Abstract
Background
Animal studies have demonstrated poor cognitive outcomes in offspring in relation to maternal vitamin D deficiency before and/or during pregnancy. Human studies linking maternal vitamin D status during pregnancy with offspring cognitive function are limited. We aimed to test the hypothesis that lower maternal vitamin D status during pregnancy is associated with poor offspring cognitive ability in an Indian population.
Methods
Cognitive function was assessed in children from the Mysore Parthenon birth cohort during childhood (age 9-10 years; n=468) and adolescence (age 13-14 years; n=472) using 3 core tests from the Kaufman Assessment Battery for children and additional tests measuring learning, long-term retrieval/storage, short-term memory, reasoning, verbal fluency, visuo-spatial ability, and attention and concentration. Maternal serum 25-hydroxyvitamin D concentration was measured at 30±2 weeks of gestation.
Results
During pregnancy 320 (68%) women had ‘vitamin D deficiency’ (serum 25-hydroxyvitamin D concentration <50 nmol/L). Girls scored better than boys in tests of short-term memory, reasoning, verbal fluency, and attention (p<0.05 for all). Maternal vitamin D status (low as well as across the entire range) was unrelated to offspring cognitive function at both ages, either unadjusted or after adjustment for the child’s current age, sex, maternal age, parity, season at the time of blood sampling, gestational age, the child’s birth and current size, socio-economic status, parents’ education, maternal intelligence and home environment.
Conclusions
In this population, despite a high prevalence of vitamin D deficiency during pregnancy, there was no evidence of an association between maternal vitamin D status and offspring cognitive function.
Keywords: Maternal Vitamin D, Pregnancy, Cognitive function, Children, India
Introduction
Vitamin D is an important micronutrient essential for bone growth and regulation of calcium homeostasis.1 Apart from its vital role in skeletal growth, vitamin D has a number of biological actions fundamental to neurodevelopment and function, including a signalling role in cell differentiation and synaptic formation,2 gene expression,2 regulation of the metabolism of neurotrophic and neurotoxic factors3 and a protective role during brain inflammation.4 The main source of vitamin D is sunlight; it is also obtained from a few foods such as oily fish and fortified margarines.5 Vitamin D deficiency is a public health problem across the globe.6 Despite abundant sunshine, there is a high prevalence of vitamin D deficiency in Indians, including pregnant women.7,8 The vitamin D supply to the growing fetus depends on maternal vitamin D status.9 Therefore maternal vitamin D deficiency during pregnancy might lead to adverse health outcomes in the offspring.10 Some studies have observed fetal growth restriction,11 reduced bone size and bone mineral content12 and recurrent wheeze13 in the offspring of mothers with vitamin D deficiency.
Interest in the relationship of maternal vitamin D status during pregnancy to offspring cognitive function is recent, and literature is limited. Animal studies have demonstrated poor learning and memory, and alterations in attention, in association with vitamin D deficiency before conception and/or during pregnancy.14,15 In humans, only five studies, all from developed populations, have examined the relationship between maternal vitamin D status and offspring cognitive function.16–20 The findings are inconsistent. Two studies, one in Spain and another in Australia, observed poor cognitive outcomes in children of deficient mothers.16,17 A study in the UK and another in Denmark found no association.18,19 The fifth study in the USA, observed an association in young children that was no longer evident when the children were older.20
In the Mysore Parthenon Study in south India, we have measured maternal serum 25-hydroxyvitamin D concentration in pregnancy using stored serum samples; more than 60% of the women had vitamin D deficiency at 30±2 weeks gestation.21 Cognitive function in the offspring was assessed during childhood and adolescence. Using these data, we aim to test the hypothesis that lower maternal vitamin D status and/or vitamin D deficiency are associated with poorer offspring cognitive ability, independent of socio-demographic factors.
Materials and Methods
Study population
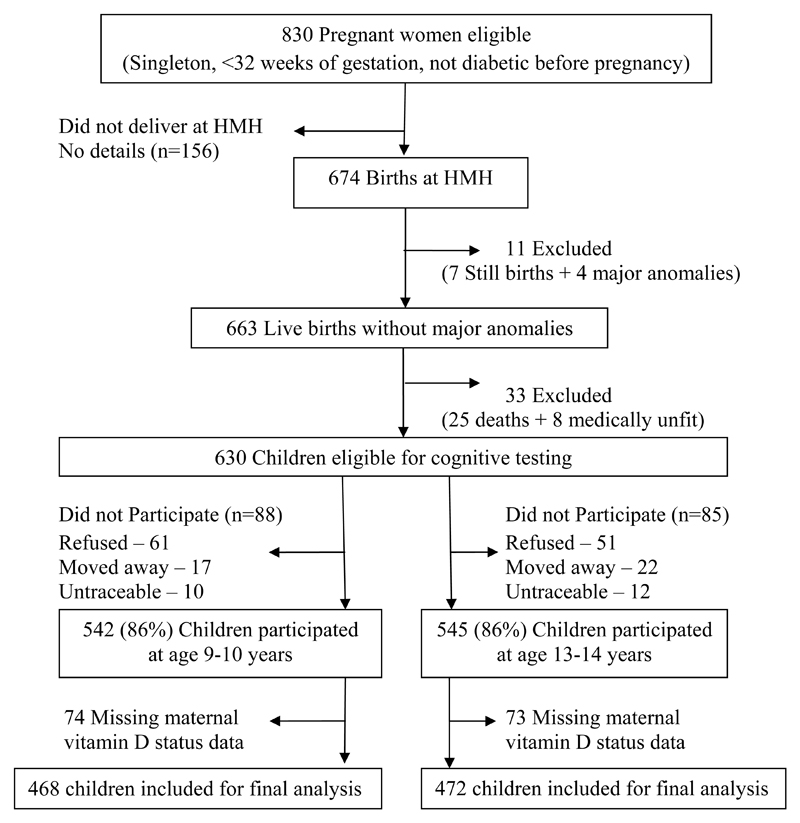
The Mysore Parthenon birth cohort was initiated in 1997-1998.22 Eight hundred and thirty women booking consecutively into the antenatal clinic at the Holdsworth Memorial Hospital (HMH), Mysore, India and satisfying the eligibility criteria (no history of diabetes before pregnancy, planning to deliver at HMH, and having a singleton pregnancy of <32 weeks gestation) participated in the study. Six hundred and seventy four women (81% of the participants) delivered their babies at HMH. Excluding 7 stillborn babies, and 4 with major congenital anomalies, detailed newborn anthropometry was performed on 663 normal live born babies according to a standard protocol, within 72 hours of birth, as reported previously.23 Excluding 25 children who died, and 8 with major medical problems, 630 healthy children were followed up with repeat anthropometry, annually till the age of 5 years and every 6 months thereafter.
