Abstract
Objective:
To report an initial experience using a primary constrained transjugular intrahepatic portosystemic shunt (TIPS) technique for treating cirrhotic patients with refractory ascites or variceal bleeding.
Methods:
All patients undergoing primary constrained (n = 9) and conventional (n = 18) TIPS between July 2014 and June 2016 were retrospectively reviewed. Preprocedure demographics, Child–Pugh, model for end-stage liver disease and technical variables were recorded. Outcomes measured included technical and clinical success, complications, 30-day mortality, as well as necessity for TIPS revision. Average (SD) and median follow-up was 237 (190) and 226 days.
Results:
All constrained and conventional TIPS were technically successful (100%). Clinical success as defined as a reduction or improvement in presenting symptoms was 88.9% (8/9) and 100% (18/18) in the constrained and conventional groups, respectively (p = 1). The average reduction in portosystemic gradient was lower in the constrained group, 6.1 mmHg compared with 10.6 mmHg in the conventional group (p = 0.73). The rate of hepatic encephalopathy following TIPS placement was higher in the conventional group [16.7% (3/18)] compared with 0% in the constrained group (p = 0.52). The percentage of patients requiring TIPS revision was lower in the constrained group, although the results were not significant (11.1 vs 22.2%, p = 0.63).
Conclusion:
Primary constrained TIPS is a feasible modification to conventional TIPS with similar technical and clinical success rates. A trend towards a smaller reduction in the portosystemic gradient and need for revision was observed in the constrained group.
Advances in knowledge:
Primary constrained TIPS allows for greater stepwise control over shunt diameter and may represent an improved technique for patients at risk for hepatic encephalopathy.
INTRODUCTION
Transjugular intrahepatic portosystemic shunt (TIPS) creation is a well-established and effective treatment for variceal bleeding and refractory ascites in patients with portal hypertension.1,2 The clinical success rates of TIPS have risen in the past decade with the routine use of extended polytetrafluoroethylene (e-PTFE) self-expanding stent grafts, as demonstrated by prolonged shunt patency and a reduced need for secondary endovascular intervention when compared with bare metal stents.3–9
While the use of e-PTFE stents has improved clinical outcomes by decreasing rates of shunt restenosis and occlusion, patients following TIPS creation remain at risk for major clinical sequela such as hepatic encephalopathy (HE). HE in the post-TIPS setting is not an uncommon event and may occur in as many as 5–35% of cases.10 The majority of cases of post-TIPS HE resolve with medication and dietary modifications. If refractive to medical management, one endovascular treatment option for HE includes shunt occlusion, a procedure that places patients at risk for recurrent ascites, variceal bleeding and severe haemodynamic instability.11,12 Alternatively, another method to mitigate HE is to reduce the stent graft diameter without causing complete occlusion. Various procedural techniques have been described to accomplish this, effectively all attempting to increase the portosystemic gradient (PSG) and alleviate symptoms of medically refractory HE.13–17 Among these, shunt reduction with placement of hourglass-shaped ePTFE balloon-expandable stent grafts into the existing TIPS have shown positive outcomes with the added advantage of creating shunts that can be further adjusted in calibre-based on the patient’s clinical condition.18 The usage of balloon-expandable stents to create primary constrained stents during initial TIPS creation is less studied but offers promise for greater initial control of the PSG.19,20 The purpose of this study is to report an initial experience using a primary constrained TIPS technique with balloon-expandable stents and to compare their outcome with conventional TIPS placement at a single academic institution.
METHODS AND MATERIALS
A single-institution, Health Insurance Portability and Accountability Act compliant, retrospective study was performed following institutional review board approval. 27 patients seen from July 2014 to July 2016 with variceal bleeding and refractory ascites who underwent TIPS placement at the same institution were evaluated. Among these patients, 9 (33%) received a primary constrained TIPS and 18 (67%) underwent conventional TIPS placement. Selection for constrained vs conventional technique was performed per operator preference and after multidisciplinary collaboration with hepatology regarding concern for potential development of HE from overshunting. No specific objective criteria were used to select or exclude patients from either technique. Data collection included patient demographic data, indication for intervention, preprocedural Child–Pugh (CP) score, model for end-stage liver disease (MELD) and procedure-specific technical variables. Primary outcomes measured included technical, clinical and haemodynamic success rates as defined by the Society for Interventional Radiology’s 2016 Quality Improvement Guidelines for TIPS.2 Per guidelines, technical success was defined as successful creation of a shunt between the hepatic vein and an intrahepatic branch of the portal vein. Clinical success was defined as resolution of the clinical indication for which the procedure was performed and the length of disease-free interval. Haemodynamic success was consequently defined as reduction of the PSG to the threshold set by the clinical setting (PSG <12 mmHg). Follow-up data recorded included presence or absence of HE, refractory ascites, shunt restenosis, 30-day mortality and necessity for TIPS revision. Average (standard deviation) and median follow-up was 224 (175) and 228 days. Patient demographic information and preprocedural data is listed in Table 1.
