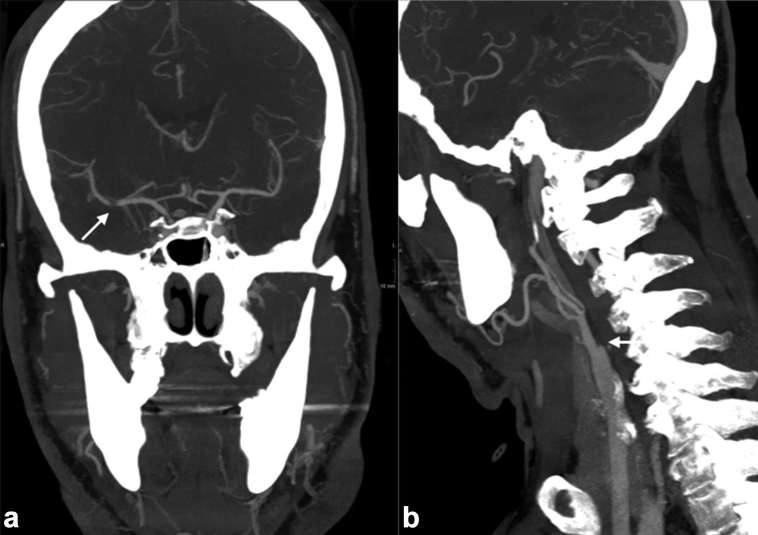
In the context of acute stroke, CTA must include the aortic arch to the vertex. The volume of thrombus demonstrated on CTA is an independent indicator of poor neurological outcome.39,40 CTA provides a target for treatment. CTA of the arch and vessels is essential to identify tandem lesions such as an ICA stenosis or arterial dissection and to aid planning of access to the intracranial circulation38 (Figure 7). Acute stenting of tandem lesions of the extracranial ICA () results in a high recanalization rate (87 vs 48% p = 0.001), favourable outcome (68 vs 15% p = 0.001) and lower mortality (18 vs 41% p = 0.048).21
Figure 7.
CT angiography with thick slice coronal (a) and sagittal (b) reformatted images demonstrating thrombus of the right MCA and occlusion in the right extracranial ICA, respectively. The patient was treated successfully with mechanical thrombectomy and stenting of the occluded ICA.
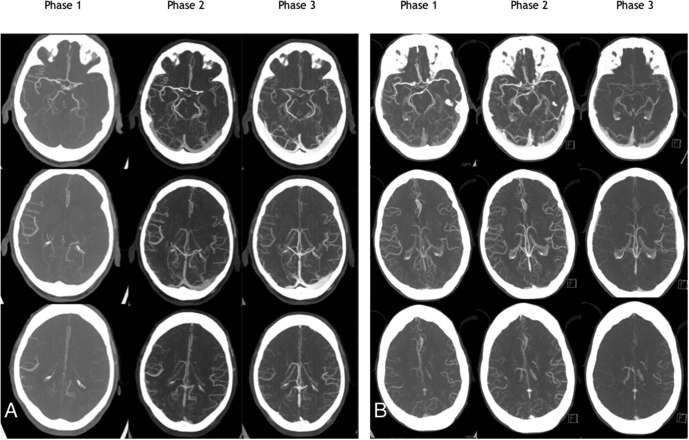
CTA also provides an assessment of the collateral circulation distal to an arterial occlusion. Assessment may be qualitative or a scoring system may be used. A good collateral circulation correlates with a positive clinical outcome. Utilizing assessment of the collateral supply can be enhanced by using a multiphase CTA technique (Figure 8). This typically comprises two additional low-dose CT scans of the head only, acquired sequentially at approximately 5 and 10 s after the initial arch to vertex acquisition. This provides a time-resolved assessment of collateral circulation and a demonstration of delayed transit time. Multiphase CTA involves a lower radiation dose than CTP and no additional intravenous contrast is required.
Figure 8.
Patient (a) has a left MCA thrombus with complete occlusion. Delayed multiphase imaging shows good collaterals, which was not shown on single-phase imaging alone. The patient underwent thrombectomy and had a good recovery. Patient (b) has a small distal MCA thrombus. Multiphase imaging demonstrates delayed washout of contrast in the left MCA territory aiding the detection and localization of thrombus. The patient was treated with i.v. tPA and made a good functional recovery.
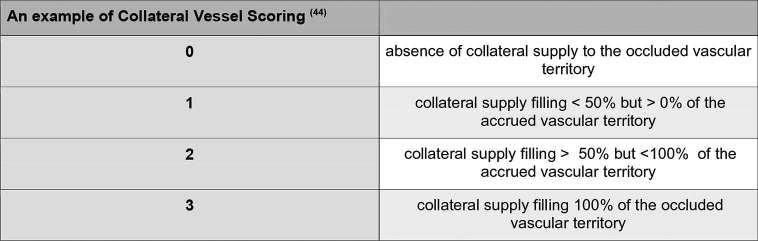
CTA allows visualization of the distal pial arteries after the occlusive thrombus, the number of which is consistent with the number of collaterals.39,41 Collateral scores were utilized in the SWIFT PRIME and ESCAPE trial for patient stratification. There are multiple collateral scores published in the literature, none of which are standardized, but some of which have demonstrated good interobserver agreement with different grading scales and a positive predictive outcome42–47(Figure 9).
Figure 9.
A single example of a scoring system that has demonstrated good interobserver agreement in the analysis of collaterals using CT angiography.
Limitations
CTA was thought to be more sensitive in detection of early irreversible ischaemia than unenhanced CT alone.48 However, in the efforts to speed up imaging and optimize arterial opacification, it has been shown to result in overestimation of infarct size, which may inadvertently exclude patients from treatment.49 It has been shown that early arterial phase CTA may underestimate thrombus length. Collateralization scores have not been standardized. In M1 branch occlusion, thrombus length discrepancy grade using dual phase CT may represent an alternative way to reflect collateral status or the presence of anterograde flow to outcome predictor than collateral scoring on CTA.50 CTA is superior for vessel luminal assessment, but there can be limitations due to artefact. Renal disease and patients with low GFR () can be relatively contraindicated to have CTA assessment.