Maternal 25-hydroxyvitamin D concentration
Maternal serum 25-hydroxyvitamin D concentration was measured in stored samples (frozen at –80°C), using radioimmunoassay (IDS Immunodiagnostics Ltd, Boldon, Tyne and Wear, UK) standardized against Nichols and Incstar methodology. Each assay complied with international DEQAS (vitamin D external quality assurance scheme) requirements.24 Intra- and inter-assay coefficients of variations were 8.8% and 10.8%, respectively. Low vitamin D status was defined as concentrations <50 nmol/L.8,25 Of 663 mothers who delivered at HMH, adequate samples were available for 568 mothers (86%).
Vitamin D and calcium supplementation
General practitioners and obstetricians routinely prescribe multivitamin supplements to pregnant women. Data on supplement use was collected at recruitment (<32 weeks of gestation) but not subsequently, and therefore no information is available on their use when blood samples were collected or at term.
Study sample for cognitive function assessment
Children were invited for assessment of their cognitive function during childhood (age 9-10 years) and adolescence (age 13-14 years). Of the 630 children, 88 were excluded (61 unwilling, 17 moved away from Mysore and 10 untraceable), and 542 (86%) underwent cognitive testing during childhood. During adolescence, 85 were excluded (51 unwilling, 22 moved away and 12 untraceable), and 545 (86%) participated in cognitive function assessment. Among the participants 74 children and 73 adolescents were excluded because maternal 25-hydroxyvitamin D concentrations were unavailable. The current analysis is restricted to 468 children (228 boys and 240 girls) and 472 adolescents (226 boys and 246 girls) (Figure 1).
Figure 1. Flow chart of the study participants.
Tests of cognitive function
These comprised a series of neuropsychological tests applicable for use in school aged children and related to specific cognitive domains (memory, attention, fluid reasoning) consistent with the Carroll model.26 They included three core tests from the Kaufman Assessment Battery for Children27 and additional tests28–31 that underwent extensive adaptation to the local cultural context and validation.32,33 The tests (Table 1) covered the domains of learning, long-term memory and retrieval ability (Atlantis), short-term memory (Word order), reasoning ability (Pattern reasoning), language production (Verbal fluency), visuo-spatial ability (Kohs’ block design) and visuo-motor processing speed and coordination, attention and concentration (coding-Wechsler Intelligence Scale for Children-III). The tests were administered in a single session of 60 to 90 minutes in a quiet room by one of 2 trained masters’ level child psychologists (unaware of maternal vitamin D status) in the local Kannada language.
Table 1. Description of the cognitive tests used in the study.
| Tests from KABC-II† | ||
|---|---|---|
| Name of the test | Description | Cognitive abilities |
| Atlantis | The child is taught nonsense names for fish, plants and shells and is asked to point to the named object among an array of pictures | Learning ability/long-term storage and retrieval, associative memory |
| Word order | The child points to a series of silhouettes of common objects in the same order as mentioned by the examiner; an interference task (color naming) is added between the stimulus and the response for the more difficult items | Memory span, short term memory, working memory |
| Pattern Reasoning | The child completes a pattern by selecting the correct image from a set of 4 to 6 options shown; most stimuli are abstract, geometric shapes and the difficulty of the task increases as the test progresses. | Reasoning abilities such as induction and deduction and fluid reasoning |
| Additional tests | ||
| Verbal fluency | The child is asked to name as many first names as possible in 1 minute. | Broad retrieval ability; speed and flexibility of verbal thought process; neuropsychological test of language production |
| Kohs block Design | A psychometric test in which the child arranges groups of 4, 9, or 16 multi-colored blocks to copy picture designs presented on test cards. | Visuo-spatial problem solving, visual perception and organization |
| Coding-WISC-III‡ | The child has to substitute specific symbols for numbers presented in boxes, and complete as many items as possible in 2 minutes. | Visual-motor processing speed and coordination, short term memory, visual perception, visual scanning, cognitive flexibility, attention |
Covariates and confounders
We considered the following as important covariates and potential confounding variables: ‘Parental factors’ included maternal age, season at the time of blood sampling, parity, maternal and paternal educational attainment (completed years), current socio-economic status (SES), assessed using the Standard of Living Index,34 maternal intelligence assessed using the Revised Bhatia’s Short battery of Performance Tests of Intelligence for Adults35 and home environment assessed using The Home Observation for Measurement of the Environment Inventory-Early Adolescent version.36 We considered season at the time of blood sampling (summer, March–June; rainy season, July–October; and winter, November-February) because exposure to sunlight tends to vary in these 3 seasons. None of the mothers had ever smoked or consumed alcohol. ‘Infant factors’ included the child’s sex, gestational age at birth, newborn weight and head circumference, and the child’s weight, length and head circumference at age 2 years. ‘Child factors’ included the current age, BMI and head circumference.
The research ethics committee of the HMH approved the study and informed verbal consent was obtained from parents and children.
Statistical methods
Variables with skewed distributions were transformed appropriately. Maternal 25-hydroxyvitamin D concentrations were log transformed; Fisher Yates transformation and square root transformation was used for Kohs block design and pattern reasoning scores respectively during childhood. To facilitate interpretation of regression models cognitive tests scores and maternal 25-hydroxyvitamin D concentrations were z-standardized. Comparisons of means and percentages between groups were made using t tests and chi-square tests, where appropriate. Associations of covariates and confounders with maternal 25-hydroxyvitamin D concentrations (exposure) and cognitive scores (outcomes) were initially examined using multiple linear regression adjusting for sex and current age. Associations of maternal 25-hydroxyvitamin D concentrations (as a binary variable (deficient compared to normal concentrations) and as a continuous variable) with cognitive scores were then examined using multiple linear regression analyses adjusting for covariates/confounders (the child’s sex, and current age, season at the time of blood sampling, gestational age at birth, newborn weight and head circumference, maternal age, parity, parents’ SES, education, maternal intelligence, home environment, and the child’s BMI and head circumference at the time of outcome assessment) that were significantly associated with either 25-hydroxyvitamin D concentrations or cognitive outcomes. Data for maternal intelligence and home environment were missing for ~7% and ~37% of the children respectively. In order to maintain the sample size and to reduce bias we imputed maternal intelligence and home environment data by replacing each of these original variables with two newly constructed variables: a) a binary variable which took the value 0 if the original variable had a known value and 1 if it was missing; b) the mean value of the original variable when it was missing. The imputed variables were used in the regression analyses. Interaction terms were used to test for differences in the associations between exposure and sex in relation to cognitive scores. After ensuring that there was no interaction between exposure and sex in predicting cognitive ability, the sexes were pooled in all analyses, with adjustment for sex. Quadratic terms were used to examine for non-linear effects. Stata (version10.0, Stata Corporation, Texas, USA) was used for all analyses.
