Abstract
Objective:
Several dose metrics in the categories—homogeneity, coverage, conformity and gradient have been proposed in literature for evaluating treatment plan quality. In this study, we applied these metrics to characterize and identify the plan quality metrics that would merit plan quality assessment in lung stereotactic body radiation therapy (SBRT) dose distributions.
Methods:
Treatment plans of 90 lung SBRT patients, comprising 91 targets, treated in our institution were retrospectively reviewed. Dose calculations were performed using anisotropic analytical algorithm (AAA) with heterogeneity correction. A literature review on published plan quality metrics in the categories—coverage, homogeneity, conformity and gradient was performed. For each patient, using dose-volume histogram data, plan quality metric values were quantified and analysed.
Results:
For the study, the radiation therapy oncology group (RTOG) defined plan quality metrics were: coverage (0.90 ± 0.08); homogeneity (1.27 ± 0.07); conformity (1.03 ± 0.07) and gradient (4.40 ± 0.80). Geometric conformity strongly correlated with conformity index (p < 0.0001). Gradient measures strongly correlated with target volume (p < 0.0001). The RTOG lung SBRT protocol advocated conformity guidelines for prescribed dose in all categories were met in ≥94% of cases. The proportion of total lung volume receiving doses of 20 Gy and 5 Gy (V20 and V5) were mean 4.8% (±3.2) and 16.4% (±9.2), respectively.
Conclusion:
Based on our study analyses, we recommend the following metrics as appropriate surrogates for establishing SBRT lung plan quality guidelines—coverage % (ICRU 62), conformity (CN or CIPaddick) and gradient (R50%). Furthermore, we strongly recommend that RTOG lung SBRT protocols adopt either CN or CIPadddick in place of prescription isodose to target volume ratio for conformity index evaluation.
Advances in knowledge:
Our study metrics are valuable tools for establishing lung SBRT plan quality guidelines.
Introduction
Stereotactic body radiation therapy (SBRT) is an excellent treatment option for inoperable early-stage non-small cell lung cancer as well as for managing metastatic lung tumours.1–6 SBRT differs from conventional fractionated radiation therapy treatment in the delivery of large doses in a small number of fractions (typically SBRT total dose is delivered in 3 to 5 treatment fractions), which results in a higher biological effective dose. To limit and minimize normal tissue toxicity, conformation of high doses to the target and rapid dose fall-off outside the target is essential in SBRT. While conventional radiation therapy typically employs homogeneous dose distributions, in SBRT heterogeneous dose distributions are clinically desirable as long as the hot spots are located within the target and there is no spillage into normal tissue in order to achieve a steep dose-fall outside the treatment target akin to intracranial stereotactic radiosurgery (SRS).7
The quality of planned dose distributions in SBRT can be evaluated by characterizing dose distributions for target coverage, homogeneity, conformity and gradient parameters. Several metrics for evaluating treatment plan quality in conventional and stereotactic planned dose distributions have been proposed in the literature.8–13Relevant plan quality metrics along with their mathematical definitions, parameters needed to calculate each index and pertinent references are summarized in Table 1. For completeness, in Table 1 we have included all the frequently cited metrics for qualitative analyses of both conventional and stereotactic dose distribution, considering neither individual metric limitation nor suitability for lung SBRT plan quality assessment. We felt it would be interesting to apply these published plan quality indices in the above categories to describe and distinguish clinical lung SBRT dose distributions.
Table 1.
Mathematical definition of plan quality metrics studied
| Dosimetric index | Definition | Reference |
| Coverage | Quality of coverage = Imin/RI | RTOG8 |
| Coverage, % = (TVPIV/TV) × 100 | ICRU 628, 9 | |
| Homogeneity | HIRTOG = Imax/RI | RTOG8 |
| HIICRU= (D2%–D98%)/D50% | ICRU 8310 | |
| CIRTOG = PIV/TVCN = (TVRI/TV) × (TVRI/VRI) | RTOG8Van’t Riet et al11 | |
| CIPaddick = (TVPIV2/TV × PIV) | Paddick12, ICRU 9114 | |
| Conformity indices | CIgeometric (g) = LUF + HTOF | SALT8 |
| CGIc = 100 × TV/PIV | Wagner et al15 | |
| CI= (TVPIV/PIV) | Lomax and Scheib16 | |
| R50% = (PIV50%PIV)/PTV | RTOG 0915 | |
| Gradient | Gradient index = (PIV50%PIV /PIV) | Paddick and Lippitz17 |
| Gradient (cm) = (Reff,50%RX –Reff,RX) | Wagner et al15 | |
| Gradienteff = 50 %/(Reff,50%RX –Reff,RX) | Mayo et al18 |
CI, conformity index; CN, confirmation number; Dx%, minimal dose to the x% highest irradiated target volume; gradienteff, effective gradient. (Note: in our study, Prescription isodose = reference isodose = 100%); HI, homogeneity index; HTOF, healthy tissue overdosage factor (HTVRI/TV); HTVRI, healthy tissue volume covered by the reference isodose; Imax, maximum isodose in the target; Imin, minimal isodose surrounding the target; LUF, lesion underdosage factor (TV<RI/TV); PIV, prescription isodose volume; PTV, planning target volume; Reff, RX, Reff, 50%RX = effective radii of 100 and 50% isodoses (Reff); RI, reference isodose; TV<RI = target volume receiving less than reference dose; VRI, volume of reference isodose .
