Abstract
Objective:
The last 20 years has seen an exponential increase in 3D printing as it pertains to the medical industry and more specifically surgery. Previous reviews in this domain have chosen to focus on applications within a specific field. To our knowledge, none have evaluated the broad applications of patient-specific or digital imaging and communications in medicine (DICOM) derived applications of this technology.
Methods:
We searched PUBMED and CINAHL from April 2012 to April 2017.
Results:
261 studies fulfilled the inclusion criteria. Proportions of articles reviewed: DICOM (5%), CT (38%), MRI (20%), Ultrasonography (28%), and Bio-printing (9%).
Conclusion:
There is level IV evidence to support the use of 3D printing for education, pre-operative planning, simulation and implantation. In order to make this technology widely applicable, it will require automation of DICOM to standard tessellation language to implant.
Advances in knowledge:
Recent lapses in intellectual property and greater familiarity with rapid prototyping in medicine has set the stage for the next generation of custom implants, simulators and autografts. Radiologists may be able to help establish reimbursable procedural terminology.
INTRODUCTION
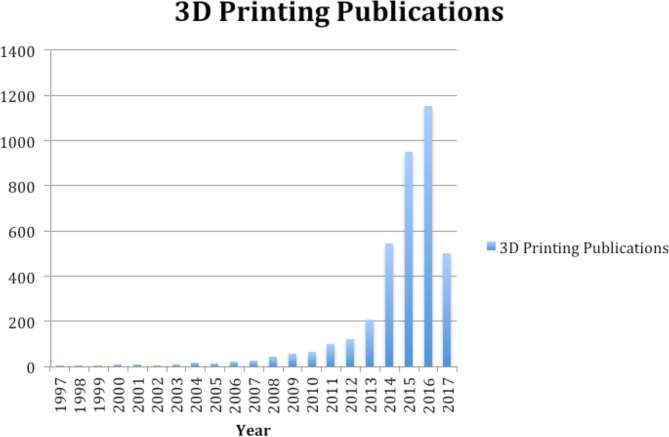
The last 20 years has seen an exponential increase of interest in 3D printing as it pertains to the medical industry and more specifically surgery. This is likely owing to the constant demand for practitioners of many specialties to mentally convert 2D images into 3D objects. This demand has increased alongside improving imaging modalities, and more importantly a dramatic decrease in cost associated with rapid prototyping owing to patent expiration. Patents protecting fused deposition modelling expired in 2009 and ushered in major desktop printer companies like MakerBot® (New York, NY) (Figure 1). Subsequently, printers fell from $10,000 per unit down to $1000 almost immediately.1 Within the year, there was a 30% increase in the number of scholarly articles applying 3D printing from the year before (Figure 2). Additional changes in intellectual property continue to disrupt this field. Powder-based or selective laser sintering patents expired in 2004 and the most recent patent that expired was in December 2016 making metal-based selective laser melting (SLM) widely available to researchers, hobbyists and entrepreneurs (Figure 3). The latter may usher in a new era of medical devices, as SLM technology is capable of creating one-off parts durable enough for automobile application. In addition to more traditional means of rapid prototyping, recently published reviews describe the use of bioprinting to produce bones, ears, vascular networks, tissues, organs and drug delivery devices.2
Figure 1.
MakerBot® (New York, NY) replicator 2 is a fused deposition modelling printer capable of 100 micron resolution.
Figure 2.
Number of publications per year, 1997–April 2017.
Figure 3.
Selective laser melting is capable of rapid prototyping metal-based models including patient specific anatomy.
Previous reviews in this domain have chosen to focus on applications within a specific field. To date, none have evaluated the broad applications of patient-specific or DICOM-derived musculoskeletal applications of this technology.
The main achievements in 3D printing are derived from translational research, whereby individual researchers can design, educate and build novel tools and tissues in real time.3 This area of research is currently limited to case reports and case series, with only a few randomized trials.4 With the massive expansion in the body of literature over the last 5 years, researchers are moving beyond proof of concept and case reports. The purpose of this manuscript is to review the recent literature regarding the current implementation of 3D printing in surgery.
METHODS AND MATERIALS
A search was performed using PUBMED and CINAHL from April 2012 to April 2017 using the terms: “3D printingℍ and ℌsurgeryℍ in combination with ℌDICOMℍ (digital imaging and communications in medicine), ℌcomputed tomographyℍ, ℌmagnetic resonance imagingℍ, ℌultrasonographyℍ and ℌbioprintingℍ. Studies published in the last 5 years were eligible for inclusion. Given the heterogeneity of the literature and the various study methodologies, quantitative data synthesis in the form of meta-analysis was deemed implausible.