Results
Characteristics of the study sample are summarized in Table 1. During pregnancy 68% of women had low 25-hydroxyvitamin D concentrations. Maternal education and SES were higher among non-participants compared to participants (p<0.05 for both); there were no differences in maternal 25-hydroxyvitamin D concentrations or the prevalence of low concentrations, maternal age, parity and the children’s birth size between participants and non-participants (data not shown).
Girls scored better than boys in tests of short-term memory, reasoning, verbal fluency, and attention and concentration at both time points (p<0.05 for all) (Table 2). Boys were heavier, and had larger head circumference at birth and at age 2 years (also taller at age 2 years) and higher home environment score compared to girls; girls had longer gestational age than boys (p<0.05 for all; Table 2). One percent of mothers were illiterate, approximately 35% had only received primary school education; 50% had completed secondary school education, and 14% were graduates or postgraduates and/or professionals. Corresponding figures for fathers were 3%, 34%, 39% and 24% respectively.
Table 2. General characteristics of the study participants.
| Participants during childhood | Participants during adolescence | |||||
|---|---|---|---|---|---|---|
| Variable | Boys (n=228) | Girls (n=240) | All (n=468) | Boys (n=226) | Girls (n=246) | All (n=472) |
| Maternal characteristics in pregnancy | ||||||
| Age (years) | 24.0 ± 4.3 | 23.8 ± 4.3 | 23.9 ± 4.3 | 23.9 ± 4.3 | 23.8 ± 4.3 | 23.8 ± 4.3 |
| Parity (n (%)) | ||||||
| 0 | 113 (49.5) | 124 (51.7) | 237 (50.6) | 114 (50.4) | 123 (50.0) | 237 (50.2) |
| 1 | 76 (33.3) | 78 (32.5) | 154 (32.9) | 74 (32.7) | 78 (32.5) | 158 (33.5) |
| ≥ 2 | 39 (17.1) | 38 (15.8) | 77 (16.4) | 38 (16.8) | 38 (15.8) | 77 (16.3) |
| Serum 25-hydroxyvitamin D concentration (nmol/L) | 38.0 (23.0, 54.0) | 40.6 (23.9, 62.1) | 38.9 (23.5, 58.3) | 37.5 (23.0, 54.0) | 39.0 (23.8, 60.0) | 38.1 (23.5, 56.8) |
| Low 25-hydroxyvitamin D concentration, <50nmol/L (n (%)) | 154 (67.5) | 159 (66.3) | 313 (66.9) | 154 (68.1) | 166 (67.5) | 320 (67.8) |
| 25-hydroxyvitamin D concentration according to season at the time of blood sampling (nmol/L) | ||||||
| Summer (March-June) | 31.0 (22.0, 46.0) | 29.0 (21.0, 44.5) | 30.0 (21.5, 45.0) | 30.0 (20.8, 44.8) | 28.0 (20.9, 42.5) | 29.0 (20.9, 43.0) |
| Rainy (July-October) | 36.6 (18.7, 52.0) | 42.5 (23.0, 71.0) | 39.1 (21.9, 62.0) | 36.0 (18.7, 52.0) | 42.0 (22.8, 71.0) | 38.9 (21.6, 62.0) |
| Winter (November-February) | 51.5 (28.0, 78.0) | 49.3 (31.2, 87.0) | 50.8 (31.0, 79.0) | 50.0 (32.8, 77.4) | 47.0 (31.0, 79.0) | 47.2 (31.1, 77.7) |
| Children’s Characteristics | ||||||
| Tests of cognitive function (score) | ||||||
| Learning, long-term retrieval/storage | 67.8 ± 18.3 | 68.4 ± 16.6 | 68.1 ± 17.4 | 80.1 ± 14.5 | 79.8 ± 14.7 | 80.0 ± 14.6 |
| Short-term memory | 16.2 ± 2.6 | 16.9 ± 2.5 | 16.5 ± 2.6 | 18.6 ± 3.6 | 19.5 ± 4.0 | 19.0 ± 3.8 |
| Reasoning | 9.0 (4.0, 13.0) | 11.0 (6.0, 14.0) | 10.0 (5.0, 14.0) | 14.8 ± 6.5 | 16.4 ± 6.7 | 15.7 ± 6.7 |
| Verbal fluency | 14.8 ± 4.2 | 17.6 ± 5.3 | 16.2 ± 5.0 | 19.6 ± 4.6 | 22.9 ± 6.2 | 21.3 ± 5.7 |
| Visuo-spatial ability | 76.8 (63.4, 87.8) | 77.0 (63.7, 89.2) | 76.9 (63.7, 88.5) | 85.5 ± 26.2 | 82.3 ± 25.4 | 83.8 ± 25.8 |
| Attention and concentration | 30.3 ± 7.8 | 35.2 ± 8.0 | 32.8 ± 8.3 | 44.5 ± 9.7 | 50.9 ± 11.2 | 47.8 ± 11.0 |
| At birth | ||||||
| Gestational age (weeks) | 39.2 ±1.4 | 39.5 ± 1.2 | 39.4 ± 1.3 | 39.2 ± 1.4 | 39.5 ±1.1 | 39.4 ±1.3 |
| Birthweight (kg) | 2.963 ± 0.424 | 2.869 ± 0.425 | 2.915 ± 0.426 | 2.948 ± 0.423 | 2.865 ± 0.417 | 2.904 ± 0.422 |
| Head circumference (cm) | 34.2 ± 1.3 | 33.6 ± 1.2 | 33.9 ± 1.3 | 34.2 ± 1.3 | 33.6 ± 1.3 | 33.9 ± 1.3 |
| At age 2 years | ||||||
| Weight (kg) | 10.8 ± 1.2 | 10.2 ± 1.3 | 10.5 ± 1.2 | 10.8 ± 1.2 | 10.2 ± 1.2 | 10.5 ± 1.2 |
| Length (cm) | 84.5 ± 3.2 | 82.9 ± 3.2 | 83.7 ± 3.3 | 84.5 ± 3.2 | 82.8 ± 3.2 | 83.6 ± 3.3 |
| Head circumference (cm) | 46.8 ± 1.4 | 45.8 ± 1.3 | 46.3 ± 1.4 | 46.9 ± 1.3 | 45.8 ± 1.3 | 46.3 ± 1.4 |
| At the time of testing | ||||||
| Age (years) | 9.7 ± 0.3 | 9.7 ± 0.3 | 9.7 ± 0.3 | 13.5 ± 0.1 | 13.5 ± 0.1 | 13.5 ± 0.1 |
| BMI (kg/m2) | 14.6 ± 1.7 | 14.7 ± 2.0 | 14.6 ± 1.9 | 17.0 ± 2.7 | 18.4 ± 3.4 | 17.8 ± 3.2 |
| Head circumference (cm) | 50.8 ± 1.4 | 50.5 ± 1.5 | 50.7 ± 1.4 | 51.5 ± 1.4 | 51.3 ± 1.4 | 51.4 ± 1.4 |
| Parents socio-economic status | ||||||
| Standard of living index (score) | 36.6 ± 7.7 | 36.7 ± 8.6 | 36.7 ± 8.2 | 38.9 ± 7.3 | 36.7 ± 7.3 | 38.8 ± 7.3 |