Table 1.
Demographic and preprocedural data
| Characteristic | TIPS creation technique | p-value | |
| Conventional (n = 18) | Primary Constrained (n = 9) | ||
| Sex | |||
| Male | n = 12, 66.7% | n = 9, 100% | 0.07 |
| Female | n = 6, 33.3% | 0% | |
| Race | |||
| White | n = 7, 38.9% | n = 8, 88.9% | 0.019a |
| Hispanic | n = 9, 50% | n = 1, 11.1% | 0.091 |
| Other | n = 2, 11.1% | 0% | 0.538 |
| Age | 55, ± 11.15 | 56, ± 13.9 | 0.915 |
| Preprocedural | |||
| CP | 9B, ± 0.6 | 9B, ± 1 | 0.868 |
| MELD | 13.5, ± 6.4 | 10, ± 4.1 | 0.796 |
| Indication for intervention | |||
| Recurrent ascites | n = 7, 38.9% | n = 8, 88.9% | 0.019a |
| Variceal haemorrhage | n = 11, 61.1% | n = 1, 11.1% | |
ap < 0.05.
CP, Child–Pugh; MELD, model for end-stage liver disease; TIPS, transjugular intrahepatic portosystemic shunt.
Primary constrained TIPS technique
All procedures were performed in the interventional radiology suite under general anaesthesia. All interventions were performed by one of two board certified interventional radiologists with at least 12 years of experience and a certificate of added qualification in vascular and interventional radiology.
A 10-Fr sheath (Flexor® Check-Flo® Performer; Cook, Bloomington, IN) was introduced into the inferior vena cava (IVC) after obtaining access into the right internal jugular vein. A 5-Fr MPA catheter (Cook, Bloomington, IN) was used to select the right hepatic vein based on institutional preference and patient anatomy to create a TIPS from this access. A right hepatic venogram was performed followed by wedged CO2 portograms in the anteroposterior and oblique projections to delineate the course of the portal vein (Figure 1). Next, a 16-gauge ross-modified Colapinto needle was advanced and used to puncture the right portal vein under fluoroscopic guidance and portal access was confirmed with contrast injection (Figure 2). Following confirmation, a hydrophilic guidewire (Terumo, Shibuya, Japan) was advanced into the main portal vein. Next, over the wire a 5-Fr pigtail catheter was inserted and placed at the splenic and superior mesenteric vein confluence. Simultaneous venograms from the pigtail catheter and sheath positioned at the hepatic vein and IVC confluence were performed to delineate the tract length necessary for TIPS placement (Figure 3). Haemodynamic pressure measurements were also obtained to calculate the pre-TIPS PSG. The pigtail catheter was exchanged over a stiff guidewire and a 6- or 7-mm balloon-expandable stent (Express®; Boston Scientific, Natick, MA) was deployed within the hepatic parenchymal tract (Figure 4). The stent diameter size between 6 and 7 mm was considered insignificant as it could be overdilated as necessary following placement. Subsequently, a 10-mm Viatorr® selfexpanding ePTFE-lined stent graft (W.L. Gore & Associates, Flagstaff, AZ) was directly advanced through the transvenous tract created by the balloon-expandable stent and deployed to create a TIPS shunt from the right portal to right hepatic vein. Balloon dilation of the stent graft was initially performed using a 7 or 8 mm high pressure balloon with the balloon-expandable stent acting to constrict and prevent further selfexpansion of the Viatorr stent (Figure 5). PSGs were calculated and titrated to a goal PSG of 5–12 mmHg by systematically increasing the size of balloon in 1 mm increments and repeating PSG measurements intraprocedure. If necessary, the balloon-expandable stent could be dilated to the 10 mm nominal size of the TIPS stent to achieve the target PSG. Completion venography was performed to confirm appropriate positioning of the TIPS (Figure 6). The sheath was subsequently removed, and haemostasis was obtained with manual compression. Conventional TIPS placement was performed in similar fashion but without the use of a balloon-expandable stent. This technique has been previously described.21,22
Figure 1.