RESULTS
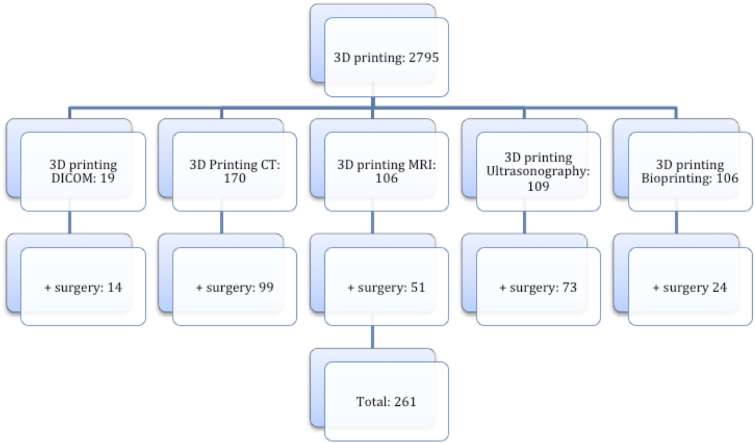
261 studies fulfilled the inclusion criteria (Figure 4). ℌ3D printingℍ and ℌsurgeryℍ when combined with the listed search terms comprised the following proportions of literature reviewed: DICOM (5%), CT (38%), MRI (20%) and ultrasonography (28%) and bioprinting (9%).
Figure 4.
Number of indexed peer-reviewed publications over the last 5 years by search term. DICOM, digital imaging and communications in medicine.
The studies were categorized by their intended applications: education, simulation, pre-operative templates, intraoperative guides/implants and biological.
Printing materials
Many materials exist that can either be extruded or controlled to create 3D objects (thermoplastics, photopolymers, metals such as titanium and alloys, polyesters and organic cells). As we move into the next generation of rapid prototyping in medicine, there are evermore materials to choose from. Each material must be considered by cost, size, temperature, durability, tolerance of complex shapes, sterility and handling among other characteristics. Prototypical desktop printers use fused deposition printing owing to its ease of use, and utilize low-cost thermoplastics such as polylactic acid. These objects have reasonable durability, very low cost and adequate 100 micron resolution.5 Thermoplastics, however, are not capable of printing a dramatic overhang (greater than 45° from vertical axis) without requiring a sacrificial construct to support the deposition while cooling. They are also tolerant of sterilization in some forms but likely not adequate for implantation.6 SLM, binder jetting and powder bed fusion are printing techniques capable of printing in advanced materials such as titanium, materials currently accepted as reliable medical grade implants. However, in order to optimize handling and surgical simulation, researchers have used novel technologies to create patient specific constructs that allow for the use of the same instruments that will be used during that patient’s operation. Realistic handling characteristics are critical to the quality of the simulation.7 Ultimately, the greatest material in medicine would allow for the printing of autologous composite tissue.
Imaging and reconstructive software
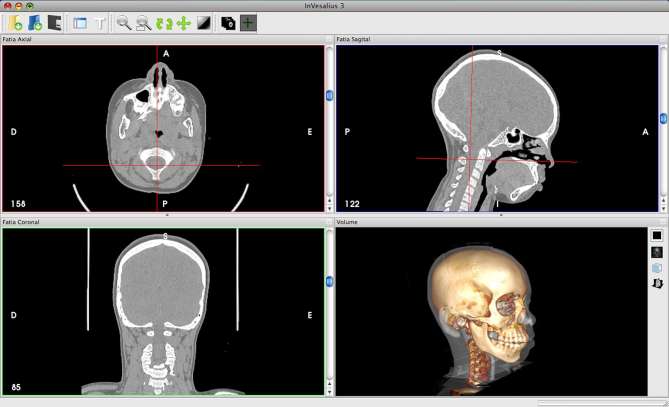
The current process of creating a 3D representation of a patient’s anatomy is far from being fully automated (Figure 5). Currently, CT is the most commonly used imaging modality for 3D reconstruction, although printing from MRI and even ultrasound have been demonstrated.8 3D printing is critically dependent on accurate volumetric representation, therefore, the slice thickness of any chosen modality is critical to creating adequate spatial resolution necessary for a smooth, natural 3D reconstruction. Whyms et al9 found that a slice thickness of 1.25 mm is the most important parameter when creating a three-dimensional render of cross-sectional imaging given the limitations of current technology. Today, clinical image acquisition can be done at ultrahigh spatial resolution (400–600 microns and higher) with good quality contrast. Slice thicknesses of less than 1 mm and isotropic voxels are important parameters to be accounted for while minimizing the partial volume effect during post-processing.5 The images obtained must go through a process termed thresholding to distinguish one tissue type from another. Specialized software such as 3D Slicer (Brigham and Females's Hospital, Boston, MA), Osirix (Pixmeo, Geneva, Switzerland) and ITK-Snap (http://www.itksnap.org), as well as commercial software such as Mimics (Materialise Interactive Medical Image Control System; Materialise, Leuven, Belgium) and Vitrea (Vital Images Inc., Minnetonka, MN) typically have preset thresholds depending on the tissue of interest, but sometimes, manual thresholding is still required to identify the optimal greyscale (Figure 6).
Figure 5.