| Maternal education (n (%)) | ||||||
| <10 completed years | 88 (38.8) | 75 (31.2) | 163 (34.9) | 84 (37.2) | 72 (29.3) | 156 (33.1) |
| -10 completed years | 69 (30.4) | 79 (32.9) | 148 (31.7) | 70 (31.0) | 87 (35.4) | 157 (33.3) |
| >10 completed years | 70 (30.8) | 86 (35.8) | 156 (33.4) | 72 (31.9) | 87 (35.4) | 159 (33.7) |
| Paternal education (n (%)) | ||||||
| <10 completed years | 90 (39.7) | 80 (33.3) | 170 (36.4) | 79 (35.0) | 69 (28.1) | 148 (31.4) |
| -10 completed years | 80 (35.2) | 103 (42.9) | 183 (39.2) | 58 (25.7) | 51 (20.7) | 109 (23.1) |
| >10 completed years | 57 (25.1) | 57 (23.8) | 114 (24.4) | 89 (39.4) | 126 (51.2) | 215 (45.6) |
| Maternal intelligence (score) | 85.9 ± 16.4 | 85.7 ± 17.2 | 85.8 ± 16.8 | 85.5 ± 16.2 | 85.7 ± 17.3 | 85.8 ± 16.8 |
| Home environment (score) | 45.1 ± 5.7 | 43.5 ± 7.0 | 44.2 ± 6.4 | 45.0 ± 5.7 | 43.5 ± 7.0 | 44.3 ± 6.5 |
Values are mean ± SD or medians (Inter quartile range) unless otherwise stated
As already reported,21 25-hydroxy vitamin D concentrations were higher among mothers whose blood sample was collected during winter compared to those whose sample was collected during the rainy (p<0.01) or summer season (p<0.001) (Table 2). Approximately 70% of women were recruited at <24 weeks gestation and 30% were recruited between 24-32 weeks. At recruitment 131 (28%) women reported taking supplements containing calcium and vitamin D3. Of these 66 (50%) were recruited at <24 weeks gestation and 65 (50%) between 24-32 weeks gestation. There were no associations of supplement use at recruitment with 25-hydroxyvitamin D concentrations at 30±2 weeks of gestation. This was true among women recruited early (<24 weeks of gestation) and those recruited later (24-32 weeks).
Associations of maternal 25-hydroxy vitamin D concentrations and cognitive outcomes with covariates and confounders
There were no associations of maternal age or parity, or the child’s size at birth, at age 2 years and at the time of outcome assessment, SES, parental education, maternal intelligence and home environment with maternal 25-hydroxyvitamin D concentrations (Table 3). Cognitive scores tended to be lower in children of mothers of higher parity and to increase with increasing maternal age and children’s birth size. The children’s weight, length and head circumference at age 2 years, current BMI and head circumference, parental educational level, SES, maternal intelligence and home environment were strongly positively related to most of the cognitive outcomes (Table 3).
Table 3. Associations of covariates or confounders with cognitive outcomes and maternal 25-hydroxyvitamin D concentrations†.
| Learning, long-term retrieval | Short-term memory | Reasoning ability | Verbal fluency | Visuo-spatial ability | Attention and concentration | Maternal 25-hydroxy vitamin D concentrations | |
|---|---|---|---|---|---|---|---|
| Covariates/confounders | β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
β (95% CI) |
| Maternal age (years) | 0.26 (-0.11, 0.63) |
0.06 (0.004, 0.11)* |
0.03 (0.01, 0.06)** |
0.01 (-0.09, 0.12) |
0.04 (0.02, 0.06)*** |
0.17 (0.01, 0.33)* |
0.01 (-0.004, 0.02) |
| Maternal parity (0, 1 and ≥2) | -2.56 (-4.41, -0.71)** |
-0.35 (-0.62, -0.07)* |
-0.15 (-0.27, -0.04)** |
-0.36 (-0.87, 0.15) |
0.04 (-0.14, 0.07) |
-0.01 (-0.83, 0.81) |
0.06 (-0.01, 0.13) |
| Birthweight (kg) | 3.09 (-0.66, 6.84) |
0.23 (-0.33, 0.78) |
0.16 (-0.07, 0.39) |
-0.19 (-1.22, 0.84) |
0.30 (0.09, 0.51)** |
1.30 (-0.35, 2.95) |
-0.08 (-0.23, 0.07) |
| Head circumference at birth (cm) | 1.86 (0.62, 3.11)** |
0.16 (-0.03, 0.34) |
0.05 (-0.02, 0.13) |
0.04 (-0.31, 0.38) |
0.09 (0.02, 0.16)* |
0.26 (-0.29, 0.82) |
-0.008 (-0.06, 0.04) |
| Weight at age 2 years (kg) | 2.31 (0.99, 3.64)** |
0.40 (0.20, 0.59)*** |
0.14 (0.07, 0.23)*** |
0.31 (-0.06, 0.67) |
0.11 (0.04, 0.19)** |
1.02 (0.45, 1.59)*** |
-0.001 (-0.05, 0.05) |
| Length at age 2 years (cm) | 1.02 (0.52, 1.52)*** |
0.15 (0.08, 0.23)*** |
0.07 (0.04, 0.10)*** |
0.15 (0.01, 0.29)* |
0.06 (0.03, 0.09)*** |
0.43 (0.21, 0.65)*** |
-0.003 (-0.02, 0.02) |
| Head circumference at age 2 years (cm) | 2.53 (1.35, 3.71)*** |
0.33 (0.16, 0.51)*** |
0.15 (0.08, 0.22)*** |
0.43 (0.10, 0.76)* |
0.11 (0.04, 0.18)** |
0.81 (0.29, 1.32)** |
-0.006 (-0.05, 0.04) |
| Child’s current BMI (kg/m2) | 1.58 (0.74, 2.43)*** |
0.18 (0.05, 0.30)** |
0.09 (0.04, 0.14)*** |
0.31 (0.07, 0.54)** |
0.04 (-0.01, 0.09) |
0.75 (0.38, 1.12)*** |
0.004 (-0.03, 0.04) |
| Child’s current head circumference (cm) | 2.37 (1.28, 3.46)*** |
0.38 (0.22, 0.53)*** |
0.15 (0.09, 0.22)*** |
0.41 (0.10, 0.71)** |