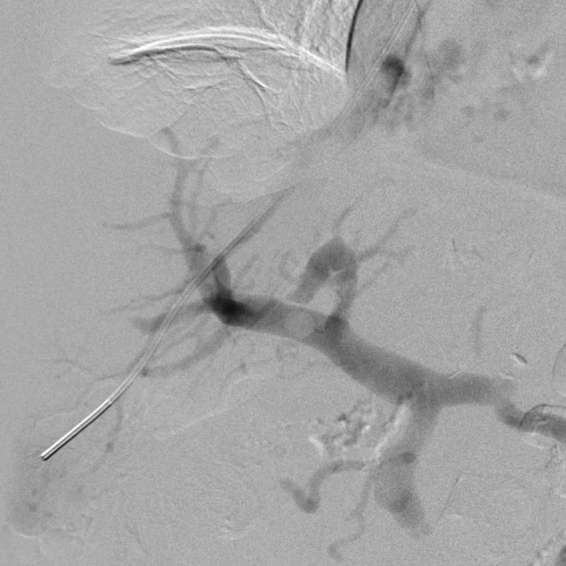
Wedged hepatic venogram utilizing CO2 delineating the course of the main portal vein and its branches.
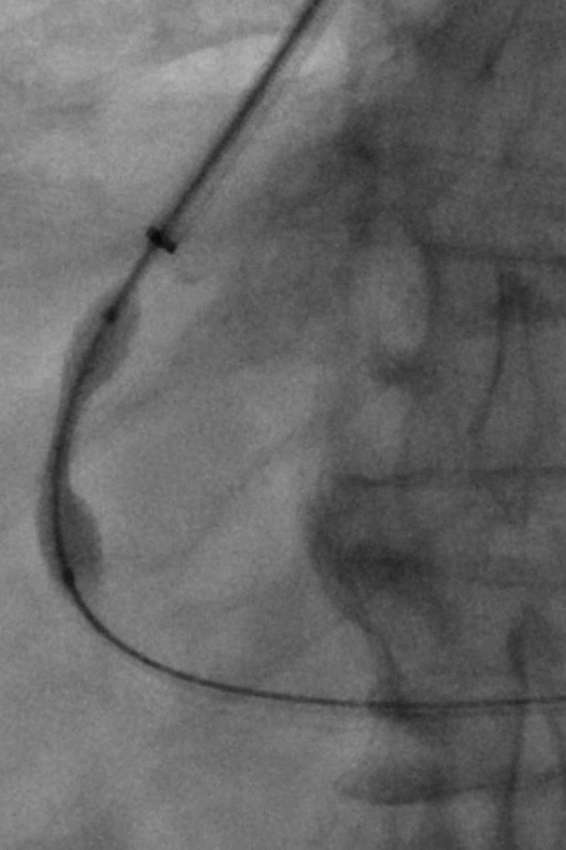
Figure 2.
Portography obtained upon puncture of the right portal vein confirms positioning of the needle tip within the portal system.
Figure 3.
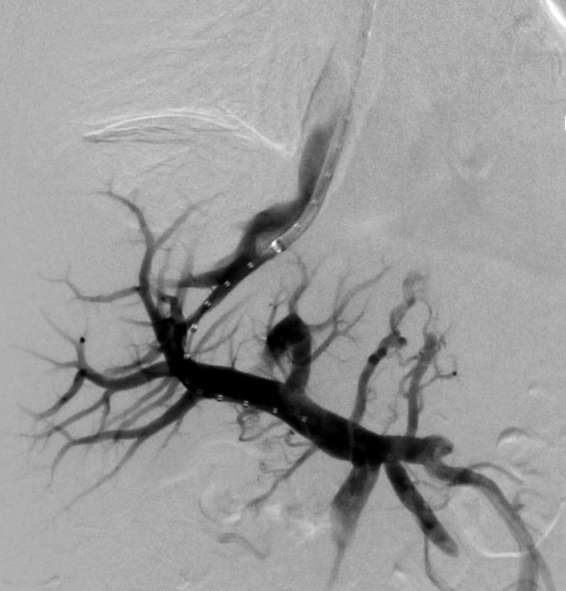
Simulatenous portogram and hepatic venogram delineates the length of the intrahepatic tract for transjugular intrahepatic portosystemic shunt placement.