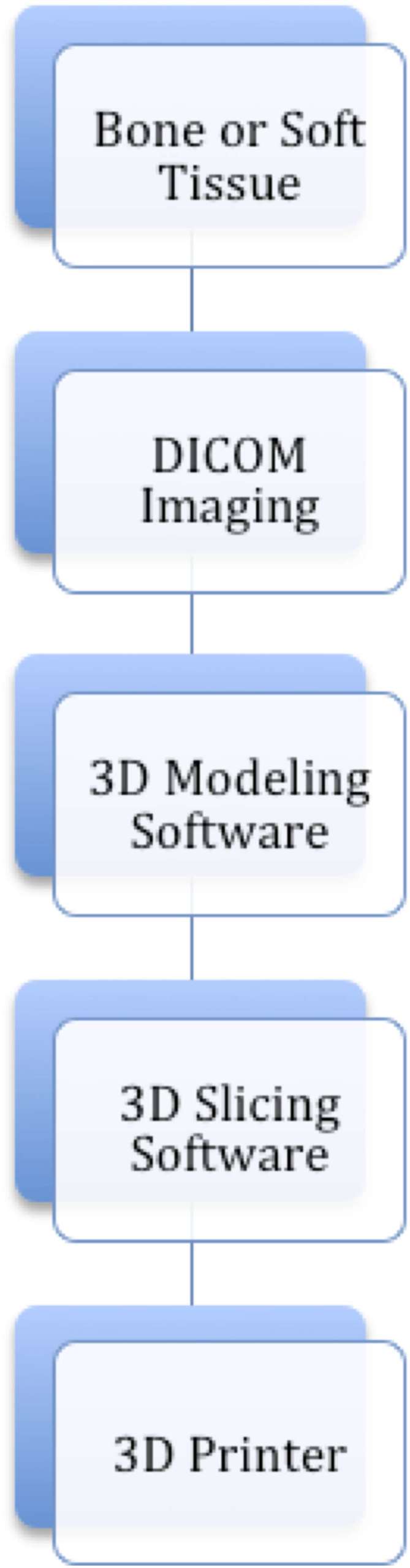
Workflow from data acquisition of patient anatomy to 3D model. DICOM, digital imaging and communications in medicine.
Figure 6.
ℌThresholdingℍ is a process whereby tissue density is digitally evaluated and used to define discreet anatomical structures that can then be used for rapid prototyping. (Invesalius, Information Technology Center Renato Archer, Campinas, Brazil)
Despite constantly improving technology, manual thresholding in multidetector row CT is still best for translation to standard tessellation language (STL) files, one of the most universal file formats for 3D image manipulation. Currently, there is too much variability in dual energy and cone beam CT to allow for universal automation.10 3D model configuration and blending can also be done via computer-aided drafting (CAD) to aid visualization of complex pathology or to combine multi-imaging modalities. Common CAD software choices include Meshmixer (Autodesk, San Rafael, CA), 3Matic (Materialise, Leuven, Belgium) and Solidworks (Waltham, MA) among many others.11 STL files are then loaded into slicing software which prepares the file for printing by converting it into G-code (numerical control programming language), a universal code for sending printer position and extrusion commands to desktop 3D printers.
Education
3D printed artefacts are a form of communication with promise in education of students, residents and their patients. Various compounds including photopolymer and thermoplastics have been favoured for this application owing to reduced costs, good resolution (20–100 micron) and ease of use.
Complex kidney stone models were printed and used to improve patient understanding of their disease process with improved satisfaction.12 Resident physicians also benefit from this technology, demonstrating improved understanding of complex renal pathology with a patient specific 3D model when compared with 2D imaging alone.13 The importance of patient education has translatable financial gains as the Center for Medicare and Medicaid Services, in the United States, have made 1–2% of reimbursements dependent on patient satisfaction.14 With improved patient understanding and satisfaction through the use of personalized 3D models during clinical explanations of complex anatomy, there could be financial benefits to implementing rapid prototyping in the office.15 A recent series highlights the utility of tangible objects as researchers recruited 10 students, 10 surgeons and 10 radiologists. The subjects were asked questions about imaging in three forms (CT axial, CT 3D digital reconstruction and 3D printed reconstruction). While computerized visual aids improved education, the addition of tangible counterparts alone significantly improved their teaching power.16
Procedural simulation
Pre-operative simulation of a procedure allows both pre-operative assessment and reproduction of complex operative steps but does not suffer the time, financial and patient constraints of a live procedure. Effective surgical simulation requires that the anatomy not only be true to life, but it must also behave in a similar manner to the native tissue. For example, a hard thermoplastic would make for a poor simulated length of bowel as a surgeon would not be able to anastomose plastic with a stapling device and an orthopaedic surgeon would not be able to realistically stabilize a pliable silicone model.
In an effort to reproduce the ideal handling properties in simulated organs, researchers are now 3D printing molds that are then used for silicone injection molding. The idea of printing a negative has also been applied in ear reconstruction.17 A study using silicone kidneys to simulate partial nephrectomy resulted in shorter in vivo operative times of 16 vs 17 min in vitro and lower ischemia times in vivo.18 Additionally, Fukushima et al7 was able to print a salt construct that allowed for the use of chisels and drills during the pre-operative planning and simulation of periacetabular osteotomies.