0.11 (0.05, 0.18)*** |
1.08 (0.60, 1.56)*** |
-0.008 (-0.05, 0.04) |
| Standard of living index (score) | 0.42 (0.23, 0.61)*** |
0.07 (0.04, 0.10)*** |
0.03 (0.02, 0.05)*** |
0.10 (0.05, 0.16)*** |
0.03 (0.02, 0.04)*** |
0.19 (0.10, 0.27)*** |
-0.004 (-0.01, 0.003) |
| Maternal education (completed years) | 1.06 (0.61, 1.51)*** |
0.22 (0.15, 0.28)*** |
0.09 (0.06, 0.11)*** |
0.25 (0.13, 0.37)*** |
0.09 (0.06, 0.11)*** |
0.44 (0.25, 0.64)*** |
-0.005 (-0.02, 0.01) |
| Paternal education (completed years) | 0.78 (0.43, 1.12)*** |
0.12 (0.07, 0.17)*** |
0.07 (0.04, 0.09)*** |
0.17 (0.08, 0.27)** |
0.06 (0.04, 0.08)*** |
0.35 (0.19, 0.50)*** |
0.008 (-0.006, 0.02) |
| Maternal intelligence (score) | 0.21 (0.12, 0.31)*** |
0.03 (0.02, 0.04)*** |
0.02 (0.01, 0.02)*** |
0.02 (-0.01, 0.05) |
0.01 (0.005, 0.02)*** |
0.05 (0.01, 0.09)* |
-0.0008 (-0.005, 0.003) |
| Home environment (score) | 0.58 (0.27, 0.89)*** |
0.08 (0.03, 0.12)*** |
0.06 (0.04, 0.08)*** |
0.18 (0.10, 0.27)*** |
0.04 (0.02, 0.06)*** |
0.36 (0.22, 0.49)*** |
-0.004 (-0.02, 0.01) |
Data presented for the participants during childhood
β is the effect size of the cognitive scores and maternal 25-hydroxy vitamin D concentrations per unit change in covariates/confounders, derived using multiple linear regression adjusted for the child’s sex and current age, and using all variables as continuous
P<0.05;
P<0.01;
P<0.001; P values derived by multiple linear regression adjusted for the child’s sex and current age
Associations of maternal 25-hydroxyvitamin D concentrations with offspring cognition
Maternal vitamin D status (both deficiency versus non-deficiency, and the continuous variable) was unrelated to offspring cognitive performance in childhood (Table 4). The findings were similar during adolescence, but there was a positive association between 25-hydroxyvitamin D concentrations and verbal fluency which became stronger and significant after adjusting for season and covariates and confounders (Table 5). The findings were similar in boys and girls.
Table 4. Associations of maternal 25-hydroxyvitamin D concentrations in pregnancy with offspring cognitive performance during childhood.
| Cognitive function tests | |||||||
|---|---|---|---|---|---|---|---|
| Maternal vitamin D concentrations | N | Learning, long-term retrieval | Short-term Memory | Reasoning Ability | Verbal Fluency | Visuo-spatial Ability | Attention and concentration |
| Vitamin D status | Score | ||||||
| Normal (>50 nmol/L) | 155 | 68.7 ± 17.8 | 16.5 ± 2.5 | 10.0 (4.0, 15.0) | 16.4 ± 5.4 | 77.5 (63.0, 89.3) | 33.2 ± 9.1 |
| Low (<50 nmol/L) | 313 | 67.8 ± 17.3 | 16.5 ± 2.6 | 10.0 (5.0, 13.0) | 16.1 ± 4.8 | 76.9 (63.7, 88.4) | 32.7 ± 7.8 |
| P† | 0.6 | 0.9 | 0.7 | 0.6 | 0.9 | 0.5 | |
| β (95% CI)‡ | |||||||
| Model 1 | 468 | -0.03 (-0.23, 0.17) | 0.04 (-0.15, 0.24) | -0.005 (-0.20, 0.19) | -0.01 (-0.20, 0.18) | -0.001 (-0.19, 0.19) | 0.03 (-0.16, 0.21) |
| Model 2 | 468 | 0.01 (-0.20, 0.21) | 0.05 (-0.16, 0.25) | 0.003 (-0.20, 0.20) | -0.02 (-0.22, 0.18) | 0.03 (-0.16, 0.23) | 0.002 (-0.19, 0.19) |
| Model 3 | 465 | -0.04 (-0.24, 0.15) | 0.01 (-0.19, 0.21) | -0.04 (-0.23, 0.16) | -0.04 (-0.25, 0.16) | 0.02 (-0.18, 0.21) | 0.04 (-0.15, 0.22) |
| Vitamin D quartiles | Score | ||||||
| < 23.5 nmol/L | 121 | 68.3 ± 16.7 | 16.5 ± 2.6 | 10.0 (6.0, 13.0) | 15.6 ± 4.3 | 77.6 (63.7, 87.8) | 32.7 ± 7.9 |
| 23.6 – 38.9 nmol/L | 113 | 67.7 ± 17.2 | 16.3 ± 2.4 | 10.0 (4.0,14.0) | 16.3 ± 5.1 | 75.0 (63.1, 89.2) | 32.0 ± 8.1 |
| 39.0 – 57.0 nmol/L | 116 | 68.9 ± 17.4 | 16.8 ± 2.9 | 11.0 (7.0,14.0) | 16.3 ± 4.9 | 76.8 (66.3, 88.2) | 33.4 ± 7.7 |
| >57.0 nmol/L | 118 | 67.4 ± 18.7 | 16.5 ± 2.4 | 10.0 (4.0,14.0) | 16.6 ± 5.6 | 77.5 (62.0, 90.2) | 33.3 ± 9.3 |
| P for trend§ | 0.7 | 0.9 | 0.2 | 0.6 | 0.9 | 0.7 | |
| β (95% CI)¶ | |||||||
| Model 1 | 468 | -0.02 (-0.11, 0.07) | -0.01 (-0.10, 0.09) | -0.06 (-0.15, 0.04) | 0.02 (-0.07, 0.12) | 0.008 (-0.08, 0.10) | -0.02 (-0.11, 0.07) |
| Model 2 | 468 | -0.03 (-0.13, 0.06) | -0.01 (-0.11, 0.09) | -0.06 (-0.16, 0.03) | 0.03 (-0.07, 0.12) | -0.006 (-0.10, 0.09) | -0.01 (-0.10, 0.08) |
| Model 3 | 465 | -0.01 (-0.11, 0.08) | 0.005 (-0.09, 0.10) | -0.05 (-0.14, 0.04) | 0.04 (-0.05, 0.14) | 0.002 (-0.09, 0.09) | -0.02 (-0.11, 0.07) |
Values are mean ± SD or medians (inter quartile range) unless otherwise stated
P value for the difference in cognitive test scores between children of mothers with normal and low 25-hydroxyvitamin D concentrations derived using t test
β (SD) is the difference in cognitive test score between children of mothers with normal and low 25-hydroxyvitamin D concentrations