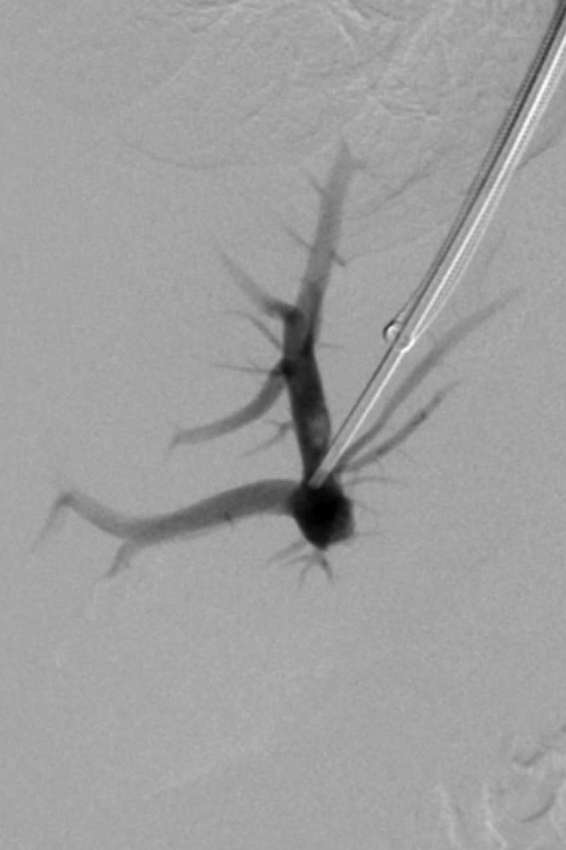
Figure 4.
Fluoroscopic image demonstrating initial placement of a 7-mm balloon expandable in the intrahepatic tract. This stent will serve to act as the primary constraining device for subsequent transjugular intrahepatic portosystemic shunt placement with the extended polytetrafluoroethylene-lined graft.
Figure 5.
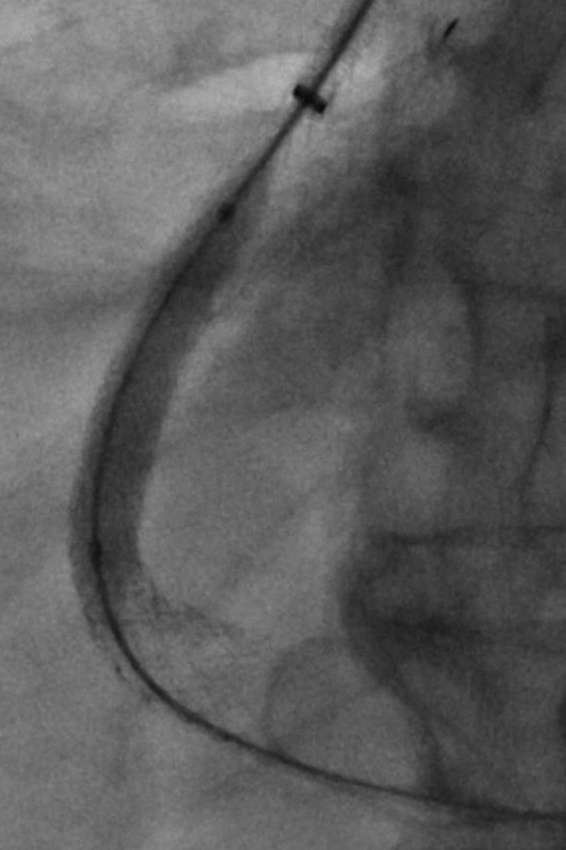
Fluoroscopic image demonstrating creation of the transjugular intrahepatic portosystemic shunt by deployment of Viatorr selfexpanding extended polytetrafluoroethylene-lined stent graft within previously placed 7-mm balloon-expandable stent. The graft is being dilated to 8 mm to further decrease the portosystemic gradient.
Figure 6.
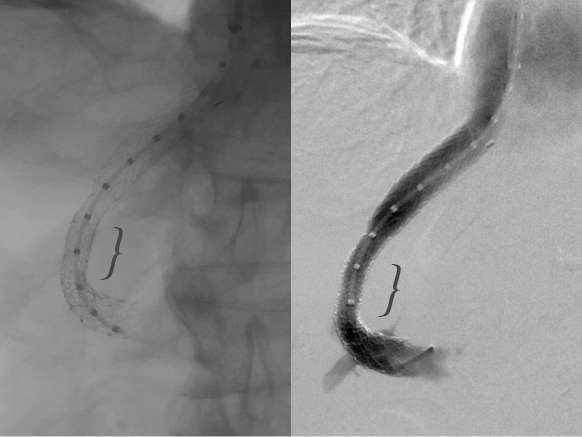
Fluoroscopic image obtained with and without hand injection of contrast demonstrating patency of transjugular intrahepatic portosystemic shunt. Arrowhead indicates the site of balloon expanded primary constraining stent.
Post procedural care and follow -up
Post-operatively, patients were monitored in the ICU for 24 h for signs or symptoms of haemodynamic instability and/or HE. Patients were scheduled for 1 month follow-up with Doppler ultrasound to assess shunt patency.