CTP increases imaging acquisition time, radiation dose and contrast dose. CTP when used in combination with unenhanced CT and CTA can the increase dose of examinations to between 9 and 10 mSv. A non-contrast CT typically measures 4 mSv with multiphase CTA introducing a 1 mSv increment of exposure in comparison.41 However, lower dose protocols for CTP have been described.54
Interpretation requires specialist training, experience and is time-consuming. When looking at the analysis of workflow for the SWIFT PRIME trial, image time from unenhanced CT head to successful post-processing for CTP was 22 min.23 There are multiple software packages that are not standardized. CTP is sensitive to patient motion artefact resulting in misregistration and unreliable data. On older CT scanners z-axis coverage is limited, typically to 8 cm, and is, thus, dependent on accurate preselection of the volume of interest.38
CTP maps may be inaccurate in those patients who have extracranial carotid occlusion/stenosis, atrial fibrillation or poor cardiac output if inadequate image acquisition time is given. Reduced perfusion may also be seen in those patients who have seizures that mimic strokes and vasospasm.57
Although MRI offers many advantages in hyperacute stroke imaging, its use in many centres may be limited by non-availability of 24/7 access, remote location of scanners from the acute stroke assessment areas and well-known safety issues that are exacerbated in the acutely unwell patient, especially if intubated.58 These issues all have a potentially deleterious effect on time to treatment and, therefore, outcome. MRI is of course contraindicated in some patients with incompatible devices and implants.
DWI is not totally specific for irreversible infarction and DWI positive lesions have been reversed with early vessel recanalization.53,71
MRA is less able to provide accurate assessment of the collateral circulation distal to an occlusions compared with CTA or as accurate assessment of luminal stenosis. When comparing CTA and MRA in the presence of T-occlusions of the distal internal carotid or basilar artery, they provide similar assessment accuracy.58
PWI demonstrates some challenges regarding penumbra measurement. This usually results because of overestimation of penumbra due to the inclusion of benign regions of oligaemia.72 PWI may help to provide delayed treatment to those potential candidates who have late presentation with slow progressive strokes. However, a meta-analysis of multiple studies with PWI assessment using delayed thrombolysis in acute stroke did not demonstrate improved clinical outcome.73 Also, a recent study has suggested clinical diffusion mismatch, where condition severity does not match with the imaging, could be used as an alternative method to predict patient outcome in M1 MCA occlusion and in small infarct cores < 25 ml.72 However, PWI/DWI mismatch modelling should be considered superior for selecting patients for reperfusion therapy where thrombus level has not been accounted for.74
Summary CT angiography
CTA is an effective method for rapid assessment of the extra- and intracranial arterial vasculature providing a target for MT, thereby assisting with preprocedural planning. Assessment of collateral circulation can be used as a positive predictor of patient outcome.
CT perfusion
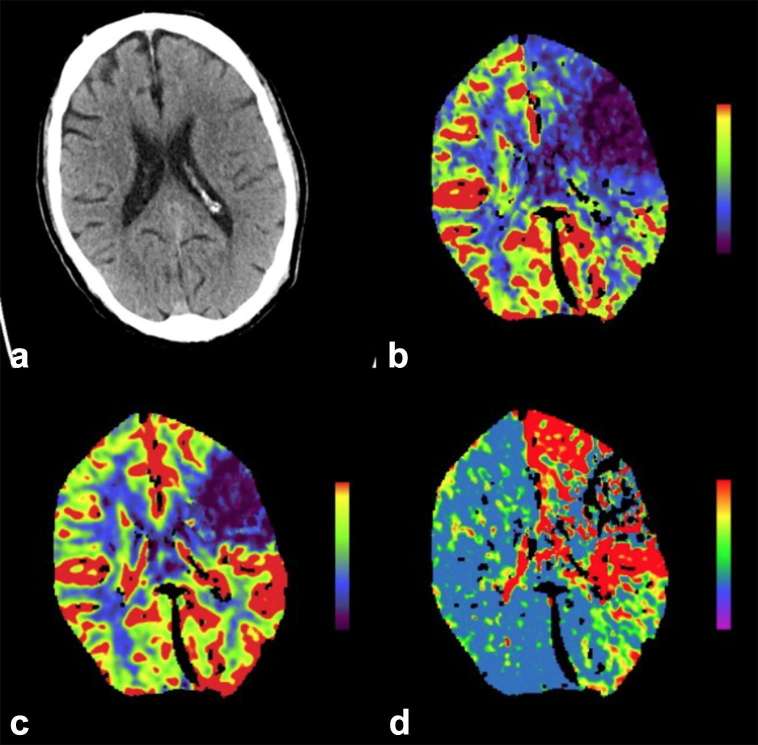
CT perfusion (CTP) produces high spatial resolution quantitative perfusion–blood volume mapping. When administering contrast, there is a linear relation of attenuation changes with contrast concentration that has been shown to produce comparable results with MR perfusion.51,52 Using automated or semi-automated software, qualitative and quantitative data for cerebral blood volume (CBV), cerebral blood flow (CBF) and mean transit time (MTT), i.e. the time difference between arterial inflow and venous outflow, and time-to-peak enhancement (TTP) are derived. Normal cerebral blood flow is defined as 45–110 ml100 g–1 min–1. This data can be applied to the three-compartment model of acute infarction and provide an estimate of the infarct core (irreversible infarction—CBF <10 ml100 g–1 min–1), potentially salvageable brain tissue if the arterial supply is restored sufficiently quickly and tissue likely to survive (CBF >25 ml100 g–1 min–1) (Figure 10). The penumbral hypothesis suggests that the greater the mismatch between the volume of irreversible ischaemia and the volume of hypoperfused but functional brain tissue, the more the patient may benefit from revascularization of the ischaemic brain.53
Figure 10.