P for trend adjusted for the child’s sex and current age derived by multiple linear regression using 25-hydroxyvitamin D concentrations as a continuous variable
β is the effect size (SD) of the cognitive test score per SD change in 25-hydroxyvitamin D concentrations (used as a continuous variable) derived by multiple linear regression
Model 1: adjusted for the child’s sex and current age
Model 2: Model 1 + season at the time of blood sampling
Model 3: Model 2 + gestational age, the child’s birthweight, head circumference at birth, weight, length and head circumference at age 2 years, current BMI and head circumference, maternal age, parity, standard of living index, maternal and paternal education, maternal intelligence (imputed) and home environment (imputed)
Table 5. Associations of maternal 25-hydroxyvitamin D concentrations in pregnancy with offspring cognitive performance during adolescence.
| Cognitive function tests | |||||||
|---|---|---|---|---|---|---|---|
| Maternal vitamin D concentrations | N | Learning, long-term retrieval | Short-term memory | Reasoning ability | Verbal fluency | Visuo-spatial ability | Attention and concentration |
| Vitamin D status | Score | ||||||
| Normal (>50 nmol/L) | 152 | 80.3 ± 15.7 | 19.1 ± 4.0 | 16.4 ± 7.0 | 21.7 ± 5.8 | 85.5 ± 25.3 | 48.6 ± 12.1 |
| Low (<50 nmol/L) | 320 | 79.9 ± 14.0 | 19.0 ± 3.7 | 15.3 ± 6.4 | 21.1 ± 5.7 | 83.0 ± 26.1 | 47.5 ± 10.4 |
| P† | 0.7 | 0.8 | 0.1 | 0.3 | 0.3 | 0.3 | |
| β (95% CI)‡ | |||||||
| Model 1 | 472 | -0.02 (-0.21, 0.18) | -0.01 (-0.21, 0.18) | -0.14 (-0.34, 0.05) | -0.09 (-0.29, 0.10) | -0.08 (-0.28, 0.11) | -0.07 (-0.25, 0.12) |
| Model 2 | 472 | 0.06 (-0.14, 0.27) | 0.02 (-0.18, 0.22) | -0.07 (-0.28, 0.13) | -0.14 (-0.33, 0.06) | -0.08 (-0.28, 0.12) | -0.11 (-0.30, 0.08) |
| Model 3 | 472 | 0.04 (-0.17, 0.24) | 0.01 (-0.20, 0.21) | -0.10 (-0.28, 0.10) | -0.12 (-0.32, 0.08) | -0.08 (-0.28, 0.12) | -0.07 (-0.26, 0.12) |
| Vitamin D quartiles | Score | ||||||
| < 23.5 nmol/L | 123 | 80.9 ± 13.4 | 19.2 ± 3.7 | 15.4 ± 6.4 | 20.7 ± 5.3 | 82.3 ± 26.1 | 47.7 ± 10.6 |
| 23.6 – 38.9 nmol/L | 118 | 79.0 ± 13.7 | 18.7 ± 3.7 | 14.8 ± 6.6 | 20.8 ± 6.1 | 83.6 ± 26.1 | 46.5 ± 11.0 |
| 39.0 – 57.0 nmol/L | 116 | 80.0 ± 15.0 | 19.0 ± 3.8 | 16.2 ± 6.6 | 22.1 ± 5.7 | 83.5 ± 25.3 | 48.9 ± 10.6 |
| >57.0 nmol/L | 115 | 79.8 ± 16.2 | 19.1 ± 4.1 | 16.3 ± 7.1 | 21.8 ± 5.8 | 86.0 ± 26.0 | 48.3 ± 11.8 |
| P for trend§ | 0.7 | 0.7 | 0.7 | 0.08 | 0.6 | 0.9 | |
| β (95% CI)¶ | |||||||
| Model 1 | 472 | -0.02 (-0.11, 0.08) | -0.02 (-0.11, 0.07) | 0.02 (-0.07, 0.11) | 0.08 (-0.01, 0.17) | 0.03 (-0.07, 0.12) | 0.003 (-0.08, 0.09) |
| Model 2 | 472 | -0.05 (-0.15, 0.04) | -0.04 (-0.13, 0.06) | -0.01 (-0.11, 0.08) | 0.10 (0.01, 0.19)* | 0.02 (-0.07, 0.12) | 0.02 (-0.07, 0.11) |
| Model 3 | 472 | -0.02 (-0.11, 0.08) | -0.02 (-0.12, 0.07) | 0.01 (-0.08, 0.10) | 0.10 (0.01, 0.20)* | 0.03 (-0.07, 0.12) | 0.02 (-0.07, 0.11) |
Values are mean ± SD unless otherwise stated
P value for the difference in cognitive test scores between children of mothers with normal and low 25-hydroxyvitamin D concentrations derived using t test
β (SD) is the difference in cognitive test score between children of mothers with normal and low 25-hydroxyvitamin D concentrations
P for trend adjusted for the child’s sex and current age derived by multiple linear regression using 25-hydroxyvitamin D concentrations as a continuous variable
β is the effect size (SD) of the cognitive test score per SD change in 25-hydroxyvitamin D concentrations (used as a continuous variable) derived by multiple linear regression
Model 1: adjusted for the child’s sex and current age
Model 2: Model 1 + season at the time of blood sampling
Model 3: Model 2 + gestational age, the child’s birthweight, head circumference at birth, weight, length and head circumference at age 2 years current BMI and head circumference, maternal age, parity, standard of living index, maternal and paternal education, maternal intelligence (imputed) and home environment (imputed)
P<0.05; P values derived by multiple linear regression
Discussion
To our knowledge, this is the first study in a developing country to examine associations between maternal 25-hydroxyvitamin D concentrations during pregnancy and cognitive performance in their children. We found a high prevalence of maternal vitamin D deficiency (68%) and there was a significant seasonal variation in 25-hydroxyvitamin D concentrations. There were no associations between maternal 25-hydroxyvitamin D concentrations and offspring cognitive ability during childhood and adolescence.