Statistical analysis
Descriptive statistics were expressed as proportions for categorical variables and means ± standard deviation for continuous variables. Patient characteristics between primary constrained and conventional groups were compared by using quantile regression on the median for continuous data and Fisher exact test for categorical data. All analyses were performed with Stata software (release 13.1; StataCorp, College Station, TX). A p value < 0.05 was considered statistically significant.
RESULTS
The technical success rate for both conventional and primary constrained TIPS groups was 100%. There was a significant difference between indications for TIPS placement between two groups, with 38.9% (n = 7) of patients undergoing TIPS placement for recurrent ascites in the conventional group, compared with 88.9% (n = 8) of patients in the constrained group. There were no significant differences between sex, age and preprocedure MELD and Child–Pugh score between the conventional and primary constrained groups (Table 1). Patients in both groups demonstrated a reduction in the PSG. The average reduction in PSG in the conventional group was 10.6 mmHg compared with 6.1 mmHg in the primary constrained group (p = 0.731). TIPS placement was 100% successful in resolution of variceal bleeding in both groups. In the conventional group, 100% (n = 7) of patients with presenting symptom of ascites experienced improvement or resolution of their ascites symptoms after TIPS placement, compared with 87.5% (n = 7) in the constrained group (p = 1.0). Clinical outcomes of TIPS placement are summarized in Table 2.
Table 2.
TIPS results
| Outcome | TIPS creation technique | p-value | |
| Conventional (n = 18) | Primary Constrained (n = 9) | ||
| Pre-TIPS PSG (mmHg) | 17.7, ±8.2 | 14, ±3 | 0.758 |
| Post-TIPS PSG (mmHg) | 6.7, ±1.84 | 7.9, ±1.96 | 0.693 |
| PSG reduction (mmHg) | 10.6, ±8.879 | 6.1, ±2.57 | 0.731 |
| Post-procedural MELD Score | 16, ±5.8 | 14, ±6.4 | 0.742 |
| Improvement of recurrent ascitesa | n = 7, 100% improvement | n = 7, 87.5% improvementb | 1.0 |
| Improvement of variceal haemorrhagea | n = 11, 100% resolution | n = 1, 100% resolution | 1.0 |
MELD, model for end-stage liver disease; PSG, portosystemic gradient; TIPS, transjugular intrahepatic portosystemic shunt.
aImprovement and resolution of clinical symptoms that were indication for TIPS placement in each group.
bBased on data from total of eight patients who underwent primary constrained TIPS placement for indication of refractory ascites.
In the conventional group, 11.1% (n = 2) of patients experienced new onset ascites after TIPS placement from worsening liver function compared with 0% in the constrained group (p = 0.53). 16.7% (n = 3) of patients in the conventional group experienced HE after TIPS procedure compared with 0% of patients in the constrained group (p = 0.52). There was one incidence of Clostridium difficile infection post-procedurally in the conventional group, and one incident of bacterial peritonitis post-procedurally in the primary constrained group (p = 1). There were no significant differences in 30-day mortality between both groups (p < 0.05). Procedure-related complications are summarized in Table 3.
Table 3.
Procedure-related complications
| Complication | TIPS creation technique | p-value | |
| Conventional (n = 18) | Primary Constrained (n = 9) | ||
| Refractory ascites | n = 2, 11.1% | 0% | 0.53 |
| Hepatic encephalopathy | n = 3, 16.7% | 0% | 0.52 |
| Other | n = 1, C. difficile infection postoperatively | n = 1, Bacterial peritonitis postoperatively | 1.0 |
| 30 day mortality | n = 2, 11.1% | 0% | 0.53 |
TIPS, transjugular intrahepatic portosystemic shunt.
30-day follow-up imaging demonstrated reduced shunt patency in 13.3% (n = 2) of conventional cases compared with 0% in the constrained group (p = 1.0). There were no significant differences shunt patency and restenosis on follow-up imaging (Table 4). During the follow-up period, 22.2% (n = 4) of patients required shunt re-intervention in the conventional group compared with 11.1% (n = 1) of patients in the constrained group (p = 0.63). Indications for re-intervention for both conventional and primary constrained groups are summarized in Table 5.
Table 4.
Follow-up shunt patency
| Doppler ultrasound (30 day) | TIPS creation technique | p-value | |
| Conventional (n = 18) | Primary Constrained (n = 9) | ||
| Availability | n = 15, 83.3% | n = 7, 77% | 1.0 |
| Demonstrate atency | n = 13, 86.7% | n = 7, 100% | 1.0 |
| Reduced flow through shunt | n = 2, 13.3% | 0% | 1.0 |
TIPS, transjugular intrahepatic portosystemic shunt.
Table 5.
