Abstract
Primary effusion lymphoma (PEL) is a common cancer in AIDS patients closely associated with Kaposi’s sarcoma-associated herpesvirus (KSHV). Previously, we showed that KSHV latency associated nuclear antigen (LANA) stabilizes intracellular activated Notch1 (ICN) involved in maintenance of the malignant phenotype of KSHV infected PEL cells in vitro. The γ-secretase inhibitor (GSI) which specifically blocks the production of ICN slows down the proliferation of the KSHV infected PEL cell lines BCBL1, BC3 as well as JSC1 in vitro. In this study, we extended these studies to explore the possibility that manipulation of the Notch signaling by GSI would prevent the growth of the PEL tumors in vivo. We observed that the onset of tumorigenesis of KSHV infected PELs was significantly delayed in GSI treated SCID mice harboring the PEL cell lines. We also found that GSI treatment resulted in necrosis as well as apoptosis in tumors generated by the xenotransplanted KSHV positive PEL cell lines. In contrast, GSI had no effect on mice harboring BJAB cells, a KSHV negative Burkitt’s lymphoma cell line where ICN levels were negligible. Our study provides further evidence to suggest that targeted downregulation of abnormal Notch signaling has therapeutic potential for KSHV related primary effusion lymphomas.
Keywords: KSHV, lymphoma, secretase inhibitor, mouse model, notch, treatment, proliferation
Introduction
Viral associated Primary effusion lymphomas (PELs) are a form of non-Hodgkin’s lymphoma which is seen quite frequently in immunocompromised AIDS patients infected with the human immunodeficiency virus.1,2 This form of lymphomas typically respond poorly to conventional chemotherapy, and almost always lead to death of the infected individuals.3,4 PELs can be closely associated with infection by one of the two known human gammaherpesvirus type-8 (HHV-8), also designated as Kaposi’s sarcoma-associated herpesvirus (KSHV) and is also frequently co-infected with the second well known human gammaherpesvirus, Epstein-Barr virus (EBV).1,2
KSHV belongs to the gamma-2 herpesvirus subfamily and is now accepted as a major contributor to the development of the human malignancies, Kaposi’s sarcoma and primary effusion lymphoma.1,5 These cancers can also be classified with a growing number of human cancers which is shown to be associated with a range of infectious agents, which includes viruses, bacteria and other parasites all possibly contributing to the initiation and development of these cancers.6 KSHV is also thought to establish and reside as a latent virus after the initial primary lytic infection, and persists in the human host for a lifetime.7–9 Ongoing studies will eventually determine the mechanisms or strategies utilized by the virus in combating the many cellular deterrents that are in place to thwart these infections.
To date a total of ninety genes are identified encoded by the KSHV genome,10 however, and similar to EBV, approximately 10% of these genes are expressed during latency which is quickly established after primary infection.11 The virus encodes functionally distinct genes that are involved in regulating the many cellular processes important for maintaining the integrity of the infected host. The broad ranging effects due to expression of these gene products allow the virus to overcome these blocks, which favors the resulting pathogenesis.
The KSHV encoded latency associated nuclear antigen (LANA) contributes to a number of viral functions and is expressed through the viral life cycle and typically seen as punctuate signals in the nucleus associated with the viral genome.12 LANA is essential for continued maintenance of viral episome, although some level of viral genomes can be maintained in cells knocked down for LANA.13–15 LANA can also interact with a number of functionally distinct cellular proteins modulating their activities.16,17 Importantly, LANA has also been shown to associate with tumor suppressors such as VHL, p53 and pRB important for regulation of cell survival in a hypoxic environment, prevention of apoptosis as well as deregulation of cell cycle, thus promoting oncogenesis.16,17 Additionally, LANA can also regulate critical cellular signaling pathways such as Wnt pathway causing a cell cycle dependent accumulation of GSK-3β.18,19 Interestingly, LANA can also upregulate the telomerase reverse transcriptase promoter, therefore contributing to the malignant phenotype.20 KSHV is also seen as a co-infection with HIV and/or Epstein Barr virus in the host cells.21–23 Studies from our group and others have reported that LANA can transactivate the long terminal repeat (LTR) of HIV as well as the EBV major latent, LMP1 and Cp promoters,24–26 which together contribute to the oncogenic process mediated by these tumor viruses. Specifically, these studies suggest that LANA contributes to oncogenic progression in KSHV infected cells.
Recently, we showed that LANA enhances the stability of intracellular Notch (ICN) in PEL cells.27 The Notch signaling pathway which is highly conserved in vertebrates and invertebrates has been shown to be critical for tissue development and homeostasis.28,29 A body of accumulating evidence suggests that deregulation of Notch signaling is tightly linked to oncogenesis. Furthermore, studies have shown that abnormally high expression of the intracellular activated Notch1 (ICN) is related to a subset of T-cell lymphomas.30–32 We have also shown that the accumulation of intracellular activated for of Notch (ICN) is responsible for the increased proliferation of KSHV infected PEL cells.33 Importantly, downregulation of ICN can slow the proliferation of these cells in vitro.27,33 This finding corroborates a body of other evidence demonstrating that KSHV can also seize control of this critical and conserved signaling pathway to drive oncogenesis, and that manipulation of this signaling activity may have the potential for management of KSHV related cancers.