Certain areas of the human anatomy are less accessible and their exposure places critical structures and patient well-being at risk. Zeng et al studied 50 patients with pelvic fractures that would require open reduction and internal fixation. Using simulation and pre-operative bending of titanium plates (as opposed to intraoperative bending), they were able to reduce operative time and decrease blood loss. Additionally, post-operative CT’s showed good fidelity between the pre-operative simulation and post-operative outcome.19 This technology also allows for experimentation of new procedures and approaches not previously feasible owing to excess risk to patient and surrounding structures. For example, high spinal cord ligamentous injuries may necessitate craniocervical fixation, but such procedures risk the vertebral artery, hypoglossal canal, condyle emissary vein canal and the atlanto-occipital joint. Researchers in China postulate that with the use of 3D printing technology, occipital condyle screws could be safely placed using a 3D printed template to guide the screws.20
Pre-operative planning (3D templates)
Pre-operative analysis may currently be one of the most useful applications of 3D printing technology. Areas where instrumentation of complex anatomy can risk function, perfusion and life will benefit the most from rapid prototyping of DICOM images. Head, spine, pelvic, hand and foot surgery are areas that stand to benefit greatly.21 Additionally, anatomy with higher degrees of interpatient variability presents an opportunity for improvement. In a minimally invasive approach, vascular surgeons are able to instrument and in some cases stent carotid arteries. However, these procedures come with a high risk of stroke, necessitating an intimate knowledge of an individual’s vascular anatomy in order to reduce intraoperative risk.22 The ability to be able to preselect an angiocatheter with the appropriate shape and characteristics could mean fewer passes and less risk of embolizing plaque.
Similarly preoperative bending of implants prior to fixation of fractures negates the need for intraoperative modification.23 When applied to spine surgery, Sugawara et al24 reduced the rate of cortical perforation with free hand screws from 43% in some reports down to 0%.
These applications are of course only as good as the patient data obtained. In an effort to improve outcomes, researchers have developed a method for measuring point inaccuracies associated with rapidly prototyped objects to measure fidelity.25 One of the larger series utilizing pre-operative simulation tracked 120 femur fractures that were virtually reduced and navigational tracking points were recorded for analysis. Post-operative comparisons showed accuracy with the anticipated, digital reductions.26
Time is critical. Financial savings have been realized with rapid prototyping but limitations remain in quantifying the opportunity cost of the time spent in rendering and building models, prototypes and implants. However, in one of the first reports of a true real time application of 3D printing in medicine, Konno and his colleagues27 showed that 3D printing is fast enough for emergency cerebral aneurysm clipping with print time averaging a mere 67 min and time to OR averaging 240 min.
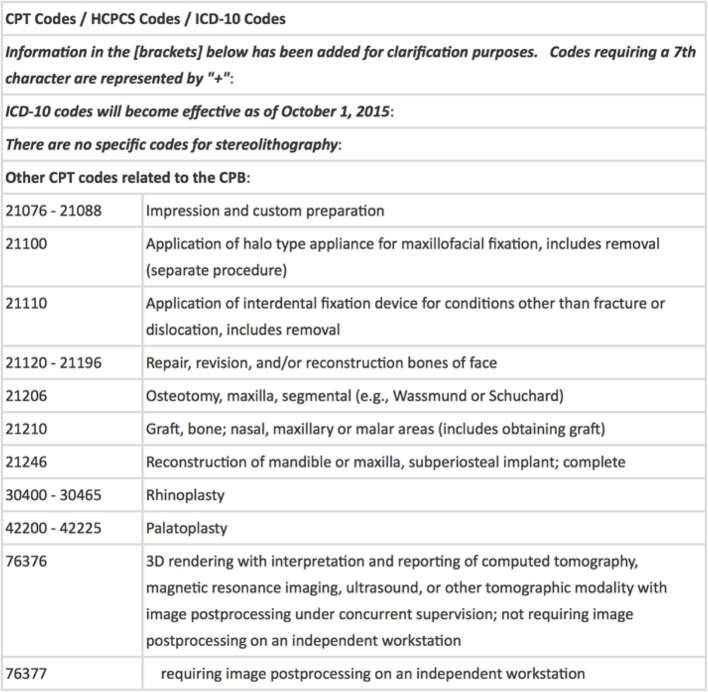
The financial implications of rapid prototyping in medicine must eventually be addressed. 3D printing of medial femoral trochlear flaps and fibula flaps were used in a series of patients for planning of osteocutaneous flaps for wrist recon on a home based printer. Researchers realized significant cost savings over industry-produced 3D templates, as they realized a total cost of roughly $92 per template, although this cost could be substantially lowered with increased volume.28 We have found that with sufficient volume, we have been able to bring our template costs down to roughly $3 per unit.6 Of note, computer-aided surgery of the liver is and has been covered by universal health care in Japan since 2012.29 Although US-based insurance companies do not support all types of computer-aided surgery at this point, some patient specific industry-made craniofacial implants, such as cranial plates for cranioplasty, are covered. Conversely, ℌ3D Printedℍ implants and templates are not currently covered as there are no specific current procedural terminology codes for additive manufacturing (Figure 7).30 There are ongoing challenges associated with keeping healthcare from falling behind rapidly advancing technology. This technology has already shown tangible utility in patient care and satisfaction, therefore, reimbursements must keep up with technology if we are to provide cutting edge care for our patients.
Figure 7.
CPT codes that may be used to approximate the applications of additive manufacturing, although there are currently no reimbursable codes for 3D printing. CPT, current procedural terminology; HCPCS, health care common procedure coding system; ICD-10, 10th revision of the International statistical classification of diseases and related health problems.