A 65-year-old male with right-sided weakness. (a) Demonstrates loss of grey white matter differentiation and cortical swelling involving the left anterior MCA territory. There is a mismatch between the CBF (b) and CBV (c) with an overall increase in MTT (d) suggesting there is ischaemic penumbra that would benefit from revascularization. (Image courtesy of Dr D Scoffings). CBF, cerebral blood flow; MTT, mean transit time.
Preservation of or an increase in regional blood volume in the presence of reduced flow is thought to be an indicator of salvageable brain tissue. Thus, the greater the mismatch, the more likely that the patient would benefit from vessel recanalization. It can be used to triage patients who present late after initial onset of symptoms.29,54 CTP has also been shown to be able to help predict haemorrhagic transformation in ischaemic stroke and recognize stroke mimics.38,55 For example, a patient with focal seizure would demonstrate decreased CBF and cerebral blood volume in the affected area in the absence of proximal occlusion, whilst a patient with an acute ischaemic stroke would have a decreased CBF with an increased time-to-peak enhancement with no sparing of the parenchymal structures in the affected vascular distribution. CTP comprised part of the imaging protocol for the SWIFT PRIME and ESCAPE trials. RAPID software has shown great accuracy in predicting final infarct volume56
Limitations
CTP increases imaging acquisition time, radiation dose and contrast dose. CTP when used in combination with unenhanced CT and CTA can the increase dose of examinations to between 9 and 10 mSv. A non-contrast CT typically measures 4 mSv with multiphase CTA introducing a 1 mSv increment of exposure in comparison.41 However, lower dose protocols for CTP have been described.54
Interpretation requires specialist training, experience and is time-consuming. When looking at the analysis of workflow for the SWIFT PRIME trial, image time from unenhanced CT head to successful post-processing for CTP was 22 min.23 There are multiple software packages that are not standardized. CTP is sensitive to patient motion artefact resulting in misregistration and unreliable data. On older CT scanners z-axis coverage is limited, typically to 8 cm, and is, thus, dependent on accurate preselection of the volume of interest.38
CTP maps may be inaccurate in those patients who have extracranial carotid occlusion/stenosis, atrial fibrillation or poor cardiac output if inadequate image acquisition time is given. Reduced perfusion may also be seen in those patients who have seizures that mimic strokes and vasospasm.57
Although MRI offers many advantages in hyperacute stroke imaging, its use in many centres may be limited by non-availability of 24/7 access, remote location of scanners from the acute stroke assessment areas and well-known safety issues that are exacerbated in the acutely unwell patient, especially if intubated.58 These issues all have a potentially deleterious effect on time to treatment and, therefore, outcome. MRI is of course contraindicated in some patients with incompatible devices and implants.
DWI is not totally specific for irreversible infarction and DWI positive lesions have been reversed with early vessel recanalization.53,71
MRA is less able to provide accurate assessment of the collateral circulation distal to an occlusions compared with CTA or as accurate assessment of luminal stenosis. When comparing CTA and MRA in the presence of T-occlusions of the distal internal carotid or basilar artery, they provide similar assessment accuracy.58
PWI demonstrates some challenges regarding penumbra measurement. This usually results because of overestimation of penumbra due to the inclusion of benign regions of oligaemia.72 PWI may help to provide delayed treatment to those potential candidates who have late presentation with slow progressive strokes. However, a meta-analysis of multiple studies with PWI assessment using delayed thrombolysis in acute stroke did not demonstrate improved clinical outcome.73 Also, a recent study has suggested clinical diffusion mismatch, where condition severity does not match with the imaging, could be used as an alternative method to predict patient outcome in M1 MCA occlusion and in small infarct cores < 25 ml.72 However, PWI/DWI mismatch modelling should be considered superior for selecting patients for reperfusion therapy where thrombus level has not been accounted for.74
Summary CT perfusion
CTP provides high spatial resolution data on blood flow and volume which can supplement the information from plain CT and CTA to optimize selection for treatment, especially in subjects who present late after symptom onset. It provides quantitative and qualitative data on the infarct core and ischaemic penumbra and is particularly useful in those patients who present 3 h after symptom onset. It may be useful in predicting haemorrhagic risk of reperfusion therapy and recognize stroke mimics in CTA negative studies.38 Providing a routine CTP service in acute stroke requires a considerable resource of equipment and expertize.
MRI
MRI presents practical difficulties for hyperacute stroke patients but can be a valuable contributor to patient work-up, particularly beyond 3 h post-symptom onset.58 Typical MRI stroke protocols can take 10–15 min to perform but rapid protocols are described allowing patient evaluation in 6 min with a mean delay of only 18 min in workflow when compared with CT scanning at very experienced acute stroke centres.24,59 The goals in MRI and CT scanning are identical. Assessment of the brain parenchyma can be performed on T2W FSE () and FLAIR (fluid attenuated inversion recovery) imaging, with assessment of haemorrhage and microhaemorrhage with gradient echo and susceptibility-weighted imaging (SWI) (Figure 11). Although there is no significant difference in patient outcome, when utilizing unenhanced CT and MR within the first 3 h post-symptom onset, use of MRI may reduce the rate of symptomatic intracerebral haemorrhage.58
Figure 11.