At present, clinical trials and protocols involving lung SBRT (such as RTOG 0915 protocol) specify plan quality and provide guidelines for treatment plan quality evaluation. However, the individual plan quality metrics being advocated in these protocols may not necessarily represent best of the published dose metrics in that evaluation category. In the published lung SBRT literature, reporting of plan quality metrics is varied and no universal standards on reporting plan quality metrics are currently followed. In an effort to standardize, ICRU report 91 proposed a set of recommendations on prescribing, recording and reporting of stereotactic treatments with small photon beams.14 However, routine use of these recommendations in SBRT literature remains to be seen.
To our knowledge, no publication exists quantifying coverage, homogeneity, conformity and dose gradient metric in lung SBRT dose distributions in a single study. Quantification of plan quality metrics is valuable as tools for establishing lung SBRT plan quality guidelines. The purpose of our study was to characterize lung SBRT target dose distributions by applying published plan quality indices, evaluate and identify which of these metrics are appropriate for lung SBRT plan quality assessment.
methods and materials
Treatment plans from 90 lung SBRT patients (comprising of 91 targets) treated at our institution since the inception of our institutional lung SBRT program in the year 2009 until 2015 were retrospectively reviewed. In each case, CT simulation was performed according to lung SBRT RTOG protocol guidelines. All patients were simulated in a supine position and patients were immobilized using BodyFix™ immobilization system (Elekta Medical Intelligence, Stockholm, Sweden). At simulation, two CT-image scans were obtained; a free breathing CT-scan and a 4-dimensional (4-D) CT-scan on a GE light-speed CT scanner with respiration phase inferred using an infrared marker and camera system [Respiratory Position Management (RPM) system, Varian Oncology, Palo Alto, CA]. Thus acquired CT-image data set comprised of images in 10 phases corresponding to equally spaced phases in the respiratory cycle. In cases, where the phase binning error was >10% but ≤20%, the CT-image data set was divided into 5 phases corresponding to equally spaced phases in the respiratory cycle The 4-D CT-scan was used to assess gross tumour volume (GTV) motion and generate internal target volume (ITV). A maximum intensity projection data set was used for ITV generation and dose was calculated on the Ave-IP (average intensity projection) data set of the 4D-CT.19,20 Additional margins to ITV for planning target volume (PTV) generation isotropically ranged from 5 to 10 mm.
For each patient, volumetric modulated arc therapy (VMAT) treatment planning was utilized. Dose optimizations were accomplished with 6 MV photons using 2 to 5 coplanar and non-coplanar partial arcs [number of partial arcs utilized: 2 (33%); 3 (53%); 4 (8%) and 5 (3%)]. Dose was prescribed to 100% isodose and generally, the prescription dose encompassed ≥95% of the PTV, largely depending on the location and proximity to critical organs. All efforts were made to constrain organ-at-risk and normal tissue doses using SBRT protocol guidelines [as outlined in RTOG protocol 0915 (radiation therapy oncology group)] based on expected normal tissue complications. Treatments were delivered on Trilogy and Truebeam STX linear accelerators, equipped with HD multileaf collimation (MLC). Patient setup and target localization was achieved using pre-treatment image guidance via cone-beam CT and kilo voltage imaging. All dose calculations were performed using the Eclipse planning system AAA (anisotropic analytical algorithm) dose calculation model (Varian Oncology, Palo Alto, CA), with heterogeneity correction and a dose calculation grid size of 1.25 mm2.
Characteristics of patients included in this study along with tumour location, target volume (GTV and PTV), dose fractionation and treatment technique applied are summarized in Table 2. At our institution, we typically prescribe 10 to 11 Gy × 5 for centrally located tumours and 18 to 20 Gy × 3 for non-central tumours. For each patient, using dose-volume histogram data from treatment plan, the necessary input values for calculating various indices in Table 1 were extracted. Data were analysed using descriptive statistics. Normally distributed variables are presented with mean and standard deviation values, whereas non-normal variables are given as median and range. A two-tailed probability t-test (Wilcoxon signed-rank test) was used to examine the data and a p-value of <0.05 was considered statistically significant.
Table 2.