Intraoperative applications
Intraoperative applications of printed implants demand sterility, strength, and acuracy. These demands have previously come with a high cost from industrial partners, but now open source, free software can be used to create devices that fit the bill.31 The first intraoperative 3D printed models served as templates, which improved understanding of complex anatomy in real time for such procedures as revision craniosynostosis.32 Moving beyond templates; cutting jigs, screw guides and graft molds may make surgical intervention more accurate and efficient. Cutting jigs for osteocutaneous reconstruction offer a huge advantage in time and accuracy during free tissue transfer that is necessary for certain head and neck pathologies (Figure 8). 3D printed reduction aids have also been used in long bone fractures. While applying hardware during tibia and femur open reduction and internal fixation, researchers are able to approximate 3D printed templates to guide osseous reduction, thereby improving reduction and shortening operative time.33 Additionally, implanted biodegradable 3D printed templates have been used for vertical alveolar ridge bone grafts. As a result, the bone graft more accurately reconstructs the missing alveolar ridge.34 Combining laser scanning of gums and teeth over CAD imaging from CT allows for accurate fabrication of dental implants as it provides a more accurate relationship between soft tissue and bony structures.35 When applied as an intraoperative guide, a case series of 12 patients requiring C1-C2 fusion benefitted from use of a personalized 3D printed template, as all 48 screws were appropriately placed with 0.7 ± 0.4 mm variation from simulated and preplanned trajectories.36
Figure 8.
A 3D printed cutting jig designed for guided osteotomies of a free fibula that will then be used from mandibular reconstruction (Embodi 3D, Biomedical 3D printing community).
The next level of 3D printing faces the rigid standards of implantable reconstruction of the load bearing axial skeleton. Micromotion of skeletal implants can ultimately lead to pain, destabilization and ultimately need for revision. Novel personalized 3D printed titanium cones show decreased micromotion compared with standard titanium cones for revision total knee arthroplasty.37 These works will need to be verified in vivo, but could potentially reduce the rate of revision in total knee arthroplasty.
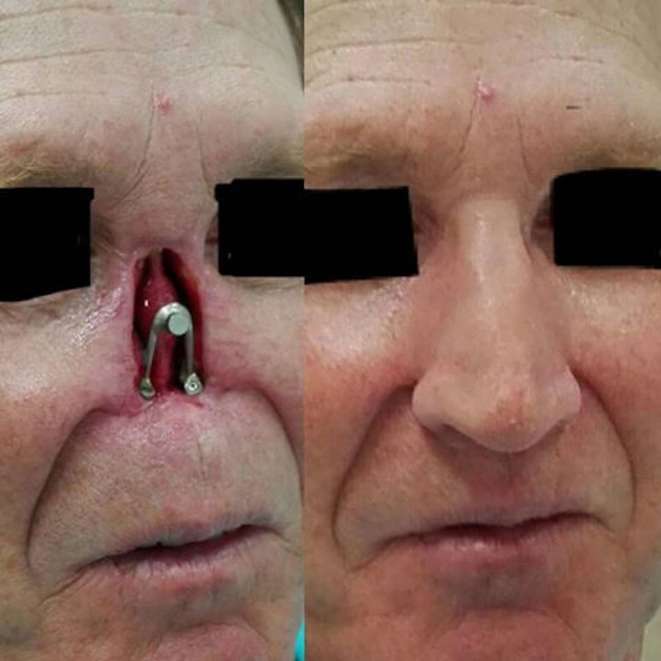
Most rapidly prototyped implants are designed to be covered with soft tissue, but this is not always the rule. The dental industry has shown a novel technology whereby aesthetic subunits of the face are printed in their entirety. Researchers identified a male who had failed multiple nasal reconstructions and were able to 3D print a composite prosthetic for total nasal reconstruction. Anaplastologists and surgeons worked together with industry to rapidly prototype a titanium implant that was secured to the skull base and midface and subsequently covered with non-biologic, artificial soft tissue (Figure 9).38
Figure 9.