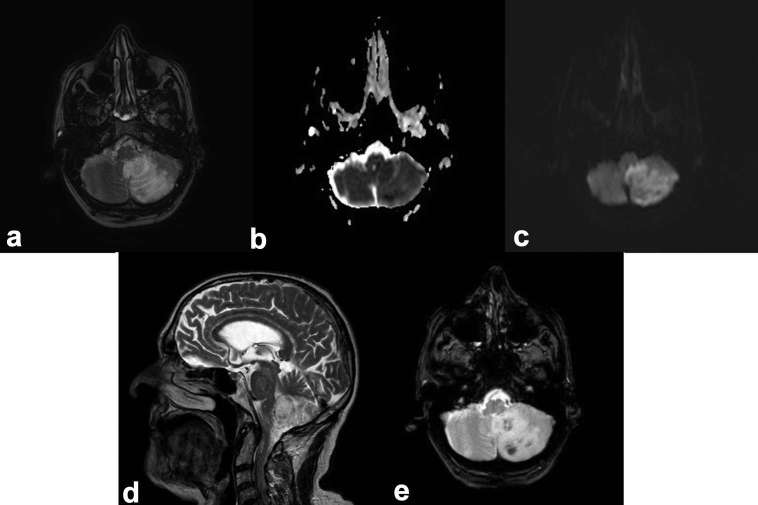
MRI of left PICA territory infarct (a) High FLAIR signal clearly identifies the region of oedema related to the infarct. (b) ADC map and (c) DWI demonstrates restricted diffusion confirming the acute nature of the pathology. (d) Sagittal T2 demonstrates high signal. (e) Gradient echo sequences demonstrates low signal confirming the presence of haemorrhage. ADC, apparent diffusioncoefficient; DWI, Diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; Posterior Inferior Cerebellar Artery.
Diffusion-weighted imaging (DWI) provides the most accurate measure of infarct core volume and is an independent predictor of clinical outcome in many trials.11,58–61 Restricted diffusion on DWI can be seen as early as 30 min post-ictus, with evolution of infarction being observed up to 4 weeks.29 DWI can identify small volume infarction that may not be appreciated on unenhanced CT alone and locates infarct position relative to region eloquence accurately. In the RECOST study, MRI imaging with DWI-derived ASPECTS scoring demonstrated effective patient selection for MT, in particular in those patients older than 70 with small volume infarcts.62 An added benefit of DWI is optimized posterior circulation evaluation. Between 6 and 10% of ischaemic strokes involve the basilar artery with patients having a poor prognosis and mortality rate of up to 85%.63 Studies have shown that a DWI Brain Stem Score and DWI ASPECTS can optimally assess small volume infarcts and predict clinical outcome.63,64 DWI is also valuable in the diagnosis of stroke mimics particularly as a negative finding in Todd’s Paraesis.
SWI is useful in assessment of thrombus length in the MCA, with a recent study having a 95.5% sensitivity on SWI imaging and strong correlations between the thrombus location identified on SWI with time-of-flight (TOF) imaging (Pearson’s correlation coefficient (PCC) 0.918, p < 0.001), contrast MRA (Magnetic Resonance Angiography) (PCC 0.887, p < 0.001) and digital subtraction angiography (PCC 0.841, p < 0.001).60 SWI imaging can give an idea of collateralization due to deoxygenated blood in the hypoperfused area. Thrombus can also be indicated by blooming on gradient echo sequences.65 SWI is very sensitive to cerebral microhaemorrhages, which may be associated with an increased risk of symptomatic intracerebral haemorrhage after thrombolysis therapy.66
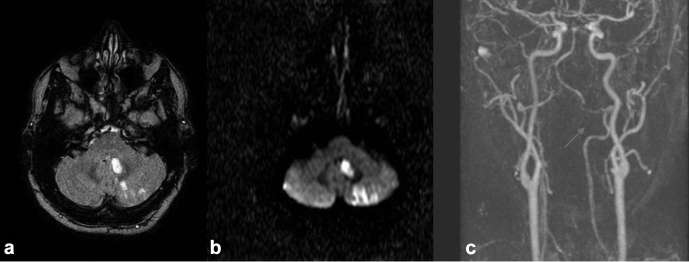
TOF and contrast enhanced MRA (CEMRA) are effective in assessment of the intracranial and extracranial vasculature (Figure 12). CEMRA requires IV Gadolinium which may be contraindicated in some patients. TOF will frequently suffice. As with CTA, MRA can identify the location and extent of occlusive thrombus as well as identify tandem lesions at the cervical level. Sensitivity of TOF MRA for total occlusion in the internal carotid artery was shown to be 92% with 100% sensitivity for near total occlusion.61
Figure 12.
There are multiple infarcts in the left cerebellar hemisphere as demonstrated on FLAIR (a) and DWI (b). Further investigation with TOF MRA demonstrates a dissection of the left vertebral artery (Arrow). DWI, Diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery, TOF, time-of-flight.
The strength of perfusion-weighted imaging (PWI) lies in its ability to detect reversible ischaemic tissue.29 PWI can be performed using contrast-enhanced (dynamic susceptibility contrast and dynamic contrast-enhanced) or non-contrast methods (arterial spin labelling). It produces a perfusion map that calculates the mean transit time through the cerebral parenchyma which can be compared directly with the DWI map measuring the infarct core. If the DWI and PWI maps match, reperfusion would not be beneficial, correlating with the DEFUSE trial findings.62 PWI readily covers the whole brain, providing a more comprehensive evaluation compared with CTP on many scanners.38
Revisiting the EPITHET and DEFUSE trial data, the use of co-registration techniques with PWI and DWI was effective in determining the presence of mismatch compared with standard volumetric imaging. This suggested that these patients significantly benefitted after treatment with Alteplase.67–69 In the acute setting, PWI combined with a DWI can offer accurate assessment for the individual patient and it could be hypothesized that the requirement of a specific time window for treatment in certain patients could be negated if significant penumbra is identified, thereby making treatment more available.70
ASPECTS has been applied to PWI and DWI as a means of producing a semi-quantitative assessment of perfusion–diffusion mismatch in acute MCA stroke. This is an alternative to less reliable visual assessment or time-consuming manual segmentation for volumetry when ultrafast post-processing software is not available. A recent single centre study showed very high interobserver agreement for the mismatch-ASPECTS method and that a mismatch-ASPECTS > 1 identified patients with a volumetric mismatch with high sensitivity and specificity.71 This study did not include correlation with patient outcome.