Previous studies from our group has shown that a gamma-secretase inhibitor34 which blocks cleavage of intracellular Notch and production of ICN can dramatically reduce the proliferation of KSHV infected PEL cells in vitro.27,33 In this study, we further tested this potential in PEL-engrafted NOD/SCID mice (PEL/SCID) with the γ-secretase inhibitor DAPT, and demonstrated a significant improvement in the mean survival time (MST). In addition, γ-secretase inhibitor treatment of these mice resulted in necrosis as well as apoptosis in the tissues formed by KSHV positive PEL cells. This study may shed new light for development of new therapeutic strategies for the treatment of KSHV associated primary effusion lymphomas.
Results
Gamma secretase inhibitor (GSI) treatment of PEL/SCID mice delayed tumor growth in vivo
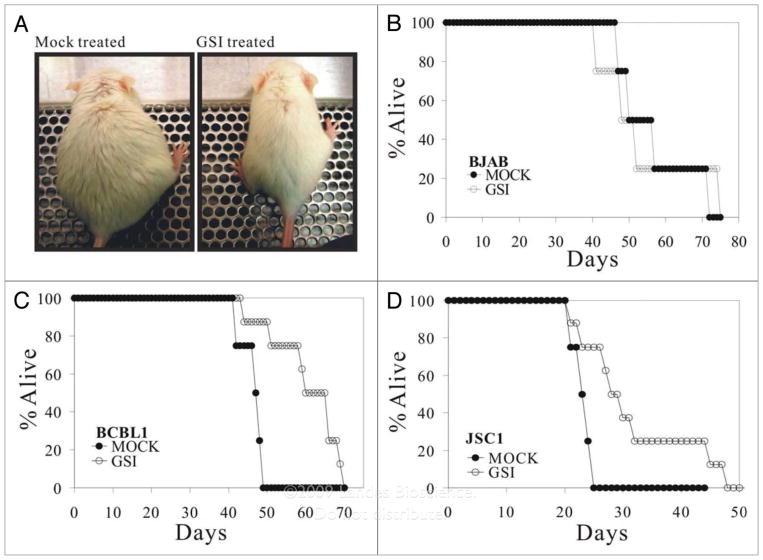
In our previous study, we showed that intracellular activated form of Notch1 (ICN) is abnormally accumulated in KSHV infected B cell lymphoma cell lines such as BCBL1, BC3 as well as JSC1.33 In addition, we also demonstrated that γ-secretase inhibitor (GSI) which blocks the production of ICN can dramatically slow the proliferation of these cell lines in vitro.33 These results suggest that manipulation of the Notch signaling pathway may be potentially useful for treatment of KSHV related B cell lymphomas. To test this possibility, we first prepared an animal model using SCID mice which allows the growth of BCBL1 and JSC1 cells in vivo. Previous studies with NOD/SCID mice showed that intraperitoneally inoculation of 1 × 107 cells results in tumor engraftment as well as ascites formation from 2 weeks to 4 weeks post inoculation.35 In this study, we randomly divided 4 weeks old mice into 3 groups, BJAB (KSHV and EBV negative), BCBL1 (KSHV positive) and JSC1 (KSHV and EBV positive, but with predominant KSHV gene expression) were inoculated intraperitoneally. 5 days post inoculation, each group was divided into two subgroups, one was mock treated, and another was treated with GSI. Interestingly, we observed that GSI treatment of PEL/SCID mice consistently delayed tumor growth in BCBL1 as well as JSC1 groups. In detail, NOD/SCID mice were inoculated with BCBL1 or JSC1 cells (1 × 107) on day 0, respectively. Daily injection of GSI (30 mg/kg) was initiated at day 5 post cell inoculation. For the BCBL1 cell transplantation group, mock-treated SCID mice receiving PBS presented with abdominal distention and were significantly heavier (Fig. 1A, left) at 25 days post cell inoculation compared to the SCID mice treated daily with GSI (Fig. 1A, right). The two mice shown are representative animals from the BCBL1 cell group. Similar results were also observed in the JSC1 group (data not shown). The results further suggest that GSI dramatically slowed the growth of these PEL cells in vivo due to the inhibition of the production of ICN. However, for BJAB cell transplantation group, there was no significant change between the mock-treated and GSI-treated mice. This can be explained as a result of the basal levels of ICN in BJAB cells are barely detected as previously shown so that GSI treatment has little or no effect for this cell line. It should be noted in both GSI and mock treated mice, that malignant ascites as well as tumor engraftment eventually developed leading to the death of the mice at different time points (Fig. 1B, C and D). To further determine if GSI can prolong the life span of mice harboring KSHV infected PEL cells, we followed the survival time of each mouse. Indeed the mean survival time (MST) of GSI treated BCBL1 and JSC1 groups were increased by 25% and 40%, respectively. Expectedly, the MST was not significantly changed between GSI treated and mock treated BJAB/SCID subgroups (Fig. 1B). The details of this study looking at tumorigenesis and MST in the different groups of mice are summarized in Table 1.