Summary of study patient characteristics
| Number | 90 (91 targets) |
| Sex | |
| Male | 38 |
| Female | 52 |
| Age median | 75 (50–92) |
| Stage | T1-T4 |
| Tumour location | |
| RUL | 27 |
| RML | 6 |
| RLL | 15 |
| LUL | 33 |
| LLL | 9 |
| LH | 1 |
| Target volume | |
| GTV | Median 5.5 cm3 (0.2–133.3) |
| PTV | Median 31.8 cm3 (5.1–252.5) |
| Dose/fractionation | |
| 20 Gy × 3 | 8 |
| 18 Gy × 3 | 31 |
| 10 Gy × 5 | 49 |
| Miscellaneous | 3 |
| Treatment technique | VMAT |
GTV, gross tumour volume; LH, left hilum; LLL, left lower lobe; LUL, left upper lobe; PTV, planning target volume; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; VMAT, volumetric modulated arc therapy.
Results
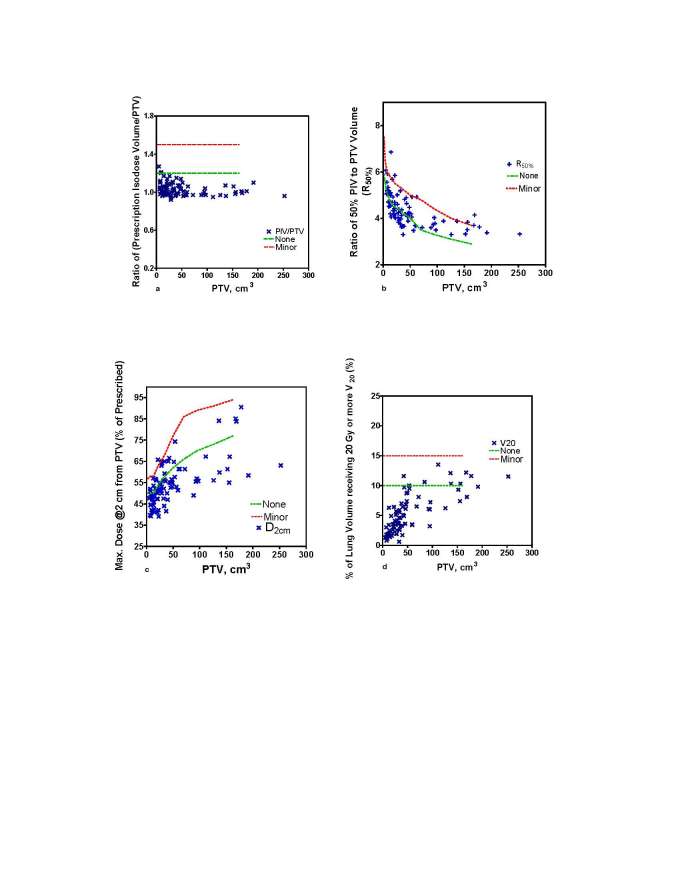
The plan quality metrics calculated for the patient population in this study are summarized in Table 3. For the study, the RTOG-defined plan quality metrics were: coverage (0.90 ± 0.08); homogeneity (1.27 ± 0.07); conformity (1.03 ± 0.07) and gradient (4.40 ± 0.80). The RTOG lung SBRT protocol advocated conformity guidelines for prescribed dose in all dosimetric evaluation categories were met in ≥94% of cases (Figure 1a–d).
Table 3.
Mean (±SD) values for the various plan quality metrics evaluated in this study
| Dosimetric index | Definition | Mean | SD | 95% CI | Correlation with PTV (p-value) | |
| Lower | Upper | |||||
| Coverage | Quality of coverage | 0.90 | 0.08 | 0.88 | 0.91 | <0.0001 |
| Coverage, % | 96.7 | 1.92 | 95.90 | 96.90 | 0.09 | |
| Homogeneity | HIRTOG | 1.27 | 0.07 | 1.26 | 1.29 | 0.02 |
| HIICRU83 | 0.21 | 0.06 | 0.20 | 0.23 | 0.08 | |
| Conformity indices | CIRTOG | 1.03 | 0.07 | 1.02 | 1.05 | 0.06 |
| CIPaddick | 0.91 | 0.04 | 0.89 | 0.93 | 0.02 | |
| CIgeometric (g) | 0.11 | 0.06 | 0.10 | 0.13 | 0.0003 | |
| CN | 0.90 | 0.06 | 0.89 | 0.91 | 0.03 | |
| CGIc | 97.4 | 5.90 | 95.5 | 98.2 | 0.05 | |
| CILomax | 0.94 | 0.05 | 0.92 | 0.94 | 0.06 | |
| Gradient | R50% (RTOG) | 4.40 | 0.70 | 4.27 | 4.58 | <0.0001 |
| Gradient index | 4.20 | 0.60 | 4.14 | 4.37 | <0.0001 | |
| Gradient (cm) | 1.20 | 0.30 | 1.21 | 1.33 | <0.0001 | |
| Gradienteff (% cm−1) | 42.3 | 9.40 | 39.60 | 43.18 | <0.0001 | |
| Low dose Spillage (%) | D2 cm (%) | 54.90 | 10.70 | 54.00 | 58.50 | <0.0001 |
| High dose spillage (%) | (V105% PD - PTV)/PTV | 0.90 | 1.70 | 0.61 | 1.27 | 0.30 |
| Total lung dose (%) | V20 Gy | 4.80 | 3.20 | 4.34 | 5.58 | <0.0001 |
| V5 Gy | 16.40 | 9.20 | 15.10 | 18.67 | <0.0001 | |
CI, conformity index; CN, confirmation number; HI, homogeneity index; PTV, planning target volume; RTOG, radiation therapy oncology group.