Limitations
Although MRI offers many advantages in hyperacute stroke imaging, its use in many centres may be limited by non-availability of 24/7 access, remote location of scanners from the acute stroke assessment areas and well-known safety issues that are exacerbated in the acutely unwell patient, especially if intubated.58 These issues all have a potentially deleterious effect on time to treatment and, therefore, outcome. MRI is of course contraindicated in some patients with incompatible devices and implants.
DWI is not totally specific for irreversible infarction and DWI positive lesions have been reversed with early vessel recanalization.53,71
MRA is less able to provide accurate assessment of the collateral circulation distal to an occlusions compared with CTA or as accurate assessment of luminal stenosis. When comparing CTA and MRA in the presence of T-occlusions of the distal internal carotid or basilar artery, they provide similar assessment accuracy.58
PWI demonstrates some challenges regarding penumbra measurement. This usually results because of overestimation of penumbra due to the inclusion of benign regions of oligaemia.72 PWI may help to provide delayed treatment to those potential candidates who have late presentation with slow progressive strokes. However, a meta-analysis of multiple studies with PWI assessment using delayed thrombolysis in acute stroke did not demonstrate improved clinical outcome.73 Also, a recent study has suggested clinical diffusion mismatch, where condition severity does not match with the imaging, could be used as an alternative method to predict patient outcome in M1 MCA occlusion and in small infarct cores < 25 ml.72 However, PWI/DWI mismatch modelling should be considered superior for selecting patients for reperfusion therapy where thrombus level has not been accounted for.74
Summary MRI
MRI provides comprehensive multiparametric assessment of the brain parenchyma and circulation in acute stroke. It is superior to CT in many respects particularly the provision of DWI in demonstration of the infarct core. DWI can be directly compared with PWI, other sequences and clinical status to assess tissue salvageability. SWI is very sensitive to microhaemorrhage, which may have proved to be significant in determining treatment. It is considerably superior to CT in posterior circulation strokes. It is likely to have a particular role in patients who present late or at an unknown interval after symptom onset to select who may benefit from reperfusion therapy. These advantages are at least in part offset by limitations in accessibility and safety and the potential for a clinically significant delay in instituting treatment.
What to do with the “wake-up stroke” (WUS) presentation
Wake-up stroke provides a diagnostic and therapeutic dilemma. These patients have an unknown time of symptom onset and can make up to 20 to 25% of stroke presentations.75 The inability to accurately identify the time of symptom onset has frequently excluded these patients from therapeutic trials and from IVT and MT according to current clinical practice guidelines.
This is an area of active research with a number of trials – DAWN, WAKE UP and EXTEND, ongoing or with preliminary data announced but as yet unpublished. T2W FLAIR and DWI are sensitive to different cerebral water changes and it has been shown that DWI positive and FLAIR negative sequences can identify acute parenchymal ischaemia within the first 3 to 4.5 h window accurately.76
The MR Witness trial findings presented at the International Stroke Conference 2016 has demonstrated that FLAIR/DWI mismatch may be utilized in initial assessment safely within 4.5 h of symptom discovery and with a median time of >11 h of last being seen well to the initialization of tPA.77
DISCUSSION
The emergence of a strong evidence base for MT as a highly effective treatment in hyperacute ischaemic stroke poses a considerable challenge to imaging services to achieve the goal of 24 h, 7-day delivery of this treatment across whole populations and large areas. The transfer of patients to suitable scanning facilities is beyond the scope of this review but nevertheless critical to organization of imaging services in any particular geographical context.
Whichever imaging modality and techniques are utilized, they must be able to accurately and safely identify those patients likely to benefit from treatment and exclude those who are not, especially those at risk of greater harm than benefit. This must be done with the greatest possible speed but not at the expense of safety. In SWIFT PRIME, there was little difference in clinical outcome for those having advanced post-processed imaging compared with those who did not, questioning the value of more advanced imaging.23 It is also important that clinical assessment and imaging are efficiently integrated within the patient pathway and one does not unnecessarily delay the other.
For MT work-up, the minimum requirement of plain CT for IVT is no longer sufficient and must be supplemented at least by single-phase CTA from the aortic arch to the vertex. There are significant staffing and training implications for providing this on an emergency basis, especially out of hours. It is fundamental that rapid, expert interpretation of these studies is available either locally or remotely. Where necessary, high-speed robust teleradiology links to the nearest thrombectomy centre are essential.
Basic CT and CTA may be supplemented by multiphase CTA and CT perfusion, which can provide key information on collateral circulation and tissue viability. The place of these techniques in routine clinical practice is yet to be fully defined, but one or both are likely to become important in future assessment, especially for those patients presenting outside current treatment guidelines. Nevertheless, any additional time taken to acquire and interpret imaging must be balanced against the need to institute treatment as rapidly as possible.
MRI can provide all the required imaging data and in particular more definitive imaging of the infarct core, thereby providing more information to identify patients who could benefit for IVT and MT. However, MRI can present greater logistic challenges and in the UK at least it is likely that CT and CTA, ± CTP will be the mainstay of hyperacute stroke imaging for the foreseeable future. In places where there is better access to MRI, such as the USA, plain CT is often supplemented by MRI and MRA.
CONCLUSION
The evidence for MT is excellent. The number needed to treat of 2.619 is much fewer than for primary coronary intervention.78 It is highly likely that the current limit of 6 h from symptom onset for consideration of MT is excluding patients who may benefit. A recent meta-analysis of five randomized trials demonstrated that MT is effective up to 7.3 h after symptom onset with functional independence gained in 64% of patients at 3 h and 46% of patients at 8 h.79 The DEFUSE III trial (NCT02586415) is assessing patients with large artery occlusion that have presented 6–16 h after symptom onset and uses multimodal CT and MRI prior to randomization to thrombectomy and best medical therapy versus best medical therapy alone. It is known that the growth rate of infarct cores is highly variable80 supporting the concept that in future optimum stroke treatment will be individualized based on clinical and imaging data.