TIPS revision
| Shunt revision | TIPS creation technique | p-value | |
| Conventional (n = 18) | Primary Constrained (n = 9) | ||
| Total incidence per group | n = 4, 22.2% | n = 1, 11.1% | 0.63 |
| Shunt expansion owing to refractory ascites | n = 3, 16.7% | n = 1, 11.1% | 1.0 |
| Shunt reduction owing to hepatic encephalopathy | n = 1, 5.6% | 0% | 1.0 |
TIPS, transjugular intrahepatic portosystemic shunt.
DISCUSSION
The efficacy for TIPS in treating complications of portal hypertension are well documented, with the largest body of evidence for its use supported in the setting of recurrent or refractory variceal bleeding and refractory ascites.23 The major limitations of TIPS include shunt restenosis and an increased risk for HE.5,12 Although the routine use of ePFTE-lined stent grafts has substantially decreased the risk of shunt restenosis and consequently increased long-term TIPS patency rates, HE remains a frequent clinical sequela and often times a contraindication to the procedure itself.10 Encephalopathy primarily occurs owing to the bypass of unfiltered portal blood through the TIPS stent graft and into the systemic circulation.24 Based on previous studies, the onset of encephalopathy after TIPS insertion occurs in 5 to 35% of cases, with 5% of cases refractory to medical management.10
An increased risk of encephalopathy following TIPS is associated with higher preprocedural MELD scores, advanced stage of hepatic fibrosis, pre-TIPS portal venous pressure, older age and pre-existing encephalopathy.24–28 Prior studies have also uncovered an association between HE and the change in PSG after shunt placement. Findings presented by Haskal et al demonstrated that higher final PSG is associated with reduced incidence of HE.29,30 Hence, along with careful patient selection, controlled reduction in the PSG gradient can potentially reduce the risk of HE after TIPS placement.17,30 With conventional TIPS technique, controlling the reduction in PSG remains a challenge during primary placement as the change in gradient is unpredictable. While various endovascular techniques have been described to decrease shunt diameter after the onset of encephalopathy, little data exists on effective modification strategies during primary placement to prevent this complication.18,19
The current study describes an endovascular technique for creation of a primary constrained TIPS that allows for greater control over shunt diameter than traditionally described methods. This constrained technique has previously been described in the literature based on two small patient case-studies.20 Compared with conventional TIPS placement, this method includes one single additional step of deploying a balloon-expandable bare metal stent into the hepatic transvenous track to function as an externally constraining device for the selfexpanding e-PTFE-lined stent graft. This technique provides a greater control over the degree of shunting during the initial TIPS creation, allowing for stepwise increments in the shunt size diameter and reduction in PSG. This is also beneficial as the potential for undershunting is also avoided as the balloon-expandable stent can be overdilated up to the nominal size of the TIPS stent. This technique may particularly be advantageous in populations at high risk for encephalopathy, in which modest reductions of the PSG may prevent the onset or exacerbation of encephalopathy. In the current study, although there were no statistically significant difference in haemodynamic outcomes between both groups, there was a trend towards a smaller change in PSG in the constrained cohort (10.6 vs 6.1 mmHg) suggesting a greater control allowed for by the constrained technique. Similarly, a trend towards a decreased rate of encephalopathic complications was noted with the primary constrained TIPS group. Although statistically insignificant, the incidence (0%, n = 8) of post-TIPS HE observed in the primary constrained group is less than those previously reported with conventional TIPS placement. The incidence (n = 3, 17%) of HE in the conventional cohort were similar to previous studies.10
An additional unique advantage of primary constrained TIPS placement is the ease of future interventions. Although a trend towards lower reintervention was observed in the constrained group, in the incident that there was insufficient shunting following TIPS placement as indicated by refractory ascites or ongoing variceal bleeding, the shunt diameter could be expanded relatively easily through balloon dilation of the stent graft up to the nominal size of the TIPS. With conventional methods, more aggressive intervention such as parallel TIPS may need to be pursued. Alternatively, shunt reduction for conventional TIPS is a technically more challenging and time consuming procedure than shunt dilation.
There are several limitations to this investigation. First, this study is limited by its retrospective nature and subject to inherent weaknesses of a non-prospective study. Second, our investigation represents the experience of a single academic institution. Third, this study was conducted with a small sample size of patients as TIPS. Fourth, there is a significance difference between TIPS indication and racial makeup of individuals in both groups which could have influenced the outcomes of the study. Fifth, data collection relied upon reviewing patient charts and clinical assessment and clinical recognition of HE and other complications may be variable among medical providers. Sixth, variability in patient adherence to medical therapy and concurrent medical illnesses could impact reported rates of procedural complications. Finally, the ability to increase the calibre of TIPS will be limited by the diameter of the constraining stent. Finally, the long-term patency of primary constrained TIPS and the dynamics of stent–stent interaction are currently unknown.