Figure 1.
Evaluation of conformality of prescribed dose per RTOG protocol guidelines: (a) PITV- ratio; (b) R50% ratio; (c) D2 cm (Gy); and (d) lung V20 (%). Dashed lines represent RTOG protocol limits for no violation and minor violation in the plan quality metric. PITV, prescription isodose to target volume; RTOG, radiation therapy oncology group.
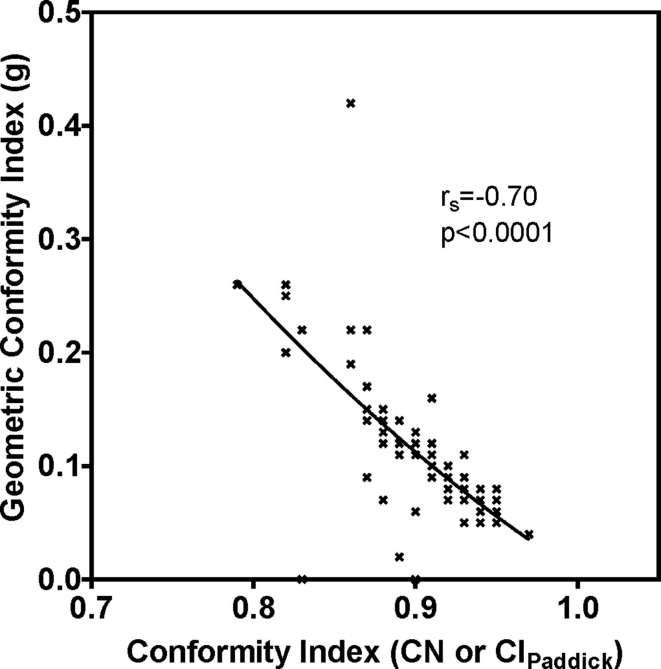
Geometric conformity index (g) (Table 1) correlated with conformity index (CN or CIPadddick) (p < 0.0001, Spearman Correlation = −0.7) (Figure 2). Gradient measures strongly correlated with PTV (p < 0.0001) (Figure 3a and b). Evaluating high dose spillage, the average cumulative volume of all tissue outside the PTV receiving a dose of >105% of prescription dose was 0.90 (±1.70) %. Considering low dose spillage, the maximum % of prescription dose to any point at 2 cm distance in any direction from PTV was 54.9 (±10.7) %. The proportion of lung volume (total lung volume—GTV) receiving doses of 20 Gy and 5 Gy (V20 and V5) were mean 4.8% (±3.2) and 16.4% (±9.2), respectively.
Figure 2.
Geometric conformity (g) vs conformity index (CN or CIPaddick).
Figure 3.
Gradient indices vs PTV: (a) gradient (cm); (b) gradientEff (% cm−1). PTV, planning target volume.
Published plan quality metrics in lung SBRT studies are summarized and presented in Table 4 for comparison along with our study data.
Table 4.
Comparison of our study data with literature published indices
| Dosimetric index | Definition | Our study(n = 91) | Kannarunimit et al21 (n = 9) | Rausche- bach et al22 (n = 25) | Weyh et al23 (n = 8) | Ding et al24 (n = 8) | Rana et al25 (n = 14) | Dickey et al26(n = 23) |
| PTV, cm3 | Range | 5.1–252.5 | 36.2 ± 18.1 | 12.65–190.7 | 7.63–83.43 | 14.6–145.4 | 3.2–43.0 | 16.0–67.0 |
| Coverage | Coverage, % | 96.7 ± 1.92 | 96.0 ± 1.0 | |||||
| Homogeneity | HIRTOG | 1.27 ± 0.07 | 1.19 ± 0.02 | |||||
| Conformity indices | CIRTOG | 1.03 ± 0.07 | 1.11 ± 0.05 | 0.99 ± 0.02 | 1.29 ± 0.02 | 1.64 ± 0.29 | 1.18 ± 0.09 | 1.03 ± 0.03 |
| CN | 0.90 ± 0.06 | 0.84 ± 0.03 | 0.88 ± 0.02 | |||||
| Gradient | R50% (RTOG) | 4.40 ± 0.70 | 4.52 ± 0.46 | 3.57 ± 0.32 | 6.53 ± 1.2 | 4.90 ± 0.56 | 4.76 ± 0.36 | |
| Low dose spillage (%) | D2 cm (%) | 54.90 ± 10.7 | 28.52 ± 2.2 | 52.0 ± 6.0 | 67.34 ± 0.02 | 50.09 ± 6.26 | 54.0 ± 3.4 | |
| High dose spillage (%) | (V105%PD–PTV)/PTV | 0.90 ± 1.70 | 0.68 ± 0.01 | |||||
| Total lung dose (%) | V20 Gy | 4.80 ± 3.20 | 4.66 ± 1.26 | 3.77 ± 2.9 | 6.32 ± 3.9 | 5.3 ± 3.6 | 5.96 ± 2.77 | |
| V5 Gy | 16.40 ± 9.20 | 23.20 ± 7.21 |
CI, conformity index; CN, confirmation number; HI, homogeneity index; PTV, planning target volume; RTOG, radiation therapy oncology group.