Contributor Information
Aubrey George Smith, Email: aubrey.smith@doctors.org.uk.
Chris Rowland Hill, Email: chris.rowland-hill@hey.nhs.uk.
REFERENCES
- 1.Stroke Association. State of the nation Stroke statistics – January 2016. 2017. Available from: https://www.stroke.org.uk/sites/default/files/state_of_the_nation_2017_final_1.pdf [Google Scholar]
- 2.World Health Organisation. The top 10 causes of death Worldwide - 2015. 2017. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/ [updated January 2017] [Google Scholar]
- 3.Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford vascular study. Neurology 2013; 81: 1588–95. doi: https://doi.org/10.1212/WNL.0b013e3182a9f45f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somford DM, Nederkoorn PJ, Rutgers DR, Kappelle LJ, Mali WP, van der Grond J. Proximal and distal hyperattenuating middle cerebral artery signs at CT: different prognostic implications. Radiology 2002; 223: 667–71. doi: https://doi.org/10.1148/radiol.2233011017 [DOI] [PubMed] [Google Scholar]
- 5.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–7. doi: https://doi.org/10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 6.Hacke W. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA 1995; 274: 1017–25. doi: https://doi.org/10.1001/jama.1995.03530130023023 [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). The Lancet 1998; 352: 1245–51. doi: https://doi.org/10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–29. doi: https://doi.org/10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 9.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000; 31: 811–6. [DOI] [PubMed] [Google Scholar]
- 10.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA 1999; 282: 2019–26. [DOI] [PubMed] [Google Scholar]
- 11.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar Imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008; 7: 299–309. doi: https://doi.org/10.1016/S1474-4422(08)70044-9 [DOI] [PubMed] [Google Scholar]
- 12.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. IST-3 collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke [he third international stroke trial (IST-3)]: a randomised controlled trial. Lancet 2012; 379: 2352–6. doi: https://doi.org/10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–35. doi: https://doi.org/10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. doi: https://doi.org/10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 15.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–30. doi: https://doi.org/10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 16.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–18. doi: https://doi.org/10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–95. doi: https://doi.org/10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 18.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–306. doi: https://doi.org/10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–31. doi: https://doi.org/10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 20.van den Berg LA, Dijkgraaf MG, Berkhemer OA, Fransen PS, Beumer D, Lingsma HF, et al. Two-Year Outcome after Endovascular Treatment for Acute Ischemic Stroke. N Engl J Med 2017; 376: 1341–9. doi: https://doi.org/10.1056/NEJMoa1612136 [DOI] [PubMed] [Google Scholar]
- 21.Wahlgren N, Moreira T, Michel P, Steiner T, Jansen O, Cognard C, et al. Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-karolinska stroke update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke 2016; 11: 134–47. doi: https://doi.org/10.1177/1747493015609778 [DOI] [PubMed] [Google Scholar]
- 22.Saver JL. Time is brain--quantified. Stroke 2006; 37: 263–6. doi: https://doi.org/10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 23.Goyal M, Jadhav AP, Bonafe A, Diener H, Mendes Pereira V, Levy E, et al. Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology 2016; 279: 888–97. doi: https://doi.org/10.1148/radiol.2016160204 [DOI] [PubMed] [Google Scholar]
- 24.Menon BK, Almekhlafi MA, Pereira VM, Gralla J, Bonafe A, Davalos A, et al. Optimal workflow and process-based performance measures for endovascular therapy in acute ischemic stroke: analysis of the solitaire FR thrombectomy for acute revascularization study. Stroke 2014; 45: 2024–9. doi: https://doi.org/10.1161/STROKEAHA.114.005050 [DOI] [PubMed] [Google Scholar]
- 25.Na DG, Kim EY, Ryoo JW, Lee KH, Roh HG, Kim SS, et al. CT sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted MR imaging. Radiology 2005; 235: 992–8. doi: https://doi.org/10.1148/radiol.2353040571 [DOI] [PubMed] [Google Scholar]
- 26.Mainali S, Wahba M, Elijovich L. Detection of early ischaemic changes in non-contrast CT head improved with "Stroke Windows". ISRN Neurosci 2014; 2014 : Article ID. doi: https://doi.org/10.1155/2014/654980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Kummer R, Allen KL, Holle R, Bozzao L, Bastianello S, Manelfe C, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology 1997; 205: 327–33. doi: https://doi.org/10.1148/radiology.205.2.9356611 [DOI] [PubMed] [Google Scholar]
- 28.Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke 2012; 43: 2319–23. doi: https://doi.org/10.1161/STROKEAHA.112.649921 [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan A, Goyal M, Al Azri F, Lum C. State-of-the-art imaging of acute stroke. Radiographics 2006; 26(Suppl 1): S75–S95. doi: https://doi.org/10.1148/rg.26si065501 [DOI] [PubMed] [Google Scholar]
- 30.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke programme early CT score. Lancet 2000; 355: 1670–4. [DOI] [PubMed] [Google Scholar]