Anaplastologists are able to work with reconstructive surgeons to design aesthetically pleasing artificial body parts or facial subunits that adhere to permanent implants if more complex reconstruction is not an option or not desired.38
Printing biological scaffolds
Bioprinting is the act of rapid prototyping with cells or biologically active matrices. The development of solvent-free, aqueous-based systems enabled the direct printing of biological materials into 3D scaffolds that could be used for transplantation with or without cells.39 Printing biologically active scaffolds, tissues or even organs is possible through various methodologies including sacrificial biodegradable constructs plus seeding, inkjet bioprinting, microextrusion bioprinting or laser-assisted bioprinting. The first method takes advantage of the lower cost of printing with thermoplastics and then overlays the construct with cells in order to create tubular structures. These sacrificial scaffolds can also be dipped into biomaterials and may be used to create a hollow viscus.40 Another group has used sacrificial scaffolds to grow cartilage. Using polycaprolactone nasal and auricular 3D printed constructs, they seeded these structures with chondrogenic growth factors and implanted in pigs. Within 2 months, researchers found that the implanted constructs had good tissue ingrowth and ℌnative-appearingℍ cartilage that grew only within the confines of the nasal or auricular construct.41
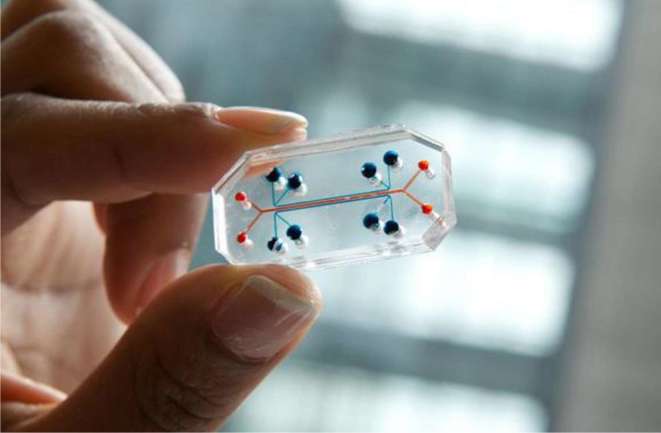
The later options use a more complex means to create biologically active tissues either by biomimicry, self-assembly or mini-tissues.42 Bioprinting, although an incredible step in the history of 3D printing has been plagued by short survival times and thin tissues. However, a group from Boston has shown fundamental progress by bioprinting complex tissue environments dependent on embedded vasculature and able to span up to a centimeter in thickness.43 Further updates from the same group have now given new hope to approximately 97,000 people awaiting kidney transplantation.44 Homan et al45 are able to create a portion of a renal tubule on a ℌchipℍ, which is a gelatin-fibrinogen extracellular matrix capable of supporting a basement membrane and polarized renal tubular cells. Once cast about a sacrificial 3D printed tubule, these tissues are capable of prolonged survival as well as drug screening (Figure 10).45
Figure 10.
ℌOrgan-on-a-chipℍ is the product of emerging bioprinting technologies. Researchers, including the Wyss Institute (Cambridge, MA), have been able to develop small, biologically active ℌchipsℍ that can be used for drug testing.
CONCLUSION
Patient specific rapid prototyping is progressing quickly but case reports and small case series still dominate the academic landscape. The outstanding questions of implant longevity and whether customized implants will truly improve outcomes still remain. Additionally, the ideal ratio of cost to spatial resolution for a given utility is unknown. In order to make this technology widely applicable, it will eventually require some level of automation of DICOM to STL and those algorithms will need to be vetted in order to ensure accurate volumetric representation. Educational outcomes are limited to subjective feedback at this time and will need to be supported by objective measures.
Radiologists need to be familiar with 3D printing, as we anticipate that there will be increased demand for pre-operative planning and implant creation.46 Radiologists may be able to guide imaging parameters, improve patient outcomes and help establish reimbursable procedural terminology. The future of all these endeavours lies in bioprinting of biocompatible custom implants or organs that will integrate into your tissues without the elevated risk of infection, rejection or extrusion.
Contributor Information
Timothy M Rankin, Email: tmrankin.md@gmail.com.
Blair A Wormer, Email: blair.a.wormer@vanderbilt.edu.
John D Miller, Email: jdmpodiatry@gmail.com.
Nicholas A Giovinco, Email: ngiovinco@gmail.com.
Salam Al Kassis, Email: salam.al.kassis@vanderbilt.edu.
David G Armstrong, Email: armstrong@usa.net.
REFERENCES
- 1.Gallego J. A Host of Soon-to-be-Expired Patents are Set to Revolutionize 3D Printing; 2016. [Google Scholar]
- 2.Ventola CL. Medical applications for 3D printing: current and projected uses. P T 2014; 39: 704–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee N. The Lancet Technology: 3D printing for instruments, models, and organs? Lancet 2016; 388: 1368. doi: https://doi.org/10.1016/S0140-6736(16)31735-4 [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Zhang G, Lin H, Lu J, Huang W, Yu Z, et al. Digital design of standard parts database for proximal tibia fractures treated with plating via three-dimensional printing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2015; 29: 704–11. [PubMed] [Google Scholar]
- 5.Gupta R, Grasruck M, Suess C, Bartling SH, Schmidt B, Stierstorfer K, et al. Ultra-high resolution flat-panel volume CT: fundamental principles, design architecture, and system characterization. Eur Radiol 2006; 16: 1191–205. doi: https://doi.org/10.1007/s00330-006-0156-y [DOI] [PubMed] [Google Scholar]