Discussion
For the patient population in this study, we observed that the RTOG lung SBRT protocol advocated conformity guidelines for prescribed dose in all dosimetric evaluation categories were met in ≥94% of cases. In ≤5 instances respectively, of a total 91 dose distributions analysed, the R50% and D2 cm dosimetric criteria were outside the RTOG compliancy criteria [classified in RTOG protocol as major violation category; none and minor protocol violation limits are shown as dashed lines in (Figure 1a–d)]; however the PITV ratio [prescription isodose volume (PIV) to target volume (TV) ratio] and total lung V20 criteria were satisfied in all 91 treatment plans included in our study.
RTOG defined quality of coverage dosimetric index (QCI) correlated with target volume (p < 0.0001; Table 3). In the RTOG definition,8 this metric is based on the minimum dose in the target. However, the ICRU 62 definition of the coverage index8 is based on the proportion of target volume receiving the prescription dose. The ICRU 62 coverage index (%) did not correlate with PTV (p = 0.09, Table 3) indicating that this target coverage parameter is influenced predominantly by the location of the target and its vicinity to organs-at-risk. A planning philosophy in which highest priority is placed on treating the whole target volume to the prescription dose (such as in conformal radiation therapy) is considered less appropriate in SRS/SBRT treatment planning, wherein it is acceptable to treat a small volume of target adjacent to a critical structure to less than prescription dose in order to respect and meet organ-at-risk dose constraints.15 For reasons of differences in treatment planning philosophies between conventional and SBRT treatments and because ICRU 62 CI has no dependency on target volume compared to dependency of RTOG QCI parameter on target volume, we believe however that the ICRU 62 CI is a more appropriate dosimetric index for target coverage metric in SBRT treatment planning.
RTOG-defined homogeneity index (HI) metric correlated with target volume (p = 0.02) but showed no significant correlation between ICRU 83 defined HI and target volume (p = 0.08). RTOG defines HI as a ratio of maximum dose to prescription dose, with the maximum dose referenced to point dosimetry.8–10 This definition of HI has been adopted in some SRS studies, but this metric while providing the highest target dose lacks information on cold spots within the target volume. Furthermore, point dosimetry is subject to dose uncertainty and is also sensitive to the resolution of the dose calculation grid. In the ICRU 83 definition,10 however, HI is based on the difference between near-maximum (D2%) and near-minimum (D98%) volumetric dose within the target volume that is normalized to target median dose (D50%) and therefore, appears to be a better-suited metric for reporting in SBRT. In our study, the mean HIICRU 83 value was 0.21 (±0.06). A HI value of 0 corresponds to a perfectly homogenous dose distribution within the target.10 While some SRS related studies27, 28 have reported that a lower HI is associated with a higher gradient index (cf. Table 1 for its definition), no recommendation for optimal value for degree of target dose heterogeneity from planning and toxicity perspective appears to have been made in SBRT literature.7 Therefore, at present HI parameter appears to have limited usefulness in SBRT lung planning optimization process.
The RTOG lung SBRT protocol defines conformity index (CI) as the PITV-ratio.8 Treatment plans with PITV-ratios <1.2 are classified as ideal and with values >1.2 but <1.5 are categorized as a minor deviation from lung SBRT protocol dose conformancy guidelines. In our study, the mean value of the RTOG defined CI was 1.03 (±0.07) (Table 3). No association was observed in our data between RTOG defined CI and PTV (p = 0.06), indicating that the prescription isodose volume parameter is influenced by the location of the target and its vicinity to organs-at-risk. However, in the RTOG defined CI, the geometric overlap of the volume receiving the prescription isodose and the target volume is not considered. So an ideal index value of 1 could be obtained even if the PIV does not geometrically coincide with PTV. Unlike the PITV-ratio, the indices proposed by van’t Riet et al (CN)11 and Paddick (CIPadddick)12 take into account the location and shape of the prescription volume by writing the index as a multiplication of target volume under-dosing (TVPIV/TV) and over-dosing (TVPIV/PIV) ratios. Indices CN and CIPadddick correlated with PTV (p = 0.02). CIPadddick did not correlate with CIRTOG (R2 = 0.02). Furthermore, we strongly recommend that RTOG lung SBRT protocols adopt either CN or CIPadddick in place of PITV ratio for conformity index evaluation.