- 31.Patel SC, Levine SR, Tilley BC, Grotta JC, Lu M, Frankel M, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 2001; 286: 2830–8. doi: https://doi.org/10.1001/jama.286.22.2830 [DOI] [PubMed] [Google Scholar]
- 32.Aviv RI, Mandelcorn J, Chakraborty S, Gladstone D, Malham S, Tomlinson G, et al. Alberta stroke program early CT scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol 2007; 28: 1975–80. doi: https://doi.org/10.3174/ajnr.A0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagel S, Sinha D, Day D, Reith W, Chapot R P. e-ASPECTS is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischaemic stroke patients. Int J Stroke 2016. doi: https://doi.org/10.1177/1747493016681020 [DOI] [PubMed] [Google Scholar]
- 34.Jensen-Kondering U, Riedel C, Jansen O. Hyperdense artery sign on computed tomography in acute ischemic stroke. World J Radiol 2010; 2: 354–7. doi: https://doi.org/10.4329/wjr.v2.i9.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molina CA, Carlos AM. Futile recanalization in mechanical embolectomy trials: a call to improve selection of patients for revascularization. Stroke 2010; 41: 842–3. doi: https://doi.org/10.1161/STROKEAHA.110.580266 [DOI] [PubMed] [Google Scholar]
- 36.Morita S, Ueno E, Masukawa A, Suzuki K, Machida H, Fujimura M. Hyperattenuating signs at unenhanced CT indicating acute vascular disease. Radiographics 2010; 30: 111–25. doi: https://doi.org/10.1148/rg.301095052 [DOI] [PubMed] [Google Scholar]
- 37.Bal S, Bhatia R, Menon BK, Shobha N, Puetz V, Dzialowski I, et al. Time dependence of reliability of noncontrast computed tomography in comparison to computed tomography angiography source image in acute ischemic stroke. Int J Stroke 2015; 10: 55–60. doi: https://doi.org/10.1111/j.1747-4949.2012.00859.x [DOI] [PubMed] [Google Scholar]
- 38.Menon BK, Goyal M. Imaging paradigms in acute ischaemic stroke: a pragmatic evidence-based approach. Radiology 2015; 277: 7–12. doi: https://doi.org/10.1148/radiol.2015151030 [DOI] [PubMed] [Google Scholar]
- 39.Wintermark M, Rowley HA, Lev MH. A cute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro CT. Radiology 2009; 251: 619–26. doi: https://doi.org/10.1148/radiol.2513081073 [DOI] [PubMed] [Google Scholar]
- 40.Sims JR, Rordorf G, Smith EE, Koroshetz WJ, Lev MH, Buonanno F, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment. AJNR Am J Neuroradiol 2005; 26: 246–51. [PMC free article] [PubMed] [Google Scholar]
- 41.Vo KD, Yoo AJ, Gupta A, Qiao Y, Vagal AS, Hirsch JA, et al. Multimodal diagnostic imaging for hyperacute stroke. AJNR Am J Neuroradiol 2015; 36: 2206–13. doi: https://doi.org/10.3174/ajnr.A4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol 2012; 33: 576–82. doi: https://doi.org/10.3174/ajnr.A2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebeskind D. A novel CT angiography scale for assessment of collaterals in acute stroke. Stroke 2003; 34: 265. [Google Scholar]
- 44.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007; 61: 533–43. doi: https://doi.org/10.1002/ana.21130 [DOI] [PubMed] [Google Scholar]
- 45.Knauth M, von Kummer R, Jansen O, Hähnel S, Dörfler A, Sartor K. Potential of CT angiography in acute ischemic stroke. AJNR Am J Neuroradiol 1997; 18: 1001–10. [PMC free article] [PubMed] [Google Scholar]
- 46.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009; 30: 525–31. doi: https://doi.org/10.3174/ajnr.A1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elijovich L, Goyal N, Mainali S, Hoit D, Arthur AS, Whitehead M, et al. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: a retrospective chart review. J Neurointerv Surg 2016; 8: 559–62. doi: https://doi.org/10.1136/neurintsurg-2015-011731 [DOI] [PubMed] [Google Scholar]
- 48.Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Cunnane ME, Halpern EF, et al. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology 2007; 244: 541–8. doi: https://doi.org/10.1148/radiol.2442061028 [DOI] [PubMed] [Google Scholar]
- 49.Pulli B, Schaefer PW, Hakimelahi R, Chaudhry ZA, Lev MH, Hirsch JA, et al. Acute ischemic stroke: infarct core estimation on CT angiography source images depends on CT angiography protocol. Radiology 2012; 262: 593–604. doi: https://doi.org/10.1148/radiol.11110896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park M, Kim KE, Shin NY, Lee SK, Lim SM, Song D, et al. Thrombus length discrepancy on dual-phase CT can predict clinical outcome in acute ischemic stroke. Eur Radiol 2016; 26: 2215–22. doi: https://doi.org/10.1007/s00330-015-4018-3 [DOI] [PubMed] [Google Scholar]
- 51.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: theoretic basis. AJNR Am J Neuroradiol 2009; 30: 662–8. doi: https://doi.org/10.3174/ajnr.A1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012; 43: 2648–53. doi: https://doi.org/10.1161/STROKEAHA.112.660548 [DOI] [PubMed] [Google Scholar]
- 53.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 2003; 34: 2729–35. doi: https://doi.org/10.1161/01.STR.0000097608.38779.CC [DOI] [PubMed] [Google Scholar]
- 54.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol 2009; 30: 885–92. doi: https://doi.org/10.3174/ajnr.A1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aviv RI, D'Esterre CD, Murphy BD, Hopyan JJ, Buck B, Mallia G, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 2009; 250: 867–77. doi: https://doi.org/10.1148/radiol.2503080257 [DOI] [PubMed] [Google Scholar]
- 56.Austein F, Riedel C, Kerby T, Meyne J, Binder A, Lindner T, et al. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke 2016; 47: 2311–7. doi: https://doi.org/10.1161/STROKEAHA.116.013147 [DOI] [PubMed] [Google Scholar]