Strengths of the study were a large sample of children and a battery of cognitive function tests specifically adapted for, and validated in, a South Indian population. The cognitive tests that we used in our study are typical tests applicable for school aged children and relevant to everyday life. These tests assess the day-to-day problem solving abilities which are more likely to be associated with academic performance and behavioural outcome of an individual. Furthermore, data on a range of important confounding factors were recorded. Missing data on maternal 25-hydroxyvitamin D concentrations in ~14% of the participants was a limitation. However, birth size, socio-demographic factors and cognitive scores were similar among those who did and did not have this data and therefore the risk of bias is low. Other important limitations were lack of information on maternal diet, sunlight exposure, and use of vitamin D supplements at the time of blood sampling and the child’s vitamin D status.
The high prevalence of maternal vitamin D deficiency in our study is consistent with findings in other Indian7,8,37–39 and western populations.17,18,20 South Asians, both in their country of origin and after migration to Europe or the USA, have lower vitamin D concentrations than white Caucasians,8,40 probably because of skin pigmentation, dress code (especially in women) and low dietary vitamin D intake. Another possible reason may be differences in vitamin D metabolism in Asian Indians; in vitro studies have shown that tissue fibroblasts have increased 25-hydroxy-24-hydroxylase activity, leading to increased catabolism of activated vitamin D and therefore an increased risk of developing vitamin D deficiency.41
We found no significant associations between intake of vitamin supplements and 25-hydroxyvitamin D concentrations. This is possibly due to a lack of complete information on supplement intake, as the study was not originally designed to examine maternal vitamin D status and supplement use was recorded only at the time of recruitment. Among women recruited between 24 and 32 week gestation, very few were taking supplements. Women who took supplements in early pregnancy might have stopped taking them by 30 week and women not taking supplements at recruitment may have been prescribed them later in pregnancy. However, despite the common practice of obstetricians prescribing calcium and vitamin D during the second trimester of pregnancy, many women had low 25-hydroxyvitamin D concentrations.
The finding of seasonal variation in 25-hydroxyvitamin D concentrations in our study is probably related to sunlight exposure. As reported earlier, although data on sunlight exposure was not available, 25-hydroxyvitamin D concentrations were lowest during the cloudy rainy season, and the summer season when people avoid the hot sun, and highest in the winter season when the weather is cooler and people go out in the sun.21 Seasonal variations in 25-hydroxyvitamin D concentrations and correlations with sunlight exposure have been reported in other Indian8 and Asian populations.42 Low 25-hydroxyvitamin D concentrations during winter have been reported among western populations.40,43
In our study, neither maternal vitamin D status (low versus normal) nor the range of 25-hydroxyvitamin D concentrations at 30±2 weeks of gestation was associated with cognitive performance in the children at either time point. Consistent with our findings, a study with a very small sample (n=178) in the UK found no associations between maternal vitamin D status at 32 weeks of gestation and offspring IQ assessed using Wechsler Abbreviated Scale of Intelligence at age 9 years.18 Similarly, a study in Denmark (n=850) found no association of maternal 25-hydroxyvitamin D concentrations at 30 weeks of gestation with children’s scholastic achievement at age 15-16 years.19 A large study in the USA (n=3896) assessed maternal 25-hydroxyvitamin D concentrations at ≤26 weeks gestation and children’s global infant development at age 8 months using the Bayley Scales of Mental and Motor Development, IQ at age 4 and 7 years using the Stanford-Binet Intelligence Scale and the Wechsler Intelligence Scale for Children respectively, and a student achievement test at 7 years.20 Findings were mostly null except for a small positive association with offspring IQ (0.10 score points per 5nmol/L increase in maternal 25-hydroxyvitamin D concentration) at age 7 years. In contrast to our findings, a study in Spain (n=1800) found a positive association between maternal 25-hydroxyvitamin D concentrations at 12-23 weeks of gestation and offspring mental and psychomotor development scores (0.8-0.9 score points (~0.06 SD) per 25nmol/L increase) assessed using the Bayley Scales of Infant Development at age 11-23 months.16 It also found higher mental and psychomotor development scores (2-3 score points (0.1-0.2 SD)) in children of mothers with normal vitamin D status (>75 nmol/L) compared to children of deficient (<50 nmol/L) mothers. A study in Australia (n=~500) observed a two-fold increase in language impairment (assessed using the Peabody Picture Vocabulary Test-Revised) in 5 and 10 years old children of mothers with vitamin D deficiency (<46 nmol/L) at 18 weeks of gestation compared to children of mothers with normal vitamin D status (>70 nmol/L).17 Comparison of our study with these studies is difficult due to differing ages of children and test batteries used, but it is notable that the two positive studies measured maternal vitamin D status during the second trimester of pregnancy, while the others (including ours) measured it in the third trimester. It is possible that there is a critical period for neurodevelopment in mid-pregnancy, when vitamin D is required. The lack of association in our study may reflect adaptation of the Indian population to low sunlight exposure and/or low dietary intakes across centuries of cultural dress codes for women and vegetarian diets. Alternatively, the positive associations between maternal vitamin D status and offspring cognitive function in two developed populations16,17 could have been due to confounding rather than a biological effect of vitamin D; these studies did not adjust for maternal intelligence or home stimulation and care.