Conformity index approaches a perfect value of 1 as the geometric conformity (g) value approaches 0 (Figure 2). The value of g is a combination of lesion under dose (LUD) and healthy tissue overdose (HTOD) factors (Table 1).8 Therefore, this index will have an ideal value of 0 when the prescription isodose is perfectly sculpted to the shape of the target and there is no spillage into healthy tissue. The mean (±SD) values of LUD and HTOD in our study were 0.03 (±0.02) and 0.07 (±0.07) respectively and the overall g value was 0.11 (±0.06). HTOD correlated with TV (p = 0.005) whereas, LUD did not correlate with TV (p = 0.19) suggesting once again that location of target in relation to organs-at-risk plays a major role in achieving the target dose coverage.
Conformity index CGIc (Table 1), is a scaled version of inverse PITV, which amplifies small differences in PITV such that CGIc = 100 corresponds to perfect conformity of the prescription isodose surface to the target.15 Conformity Indices CGIc and RTOG are therefore, inversely related. In our study, the average value of CGIc was 97.4 (±5.9) (Table 3). The index, CILomax,16 is based on the proportion of the target volume receiving at least the prescription dose and the index value can range from 0 to an ideal value of 1 when the target volume in its entirety is covered by at least the prescription dose. The average value of this index in our study was 0.93 (±0.06). The indices, CIRTOG, CIPaddick and CILomax are related as follows CIRTOG = (CIPaddick or CN)/(CILomax)2. Index CGIc, similar to the RTOG defined CI, does not consider the geometric overlap of the volume receiving the prescription isodose and the target volume and in our opinion, therefore, is not appropriate for SBRT lung plan dose conformity evaluation. On the other hand, index CILomax considers only target over-dosing and does not consider target under-dosing component and hence is an inferior metric compared to CN or CIPaddick.
In our study, gradient measures correlated strongly with target volume (p < 0.0001, Table 3). The absolute distance for the dose gradient (difference in effective radii between 50 and 100% isodose volumes) increased as a function of the target volume with the trend described well by a logarithmic fit. At smaller target volumes, there appears to be a linear and steeper relationship with gradient measures (target volumes <50 cm3) and then a plateau type relationship in gradient measures at large target volumes (Figure 3a,b). For our patient population, the mean values of gradient were (1.20 ± 0.30 cm) and (42.3 ± 9.4 % cm–1) respectively. In the RTOG definition of gradient (R50%) (low dose spillage volume), the ratio of 50% of the prescription dose isodose volume to the PTV is considered whereas in the gradient index proposed by Paddick and Lippitz17 the ratio of 50% of the prescription dose isodose volume to the PIV is considered. RTOG Gradient R50% and GIPaddick and Lippitz are related as follows RTOG R50% = CIRTOG × GIPaddick and Lippitz. Therefore, these two indices have similar magnitude and vary by the degree of difference between PTV and PIV (Table 3). Because the RTOG R50% takes into consideration the degree of dose conformity in addition to target volume dependency of dose fall-off, we feel this index is more preferable for gradient evaluation in lung SBRT. Gradient (cm) and Gradienteff indices are applied more widely in SRS treatment plan evaluation wherein the dose-fall of is much sharper compared to SBRT treatment plans.
Our study average values for high dose spillage outside the PTV [0.90% (±1.70)] and the percent of total lung volume receiving 20 Gy [4.8% (±3.2)] were well below RTOG protocol limits of <15% of the PTV and <10% of the total lung volume respectively. In a recent study, Faught et al29 , reported that the probabilities of Grade 3 + radiation pneumonitis of 20 and 10% correspond to V20 Gy correspond to functional lung sub—volumes of 26.8 and 9.3% respectively.
As previously stated, to our knowledge there are no published studies that have focused exclusively on applying various dosimetric indices for characterizing dose coverage, homogeneity, conformity and dose gradient in clinical lung SBRT dose distributions. In summarizing the available literature data on plan quality metrics in lung SBRT (Table 4), we have extracted indices pertaining to VMAT from the published data as all of our study patients were planned using VMAT technique.21, 22, 30 It is evident from Table 4, that the dosimetric indices in our study are in the range and comparable to literature data and also that our study data size (90 patients comprising 91 targets, Table 3) far exceeds the data currently available in the literature.
All our dose computations during treatment planning are based on AAA dose calculation algorithm with heterogeneity correction, which is in compliance with RTOG protocol requirements. Recently, some studies have focused on the linearized Boltzmann transport equation based Acuros XB (AXB) algorithm for dose calculation accuracy in heterogeneous media such as in lung tumours.24, 28, 30 Routinely adapting to AXB for dose calculations in lung SBRT planning may improve dose calculation accuracy, as AXB-based calculations have been shown to be closer to Monte Carlo based dose predictions in accuracy and with relatively faster computational time. Since AAA dose calculation algorithm is still being widely used in clinics for dose computations and the published lung SBRT plan metric data are using AAA calculations, we have not adapted AXB algorithm for dose calculation in this study.