- 57.Allmendinger AM, Tang ER, Lui YW, Spektor V. Imaging of stroke: part 1, perfusion CT--overview of imaging technique, interpretation pearls, and common pitfalls. AJR Am J Roentgenol 2012; 198: 52–62. doi: https://doi.org/10.2214/AJR.10.7255 [DOI] [PubMed] [Google Scholar]
- 58.Köhrmann M, Schellinger PD. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro MR imaging. Radiology 2009; 251: 627–33. doi: https://doi.org/10.1148/radiol.2513081074 [DOI] [PubMed] [Google Scholar]
- 59.Nael K, Khan R, Choudhary G, Meshksar A, Villablanca P, Tay J, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke 2014; 45: 1985–91. doi: https://doi.org/10.1161/STROKEAHA.114.005305 [DOI] [PubMed] [Google Scholar]
- 60.Weisstanner C, Gratz PP, Schroth G, Verma RK, Köchl A, Jung S, et al. Thrombus imaging in acute stroke: correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol 2014; 24: 1735–41. doi: https://doi.org/10.1007/s00330-014-3200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11: 860–7. doi: https://doi.org/10.1016/S1474-4422(12)70203-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danière F, Lobotesis K, Machi P, Eker O, Mourand I, Riquelme C, et al. Patient selection for stroke endovascular therapy--DWI-ASPECTS thresholds should vary among age groups: insights from the RECOST study. AJNR Am J Neuroradiol 2015; 36: 32–9. doi: https://doi.org/10.3174/ajnr.A4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mourand I, Machi P, Nogué E, Arquizan C, Costalat V, Picot MC, et al. Diffusion-weighted imaging score of the brain stem: a predictor of outcome in acute basilar artery occlusion treated with the solitaire FR device. AJNR Am J Neuroradiol 2014; 35: 1117–23. doi: https://doi.org/10.3174/ajnr.A3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tei H, Uchiyama S, Usui T, Ohara K. Posterior circulation ASPECTS on diffusion-weighted MRI can be a powerful marker for predicting functional outcome. J Neurol 2010; 257: 767–73. doi: https://doi.org/10.1007/s00415-009-5406-x [DOI] [PubMed] [Google Scholar]
- 65.Gasparian GG, Sanossian N, Shiroishi MS, Liebeskind DS. Imaging of occlusive thrombi in acute ischemic stroke. Int J Stroke 2015; 10: 298–305. doi: https://doi.org/10.1111/ijs.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charidimou A, Shoamanesh A, Wilson D, Gang Q, Fox Z, Jäger HR, et al. Cerebral microbleeds and postthrombolysis intracerebral hemorrhage risk updated meta-analysis. Neurology 2015; 85: 927–34. doi: https://doi.org/10.1212/WNL.0000000000001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–17. doi: https://doi.org/10.1002/ana.20976 [DOI] [PubMed] [Google Scholar]
- 68.Nagakane Y, Christensen S, Brekenfeld C, Ma H, Churilov L, Parsons MW, et al. EPITHET: positive result after reanalysis using baseline diffusion-weighted imaging/perfusion-weighted imaging co-registration. Stroke 2011; 42: 59–64. doi: https://doi.org/10.1161/STROKEAHA.110.580464 [DOI] [PubMed] [Google Scholar]
- 69.Ogata T, Christensen S, Nagakane Y, Ma H, Campbell BC, Churilov L, et al. The effects of alteplase 3 to 6 hours after stroke in the EPITHET-DEFUSE combined dataset: post hoc case-control study. Stroke 2013; 44: 87–93. doi: https://doi.org/10.1161/STROKEAHA.112.668301 [DOI] [PubMed] [Google Scholar]
- 70.Copen WA, Schaefer PW, Wu O. MR perfusion imaging in acute ischemic stroke. Neuroimaging Clin N Am 2011; 21: 259–83. doi: https://doi.org/10.1016/j.nic.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lassalle L, Turc G, Tisserand M, Charron S, Roca P, Lion S, et al. ASPECTS (Alberta Strole Program Early CT Score) assessment of the perfusion – diffusion mismatch. 2016; 47: 2553–8. doi: https://doi.org/10.1161/STROKEAHA.116.013676 [DOI] [PubMed] [Google Scholar]
- 72.Nogueira RG, Kemmling A, Souza LM, Payabvash S, Hirsch JA, Yoo AJ, et al. Clinical diffusion mismatch better discriminates infarct growth than mean transit time-diffusion weighted imaging mismatch in patients with middle cerebral artery-M1 occlusion and limited infarct core. J Neurointerv Surg 2017; 9: 127–30. doi: https://doi.org/10.1136/neurintsurg-2014-011602 [DOI] [PubMed] [Google Scholar]
- 73.Mishra NK, Albers GW, Davis SM, Donnan GA, Furlan AJ, Hacke W, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke 2010; 41: e25–e33. doi: https://doi.org/10.1161/STROKEAHA.109.566869 [DOI] [PubMed] [Google Scholar]
- 74.Ebinger M, Iwanaga T, Prosser JF, De Silva DA, Christensen S, Collins M, et al. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke 2009; 40: 2572–4. doi: https://doi.org/10.1161/STROKEAHA.109.548073 [DOI] [PubMed] [Google Scholar]
- 75.Rimmele DL, Thomalla G. Wake-up stroke: clinical characteristics, imaging findings, and treatment option - an update. Front Neurol 2014; 5: . doi: https://doi.org/10.3389/fneur.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubin MN, Barrett KM. What to do with wake-up stroke. Neurohospitalist 2015; 5: 161–72. doi: https://doi.org/10.1177/1941874415576204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwamm L, American Heart Association. IV alteplase in MR-selected patients with stroke of unknown onset is safe and feasible: results of the multicenter MR WITNESS trial (NCT01282242). Los Angeles, USA: International Stroke Conference (ISC); 2016. [Google Scholar]
- 78.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361: 13–20. doi: https://doi.org/10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 79.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–88. doi: https://doi.org/10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 80.Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke 2015; 10: 723–9. doi: https://doi.org/10.1111/ijs.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]