- 6.Rankin TM, Giovinco NA, Cucher DJ, Watts G, Hurwitz B, Armstrong DG. Three-dimensional printing surgical instruments: are we there yet? J Surg Res 2014; 189: 193–7. doi: https://doi.org/10.1016/j.jss.2014.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushima K, Takahira N, Uchiyama K, Moriya M, Takaso M. Pre-operative simulation of periacetabular osteotomy via a three-dimensional model constructed from salt. Sicot J 2017; 3: 14. doi: https://doi.org/10.1051/sicotj/2016051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuel BP, Pinto C, Pietila T, Vettukattil JJ. Ultrasound-derived three-dimensional printing in congenital heart disease. J Digit Imaging 2015; 28: 459–61. doi: https://doi.org/10.1007/s10278-014-9761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115: 682–91. doi: https://doi.org/10.1016/j.oooo.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eijnatten M, Koivisto J, Karhu K, Forouzanfar T, Wolff J. The impact of manual threshold selection in medical additive manufacturing. Int J Comput Assist Radiol Surg 2017; 12: 607–15. doi: https://doi.org/10.1007/s11548-016-1490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripley B, Levin D, Kelil T, Hermsen JL, Kim S, Maki JH, et al. 3D printing from MRI Data: Harnessing strengths and minimizing weaknesses. J Magn Reson Imaging 2017; 45: 635–45. doi: https://doi.org/10.1002/jmri.25526 [DOI] [PubMed] [Google Scholar]
- 12.Atalay HA, Canat HL, Ülker V, Alkan İ, Ökuvanci Ü, Altunrende F. Impact of personalized three-dimensional -3D- printed pelvicalyceal system models on patient information in percutaneous nephrolithotripsy surgery: a pilot study. Int Braz J Urol 2017; 43: 470–5. doi: https://doi.org/10.1590/s1677-5538.ibju.2016.0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atalay HA, Ülker V, Alkan İ, Canat HL, Ökuvancı Ü, Altunrende F. Impact of three-dimensional printed pelvicaliceal system models on residents' understanding of pelvicaliceal system anatomy before percutaneous nephrolithotripsy surgery: a pilot study. J Endourol 2016; 30: 1132–7. doi: https://doi.org/10.1089/end.2016.0307 [DOI] [PubMed] [Google Scholar]
- 14.Letourneau R. Better HCAHPS Scores Protect Revenue; 2016. [Google Scholar]
- 15.Sander IM, Liepert TT, Doney EL, Leevy WM, Liepert DR. Patient education for endoscopic sinus surgery: preliminary experience using 3D-printed clinical imaging data. J Funct Biomater 2017; 8: 13. doi: https://doi.org/10.3390/jfb8020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marconi S, Pugliese L, Botti M, Peri A, Cavazzi E, Latteri S, et al. Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc 2017; 31: 4102–10. doi: https://doi.org/10.1007/s00464-017-5457-5 [DOI] [PubMed] [Google Scholar]
- 17.Rankin TM, Mailey B, Cucher D, Giovinco NA, Armstrong DG, Gosman A. Use of 3D printing for auricular template molds in first stage microtia. Plast Reconstr Surg 2014; 134: 16–17. doi: https://doi.org/10.1097/01.prs.0000455334.62912.bc [Google Scholar]
- 18.Golab A, Smektala T, Kaczmarek K, Stamirowski R, Hrab M, Slojewski M. Laparoscopic partial nephrectomy supported by training involving personalized silicone replica poured in three-dimensional printed casting mold. J Laparoendosc Adv Surg Tech A 2017; 27: 420–2. doi: https://doi.org/10.1089/lap.2016.0596 [DOI] [PubMed] [Google Scholar]
- 19.Zeng CJ, Tan XY, Huang HJ, Huang WQ, Li T, Jin DD, et al. Clincial effect of 3D printing-assisted minimal invasive surgery through a small incision lateral to the rectus abdominis for pelvic fracture. Nan Fang Yi Ke Da Xue Xue Bao 2016; 36: 220–5. [PubMed] [Google Scholar]
- 20.Huang X, Li F, Zhang F, Wang K, Yang Q, Dang R, et al. A cadaveric study on establishing an individualized navigation template for the placement of occipital condyle screws using a three-dimensional printing technique. Zhonghua Wai Ke Za Zhi 2014; 52: 523–8. [PubMed] [Google Scholar]
- 21.Giovinco NA, Dunn SP, Dowling L, Smith C, Trowell L, Ruch JA, et al. A novel combination of printed 3-dimensional anatomic templates and computer-assisted surgical simulation for virtual preoperative planning in Charcot foot reconstruction. J Foot Ankle Surg 2012; 51: 387–93. doi: https://doi.org/10.1053/j.jfas.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 22.Govsa F, Yagdi T, Ozer MA, Eraslan C, Alagoz AK. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur Arch Otorhinolaryngol 2017; 274: 1097–102. doi: https://doi.org/10.1007/s00405-016-4355-0 [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Xing W, Wu Z, Huang H, Huang W. A combination of three-dimensional printing and computer-assisted virtual surgical procedure for preoperative planning of acetabular fracture reduction. Injury 2016; 47: 2223–7. doi: https://doi.org/10.1016/j.injury.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 24.Sugawara T, Higashiyama N, Kaneyama S, Takabatake M, Watanabe N, Uchida F, et al. Multistep pedicle screw insertion procedure with patient-specific lamina fit-and-lock templates for the thoracic spine: clinical article. J Neurosurg Spine 2013; 19: 185–90. doi: https://doi.org/10.3171/2013.4.SPINE121059 [DOI] [PubMed] [Google Scholar]