As it was beyond the objectives of this study, we have not presented critical organ dose-volume data (other than for total lung), however, organ-at-risk and normal tissue doses were constrained in accordance with RTOG lung SBRT protocol guidelines. In a preliminary outcome data based on the patient population in this study, we reported excellent local control (92% at 2 years).25 We are working towards a long-term follow-up clinical study on reporting our outcome and toxicity data and possibly correlating our study plan quality metrics.
In summary, plan quality metrics are added valuable tools for grading and ranking plan quality in lung SBRT that is often judged on the basis of clinical expertise, acceptable dose distributions and dose gradients, while respecting critical organ and normal structure doses. Based on our study data and analyses, we recommend the use of the following dosimetric indices as surrogates for establishing plan quality metrics in lung SBRT—coverage % (ICRU 62), conformity (CN or CIPaddick) and gradient (R50%, RTOG).
Contributor Information
Ravindra Yaparpalvi, Email: ryaparpa@montefiore.org.
Madhur K Garg, Email: mgarg@montefiore.org.
Jin Shen, Email: jshen@montefiore.org.
William R Bodner, Email: wbodner@montefiore.org.
Dinesh K Mynampati, Email: dmynampa@montefiore.org.
Aleiya Gafar, Email: agafar@montefiore.org.
Hsiang-Chi Kuo, Email: hskuo@montefiore.org.
Amar K Basavatia, Email: abasavat@montefiore.org.
Nitin Ohri, Email: nohri@montefiore.org.
Linda X Hong, Email: lhong@mskcc.org.
Shalom Kalnicki, Email: skalnick@montefiore.org.
Wolfgang A Tome, Email: wtome@montefiore.org.
REFERENCES
- 1.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014; 32: 2847–54. doi: https://doi.org/10.1200/JCO.2014.55.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: https://doi.org/10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010; 94: 1–11. doi: https://doi.org/10.1016/j.radonc.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2(7 Suppl 3): S94–S100. doi: https://doi.org/10.1097/JTO.0b013e318074de34 [DOI] [PubMed] [Google Scholar]
- 5.Ricardi U, Filippi AR, Guarneri A, Ragona R, Mantovani C, Giglioli F, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012; 75: 77–81. doi: https://doi.org/10.1016/j.lungcan.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 6.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81: 1352–8. doi: https://doi.org/10.1016/j.ijrobp.2009.07.1751 [DOI] [PubMed] [Google Scholar]
- 7.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys 2010; 37: 4078–101. doi: https://doi.org/10.1118/1.3438081 [DOI] [PubMed] [Google Scholar]
- 8.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006; 64: 333–42. doi: https://doi.org/10.1016/j.ijrobp.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 9.International Commission on Radiation Units and Measurements. ICRU 62. Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50) Report No: 62 Washington DC: 1999. [Google Scholar]
- 10.International Commission on Radiation Units and Measurements. ICRU 83. Prescribing, recording and reporting photon beam intensity-Modulated Radiation therapy (IMRT). Report No: 83 Washington DC: 2010. [Google Scholar]
- 11.van’t Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997; 37: 731–6. doi: https://doi.org/10.1016/S0360-3016(96)00601-3 [DOI] [PubMed] [Google Scholar]
- 12.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 2000; 93(Suppl 3): 219–22. doi: https://doi.org/10.3171/jns.2000.93.supplement [DOI] [PubMed] [Google Scholar]
- 13.Hong LX, Shankar V, Shen J, Kuo HC, Mynampati D, Yaparpalvi R, et al. Spine stereotactic body radiation therapy plans: achieving dose coverage, conformity, and dose falloff. Med Dosim 2015; 40: 181–5. doi: https://doi.org/10.1016/j.meddos.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Radiation Units and Measurements ICRU Report 91. Prescribing, recording and reporting of stereotactic treatments with small photon beams. Journal of the ICRU 2014; 14: 1–160. [DOI] [PubMed] [Google Scholar]
- 15.Wagner TH, Bova FJ, Friedman WA, Buatti JM, Bouchet LG, Meeks SL. A simple and reliable index for scoring rival stereotactic radiosurgery plans. Int J Radiat Oncol Biol Phys 2003; 57: 1141–9. doi: https://doi.org/10.1016/S0360-3016(03)01563-3 [DOI] [PubMed] [Google Scholar]
- 16.Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003; 55: 1409–19. doi: https://doi.org/10.1016/S0360-3016(02)04599-6 [DOI] [PubMed] [Google Scholar]
- 17.Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg 2006; 105: 194–201. doi: https://doi.org/10.3171/sup.2006.105.7.194 [DOI] [PubMed] [Google Scholar]