In conclusion, in this Indian population, despite a wide variation in maternal vitamin D concentrations and a high prevalence of low maternal 25-hydroxyvitamin D concentrations, maternal vitamin D status was unrelated to the children’s cognitive function. Our findings add to a very small literature on this topic; randomized controlled trials of vitamin D supplementation in pregnancy would be valuable in clarifying the importance of maternal vitamin D status for offspring cognitive function.
Acknowledgements
We are grateful to the families who participated in the study and to the Hospital Medical Director and staff of Obstetrics and Gynecology department for their support. We acknowledge the substantial contribution made to the study by research unit staff and Sneha-India for its support.
Footnotes
Conflict of interest and funding disclosure
The study was supported by the Medical Research Council, UK; the Wellcome Trust, UK; the Parthenon Trust, Switzerland and the Department for International Development, UK.
All authors have no conflicts of interest to declare.
References
- 1.Bikle DD. Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocr Rev. 1992;13:765–784. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- 2.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 3.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 4.Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Protective effects of 1 alpha, 25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Harinarayan CV, Joshi SR. Vitamin D status in India--its implications and remedial measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 8.Goswami R, Kochupillai N, Gupta N, Goswami D, Singh N, Dudha A. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J Assoc Physicians India. 2008;56:755–757. [PubMed] [Google Scholar]
- 9.Lewis S, Lucas RM, Halliday J, Ponsonby AL. Vitamin D deficiency and pregnancy: from preconception to birth. Mol Nutr Food Res. 2010;54:1092–1102. doi: 10.1002/mnfr.201000044. [DOI] [PubMed] [Google Scholar]
- 10.Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74:71–75. doi: 10.1016/j.mehy.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 12.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C, Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 13.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 years of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161:306–312. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes de Abreu DA, Nivet E, Baril N, Khrestchatisky M, Roman F, Féron F. Developmental vitamin D deficiency alters learning in C57Bl/6J mice. Behav Brain Res. 2010;208:603–608. doi: 10.1016/j.bbr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Morales E, Guxens M, Llop S, Rodríguez-Bernal CL, Tardón A, Riaño I, et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130:e913–920. doi: 10.1542/peds.2011-3289. [DOI] [PubMed] [Google Scholar]
- 17.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–493. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- 18.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C, Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strøm M, Halldorsson TI, Hansen S, Granström C, Maslova E, Petersen SB, Cohen AS, Olsen SF. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab. 2014;64:254–261. doi: 10.1159/000365030. [DOI] [PubMed] [Google Scholar]
- 20.Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol. 2014;28:434–444. doi: 10.1111/ppe.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, Osmond C, Veena SR, Fall CH. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CHD. Glucose tolerance in pregnancy in South India: Relationships to neonatal anthropometry. Acta Obstet Gynecol Scand. 2005;84:159–165. doi: 10.1111/j.0001-6349.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 23.Krishnaveni GV, Hill JC, Veena SR, Leary SD, Saperia J, Chachyamma KJ, Karat SC, Fall CH. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatrics. 2005;42:527–538. [PubMed] [Google Scholar]
- 24.Binkley N, Krueger D, Gemar D, Drezner MK. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab. 2008;93:1804–1809. doi: 10.1210/jc.2007-2340. [DOI] [PubMed] [Google Scholar]
- 25.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D deficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 26.Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York: Cambridge University Press; 1993. [Google Scholar]
- 27.Kaufman AS, Kaufman LN. Kaufman Assessment Battery for Children, Second Edition: Manual. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 28.Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: A cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20:331–354. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- 29.Kohs SC. Intelligence measurement: a psychological and statistical study based upon the Block-design test. New York: Macmillan; 1923. [Google Scholar]
- 30.Wigg CM, Duro LA. The Koh’s block tests as an important instrument to investigate the visuo-spatial impairments in myotonic dystrophy. Part I. Quantitative and qualitative analysis. Arq Neuropsiquiatr. 1999;57:547–555. doi: 10.1590/s0004-282x1999000400002. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 32.Malda M, van de Vijver FJR, Srinivasan K, Transler C, Sukumar P, Rao K. Adapting a cognitive test for a different cultures: An illustration of qualitative procedures. Psychol Sci Quarterly. 2008;50:451–468. [Google Scholar]
- 33.Malda M, van de Vijver FJR, Srinivasan K, Transler C, Sukumar P. Travelling with cognitive tests: testing the validity of a KABC-II adaptation in India. Assessment. 2010;17:107–115. doi: 10.1177/1073191109341445. [DOI] [PubMed] [Google Scholar]
- 34.International Institute for Population Sciences (IIPS) and Operations Research Centre (ORC) Macro. National Family Health Survey (NFHS-2), India 1998-1999. IIPS; Maharashtra, Mumbai: 2001. [Google Scholar]
- 35.Verma SK, Pershad D, Malhotra A, Arunima . The Revised Bhatia’s Short Battery of Performance Test of Intelligence for Adults (A Handbook) Agra: National Psychological Corporation; 1988. [Google Scholar]
- 36.Caldwell BM, Bradley RH. Home observation for measurement of the environment: Administration manual. Tempe, AZ: Family and Human Dynamics Research Institute, Arizona State University; 2003. [Google Scholar]
- 37.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 38.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, Das V. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf) 2009;70:680–684. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 39.Marwaha RK, Tandon N, Chopra S, Agarwal N, Garg MK, Sharma B, Kanwar RS, Bhadra K, Singh S, Mani K, Puri S. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–1389. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 40.Sherman SS, Hollis BW, Tobin JD. Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab. 1990;1:405–413. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- 41.Awumey EMK, Mitra DA, Hollis BW, Kumar R, Bell NH. Vitamin D metabolism is altered in Asian Indians in the southern United States: A Clinical Research Center Study. J Clin Endocrinol Metab. 1998;83:169–173. doi: 10.1210/jcem.83.1.4514. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Nashimoto M, Yamamoto M. Summer/winter differences in the serum 25-hydroxyvitamin D3 and parathyroid hormone levels of Japanese women. Int J Biometeorology. 2000;44:186–189. doi: 10.1007/s004840000067. [DOI] [PubMed] [Google Scholar]
- 43.Van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;345:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]