In conclusion, while TIPS placement is a safe and effective therapy for treatment of portal hypertension, it places patients at increased risk for HE. The current study demonstrates that primary constrained shunt creation offers a promising and feasible alternative to conventional TIPS placement with similar technical and clinical success rates, with a trend towards tighter control of PSG and decreased risk of HE. These findings support a broader utilization of primary constrained technique, particularly in patients at increased risk for HE. Future efforts with larger patient size are required to determine the appropriate patient selection and clinical effectiveness of this procedure.
Contributor Information
R Rabei, Email: rana.rabei@my.rfums.org.
S Mathesovian, Email: Sipan.mathevosian@my.rfums.org.
S Madassery, Email: kmadassery@gmail.com.
U Turba, Email: ulku_c_turba@rush.edu.
O Ahmed, Email: Osman1423@gmail.com.
Conflict of interest
Author 5 is a speaker and advisory board member for Penumbra®, Medtronic/Covidien®, and speaker for Cook®, WL Gore®, Guerbet® and CR Bard®. Author 7 is a paid speaker for Cook® and common stockholder for Penumbra®.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Fanelli F. The evolution of transjugular intrahepatic portosystemic shunt: tips. ISRN Hepatol 2014; 2014: 1–12. doi: https://doi.org/10.1155/2014/762096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dariushnia SR, Haskal ZJ, Midia M, Martin LG, Walker TG, Kalva SP, et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 2016; 27: 1–7. doi: https://doi.org/10.1016/j.jvir.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 3.Otal P, Smayra T, Bureau C, Peron JM, Chabbert V, Chemla P, et al. Preliminary results of a new expanded-polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt procedures. AJR Am J Roentgenol 2002; 178: 141–7. doi: https://doi.org/10.2214/ajr.178.1.1780141 [DOI] [PubMed] [Google Scholar]
- 4.Rössle M, Siegerstetter V, Euringer W, Olschewski M, Kromeier J, Kurz K, et al. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long-term follow-up of 100 patients. Acta Radiol 2006; 47: 660–6. doi: https://doi.org/10.1080/02841850600806324 [DOI] [PubMed] [Google Scholar]
- 5.Bureau C, García-Pagán JC, Layrargues GP, Metivier S, Bellot P, Perreault P, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int 2007; 27: 742–7. doi: https://doi.org/10.1111/j.1478-3231.2007.01522.x [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Han G, Wu Q, Ye X, Jin Z, Yin Z, et al. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol 2010; 25: 1718–25. doi: https://doi.org/10.1111/j.1440-1746.2010.06400.x [DOI] [PubMed] [Google Scholar]
- 7.Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017; 152: 157–63. doi: https://doi.org/10.1053/j.gastro.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 8.Wang CM, Li X, Fu J, Luan JY, Li TR, Zhao J, et al. Construction of transjugular intrahepatic portosystemic shunt: bare metal stent/stent-graft combination versus single stent-graft, a prospective randomized controlled study with long-term patency and clinical analysis. Chin Med J 2016; 129: 1261–7. doi: https://doi.org/10.4103/0366-6999.182830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller FS, Farsad K, Rösch J. The transjugular intrahepatic portosystemic shunt: technique and instruments. Tech Vasc Interv Radiol 2016; 19: 2–9. doi: https://doi.org/10.1053/j.tvir.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Ripamonti R, Ferral H, Alonzo M, Patel NH. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol 2006; 23: 165–76. doi: https://doi.org/10.1055/s-2006-941447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blei AT, Córdoba J, Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 2001; 96: 1968–76. doi: https://doi.org/10.1111/j.1572-0241.2001.03964.x [DOI] [PubMed] [Google Scholar]
- 12.Madoff DC, Wallace MJ, Ahrar K, Saxon RR. TIPS-related hepatic encephalopathy: management options with novel endovascular techniques. Radiographics 2004; 24: 21–36. doi: https://doi.org/10.1148/rg.241035028 [DOI] [PubMed] [Google Scholar]
- 13.Quaretti P, Michieletti E, Rossi S. Successful treatment of TIPS-induced hepatic failure with an hourglass stent-graft: a simple new technique for reducing shunt flow. J Vasc Interv Radiol 2001; 12: 887–90. doi: https://doi.org/10.1016/S1051-0443(07)61516-4 [DOI] [PubMed] [Google Scholar]