- 25.Olszewski R, Szymor P, Kozakiewicz M. Accuracy of three-dimensional, paper-based models generated using a low-cost, three-dimensional printer. J Craniomaxillofac Surg 2014; 42: 1847–52. doi: https://doi.org/10.1016/j.jcms.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Huang W, Chen X, Zhang G, Yu Z, Wu X, et al. Digital design of internal fixation for distal femoral fractures via 3D printing and standard parts database. Zhonghua Yi Xue Za Zhi 2016; 96: 344–8. [DOI] [PubMed] [Google Scholar]
- 27.Konno T, Mashiko T, Oguma H, Kaneko N, Otani K, Watanabe E. Rapid 3-dimensional models of cerebral aneurysm for emergency surgical clipping. No Shinkei Geka 2016; 44: 651–60. doi: https://doi.org/10.11477/mf.1436203350 [DOI] [PubMed] [Google Scholar]
- 28.Taylor EM, Iorio ML. Surgeon-based 3D printing for microvascular bone flaps. J Reconstr Microsurg 2017; 33: 441–5. doi: https://doi.org/10.1055/s-0037-1600133 [DOI] [PubMed] [Google Scholar]
- 29.Oshiro Y, Ohkohchi N. 3D liver surgery simulation: computer-assisted surgical planning with 3D simulation software and 3D printing. Tissue Eng Part A 2017; 23: 474–80. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30. Aetna Inc. Clinical Policy Bulletins In: Stereolithography; 2016. Available from: . www.aetna.com/cpb/medical/data/600_699/0613.html. [Google Scholar]
- 31.Ganry L, Hersant B, Quilichini J, Leyder P, Meningaud JP. Use of the 3D surgical modelling technique with open-source software for mandibular fibula free flap reconstruction and its surgical guides. J Stomatol Oral Maxillofac Surg 2017; 118: 197–202. doi: https://doi.org/10.1016/j.jormas.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 32.LoPresti M, Daniels B, Buchanan EP, Monson L, Lam S. Virtual surgical planning and 3D printing in repeat calvarial vault reconstruction for craniosynostosis: technical note. J Neurosurg Pediatr 2017; 19: 490–4. doi: https://doi.org/10.3171/2016.10.PEDS16301 [DOI] [PubMed] [Google Scholar]
- 33.Omar M, Zeller AN, Gellrich NC, Rana M, Krettek C, Liodakis E. Application of a customized 3D printed reduction aid after external fixation of the femur and tibia: technical note. Int J Med Robot 2017; e1803 Epub ahead of print. doi: https://doi.org/10.1002/rcs.1803 [DOI] [PubMed] [Google Scholar]
- 34.Draenert FG, Gebhart F, Mitov G, Neff A. Biomaterial shell bending with 3D-printed templates in vertical and alveolar ridge augmentation: a technical note. Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 123: 651–60. doi: https://doi.org/10.1016/j.oooo.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 35.Wang YT, Yu JH, Lo LJ, Hsu PH, Lin CL, Lin CHun-L. Developing customized dental miniscrew surgical template from thermoplastic polymer material using image superimposition, CAD system, and 3D printing. Biomed Res Int 2017; 2017: 1–8. doi: https://doi.org/10.1155/2017/1906197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugawara T, Higashiyama N, Kaneyama S, Sumi M. Accurate and simple screw insertion procedure with patient-specific screw guide templates for posterior C1-C2 fixation. Spine 2017; 42: E340–E346. doi: https://doi.org/10.1097/BRS.0000000000001807 [DOI] [PubMed] [Google Scholar]
- 37.Faizan A, Bhowmik-Stoker M, Alipit V, Kirk AE, Krebs VE, Harwin SF, et al. Development and verification of novel porous titanium metaphyseal cones for revision total knee arthroplasty. J Arthroplasty 2017; 32: 1946–53. doi: https://doi.org/10.1016/j.arth.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 38.Toso SM, Menzel K, Motzkus Y, Adolphs N, Hoffmeister B, Raguse JD. Patient-specific implant in prosthetic craniofacial reconstruction: first report of a novel technique with far-reaching perspective. J Craniofac Surg 2015; 26: 2133–5. doi: https://doi.org/10.1097/SCS.0000000000002142 [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Iwanaga S, Henmi C, Arai K, Nishiyama Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010; 2: 014110. doi: https://doi.org/10.1088/1758-5082/2/1/014110 [DOI] [PubMed] [Google Scholar]
- 40.Park SH, Kang BK, Lee JE, Chun SW, Jang K, Kim YH, et al. Design and fabrication of a thin-walled free-form scaffold on the basis of medical image data and a 3D printed template: its potential use in bile duct regeneration. ACS Appl Mater Interfaces 2017; 9: 12290–8. doi: https://doi.org/10.1021/acsami.7b00849 [DOI] [PubMed] [Google Scholar]
- 41.Zopf DA, Mitsak AG, Flanagan CL, Wheeler M, Green GE, Hollister SJ. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg 2015; 152: 57–62. doi: https://doi.org/10.1177/0194599814552065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014; 32: 773–85. doi: https://doi.org/10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 43.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016; 113: 3179–84. doi: https://doi.org/10.1073/pnas.1521342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Organ Procurement and Transplantation Network. U.S. Department of health and human services. Data 2017. 2017. Available from: https://optn.transplant.hrsa.gov/data/
- 45.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 2016; 6: 34845. doi: https://doi.org/10.1038/srep34845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, et al. Medical 3D printing for the radiologist. Radiographics 2015; 35: 1965–88. doi: https://doi.org/10.1148/rg.2015140320 [DOI] [PMC free article] [PubMed] [Google Scholar]