- 18.Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald TJ, Moser R. Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010; 78: 1457–66. doi: https://doi.org/10.1016/j.ijrobp.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Ehler ED, Tomé WA. Lung 4D-IMRT treatment planning: an evaluation of three methods applied to four-dimensional data sets. Radiother Oncol 2008; 88: 319–25. doi: https://doi.org/10.1016/j.radonc.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 20.Ehler ED, Bzdusek K, Tomé WA. A method to automate the segmentation of the GTV and ITV for lung tumors. Med Dosim 2009; 34: 145–53. doi: https://doi.org/10.1016/j.meddos.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 21.Ojala JJ, Kapanen MK, Hyödynmaa SJ, Wigren TK, Pitkänen MA. Performance of dose calculation algorithms from three generations in lung SBRT: comparison with full Monte Carlo-based dose distributions. J Appl Clin Med Phys 2014; 15: 4–18. doi: https://doi.org/10.1120/jacmp.v15i2.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannarunimit D, Descovich M, Garcia A, Chen J, Weinberg V, Mcguinness C, et al. Analysis of dose distribution and risk of pneumonitis in stereotactic body radiation therapy for centrally located lung tumors: a comparison of robotic radiosurgery, helical tomotherapy and volumetric modulated arc therapy. Technol Cancer Res Treat 2015; 14: 49–60. doi: https://doi.org/10.7785/tcrt.2012.500394 [DOI] [PubMed] [Google Scholar]
- 23.Rauschenbach BM, Mackowiak L, Malhotra HK. A dosimetric comparison of three-dimensional conformal radiotherapy, volumetric-modulated arc therapy, and dynamic conformal arc therapy in the treatment of non-small cell lung cancer using stereotactic body radiotherapy. J Appl Clin Med Phys 2014; 15: 147–61. doi: https://doi.org/10.1120/jacmp.v15i5.4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weyh A, Konski A, Nalichowski A, Maier J, Lack D. Lung SBRT: dosimetric and delivery comparison of rapidArc, tomotherapy, and IMRT. J Appl Clin Med Phys 2013; 14: 3–13. doi: https://doi.org/10.1120/jacmp.v14i4.4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong LX, Garg M, Lasala P, Kim M, Mah D, Chen CC, et al. Experience of micromultileaf collimator linear accelerator based single fraction stereotactic radiosurgery: tumor dose inhomogeneity, conformity, and dose fall off. Med Phys 2011; 38: 1239–47. doi: https://doi.org/10.1118/1.3549764 [DOI] [PubMed] [Google Scholar]
- 26.Balagamwala EH, Suh JH, Barnett GH, Khan MK, Neyman G, Cai RS, et al. The importance of the conformality, heterogeneity, and gradient indices in evaluating gamma knife radiosurgery treatment plans for intracranial meningiomas. Int J Radiat Oncol Biol Phys 2012; 83: 1406–13. doi: https://doi.org/10.1016/j.ijrobp.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 27.Faught AM, Yamamoto T, Castillo R, Castillo E, Zhang J, Miften M, et al. Evaluating which dose-function metrics are most critical for functional-guided radiation therapy. Int J Radiat Oncol Biol Phys 2017; 99: 202–9. doi: https://doi.org/10.1016/j.ijrobp.2017.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding C, Chang CH, Haslam J, Timmerman R, Solberg T. A dosimetric comparison of stereotactic body radiation therapy techniques for lung cancer: robotic versus conventional linac-based systems. J Appl Clin Med Phys 2010; 11: 212–24. doi: https://doi.org/10. 1120/jacmp.v11i3.3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana S, Rogers K, Pokharel S, Cheng C. Evaluation of Acuros XB algorithm based on RTOG 0813 dosimetric criteria for SBRT lung treatment with RapidArc. J Appl Clin Med Phys 2014; 15: 118–29. doi: https://doi.org/10.1120/jacmp.v15i1.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickey M, Roa W, Drodge S, Ghosh S, Murray B, Scrimger R, et al. A planning comparison of 3-dimensional conformal multiple static field, conformal arc, and volumetric modulated arc therapy for the delivery of stereotactic body radiotherapy for early stage lung cancer. Med Dosim 2015; 40: 347–51. doi: https://doi.org/10.1016/j.meddos.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 31.Kroon PS, Hol S, Essers M. Dosimetric accuracy and clinical quality of Acuros XB and AAA dose calculation algorithm for stereotactic and conventional lung volumetric modulated arc therapy plans. Radiat Oncol 2013; 8: 149–57. doi: https://doi.org/10.1186/1748-717X-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A, Ohri N, Purswani J, Yaparpalvi R, Bodner WR, Garg MK, et al. Local control after stereotactic body radiation therapy for early-stage non-small cell lung cancer: a tale of 2 schedules? Int J Radiat Oncol Biol Phys 2016; 96: E483: E483–E484. doi: https://doi.org/10.1016/j.ijrobp.2016.06.1843 [Google Scholar]