- 14.Kroma G, Lopera J, Cura M, Suri R, El-Merhi F, Reading J. Transjugular intrahepatic portosystemic shunt flow reduction with adjustable polytetrafluoroethylene-covered balloon-expandable stents. J Vasc Interv Radiol 2009; 20: 981–6. doi: https://doi.org/10.1016/j.jvir.2009.03.042 [DOI] [PubMed] [Google Scholar]
- 15.Monnin-Bares V, Thony F, Sengel C, Bricault I, Leroy V, Ferretti G. Stent-graft narrowed with a lasso catheter: an adjustable TIPS reduction technique. J Vasc Interv Radiol 2010; 21: 275–80. doi: https://doi.org/10.1016/j.jvir.2009.10.019 [DOI] [PubMed] [Google Scholar]
- 16.Madoff DC, Wallace MJ. Reduced stents and stent-grafts for the management of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. Semin Intervent Radiol 2005; 22: 316–28. doi: https://doi.org/10.1055/s-2005-925558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhocki PV, Lungren MP, Kapoor B, Kim CY. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol 2015; 32: 123–32. doi: https://doi.org/10.1055/s-0035-1549376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanelli F, Salvatori FM, Rabuffi P, Boatta E, Riggio O, Lucatelli P, et al. Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglass-shaped balloon-expandable stent-graft. AJR Am J Roentgenol 2009; 193: 1696–702. doi: https://doi.org/10.2214/AJR.09.2968 [DOI] [PubMed] [Google Scholar]
- 19.Kharoti Y, Ahuja C, Timmermans H, Schenning R, Priest R, Peterson B. Educational Exhibit Abstract No. 354 - primary placement of a constrained transjugular intrahepatic portosystemic shunt: how we do it. J Vasc Interv Radiol 2013; 24: S152–S153. [Google Scholar]
- 20.Farsad K, Kolbeck KJ, Keller FS, Barton RE, Kaufman JA. Primary creation of an externally constrained TIPS: a technique to control reduction of the portosystemic gradient. AJR Am J Roentgenol 2015; 204: 868–71. doi: https://doi.org/10.2214/AJR.14.13104 [DOI] [PubMed] [Google Scholar]
- 21.Saxon RR, Keller FS. Technical aspects of accessing the portal vein during the TIPS procedure. J Vasc Interv Radiol 1997; 8: 733–44. doi: https://doi.org/10.1016/S1051-0443(97)70655-9 [DOI] [PubMed] [Google Scholar]
- 22.LaBerge JM, Ring EJ, Gordon RL, Lake JR, Doherty MM, Somberg KA, et al. Creation of transjugular intrahepatic portosystemic shunts with the wallstent endoprosthesis: results in 100 patients. Radiology 1993; 187: 413–20. doi: https://doi.org/10.1148/radiology.187.2.8475283 [DOI] [PubMed] [Google Scholar]
- 23.Patidar KR, Sydnor M, Sanyal AJ. Transjugular intrahepatic portosystemic shunt. Clin Liver Dis 2014; 18: 853–76. doi: https://doi.org/10.1016/j.cld.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suraweera D, Sundaram V, Saab S. Evaluation and management of hepatic encephalopathy: current status and future directions. Gut Liver 2016; 10: 509–19. doi: https://doi.org/10.5009/gnl15419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Routhu M, Safka V, Routhu SK, Fejfar T, Jirkovsky V, Krajina A, et al. Observational cohort study of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS). Ann Hepatol 2017; 16: 140: –8. doi: https://doi.org/10.5604/16652681.1226932 [DOI] [PubMed] [Google Scholar]
- 26.He F, Dai S, Xiao Z, Wang L, Yue Z, Zhao H, et al. Pathological predictors of shunt stenosis and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Biomed Res Int 2016; 2016: 1–8. doi: https://doi.org/10.1155/2016/3681731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaba RC, Couture PM, Bui JT, Knuttinen MG, Walzer NM, Kallwitz ER, et al. Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol 2013; 24: 411–20. doi: https://doi.org/10.1016/j.jvir.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 28.Parvinian A, Shah KD, Couture PM, Minocha J, Knuttinen MG, Bui JT, et al. Older patient age may predict early mortality after transjugular intrahepatic portosystemic shunt creation in individuals at intermediate risk. J Vasc Interv Radiol 2013; 24: 941–6. doi: https://doi.org/10.1016/j.jvir.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 29.Patidar KR, Sydnor M, Sanyal AJ. Transjugular intrahepatic portosystemic shunt. Clin Liver Dis 2014; 18: 853–76. doi: https://doi.org/10.1016/j.cld.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haskal ZJ, Duszak R, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol 1996; 7: 139–42. doi: https://doi.org/10.1016/S1051-0443(96)70750-9 [DOI] [PubMed] [Google Scholar]