Figure 1.
Survival curves for GSI treated NOD/SCID mice inoculated with KSH V positive PE Ls. (A) Gamma secretase inhibitor (GSI) treatment of PEL/SCID mice delayed tumor growth. NOD/SCID mice were inoculated with BCBL1 cells (1 × 107) on day 0 respectively, KSH V-negative Burkitt lymphoma BJAB was used as a cell line control. Daily injection of GSI (30 mg/kg) was initiated at day 5 post cell inoculation. For BCBL1 and JSC1 cell transplantation group, mock-treated SCID mice (left) receiving DMSO presented with abdominal distention and were significantly heavier at 25 days post cell inoculation than SCID mice treated daily with GSI (right). These two mice are the representatives from BCBL1 cell group. For BJAB cell transplantation group, there is not significant change between the mock-treated and GSI-treated mice. (B, C and D) The survival curves for GSI-treated and mock-treated SCID mice. NOD/SCID mice were inoculated with BJAB (1 × 107 cells/mouse), BCBL-1 (1 × 107 cells/mouse), or JSC1 cells (1 × 107 cells/mouse). Daily i.p. injection of GSI (30 mg/kg) per mouse was initiated at 5 days post-inoculation. 8 mice were used per group. Mean survive time (MST) was increased by 25% and 40% for BCBL1/SCID, JSC1/SCID, respectively. The formula for calculating MST is as following: %MST increase = (MSTGSI treated − MSTmock)/MSTmock.
Table 1.
Tumorigenesis in NOD/SCID mice
| ID | Cell line | Mouse NO. | GSI treatment | Ascites | Tumor | Survival time (d) |
|---|---|---|---|---|---|---|
| A1 | BJAB | 1 | − | + | + | 46 |
| A2 | BJAB | 2 | − | + | + | 51 |
| A3 | BJAB | 3 | − | + | + | 56 |
| A4 | BJAB | 4 | − | + | + | 72 |
| A5 | BJAB | 5 | − | + | + | 55 |
| A6 | BJAB | 6 | − | + | + | 50 |
| A7 | BJAB | 7 | − | + | + | 71 |
| A8 | BJAB | 8 | − | + | + | 47 |
| A9 | BJAB | 1 | + | + | + | 74 |
| A10 | BJAB | 2 | + | + | + | 50 |
| A11 | BJAB | 3 | + | + | + | 42 |
| A12 | BJAB | 4 | + | + | + | 52 |
| A13 | BJAB | 5 | + | + | + | 44 |
| A14 | BJAB | 6 | + | + | + | 48 |
| A15 | BJAB | 7 | + | + | + | 53 |
| A16 | BJAB | 8 | + | + | + | 71 |
| A17 | 1 | − | + | + | 48 | |
| A18 | 2 | − | + | + | 42 | |
| A19 | 3 | − | + | + | 47 | |
| 4 | − | + | + | 42 | ||
| A21 | 5 | − | + | + | 50 | |
| 6 | − | + | + | 47 | ||
| 7 | − | + | + | 48 | ||
| 8 | − | + | + | 50 | ||
| 1 | + | + | + | 60 | ||
| 2 | + | + | + | 51 | ||
| 3 | + | + | + | 44 | ||
| 4 | + | + | + | 70 | ||
| 5 | + | + | + | 66 | ||
| 6 | + | + | + | 59 | ||
| A31 | 7 | + | + | + | 66 | |
| 8 | + | + | + | 69 | ||
| JSC1 | 1 | − | + | + | 23 | |
| JSC1 | 2 | − | + | + | 24 | |
| JSC1 | 3 | − | + | + | 21 | |
| JSC1 | 4 | − | + | + | 24 | |
| JSC1 | 5 | − | + | + | 21 | |
| JSC1 | 6 | − | + | + | 23 | |
| JSC1 | 7 | − | + | + | 25 | |
| JSC1 | 8 | − | + | + | 25 | |
| A41 | JSC1 | 1 | + | + | + | 21 |
| A42 | JSC1 | 2 | + | + | + | 26 |
| A43 | JSC1 | 3 | + | + | + | 29 |
| JSC1 | 4 | + | + | + | 27 | |
| JSC1 | 5 | + | + | + | 23 | |
| JSC1 | 6 | + | + | + | 31 | |
| A47 | JSC1 | 7 | + | + | + | 44 |
| JSC1 | 8 | + | + | + | 48 |
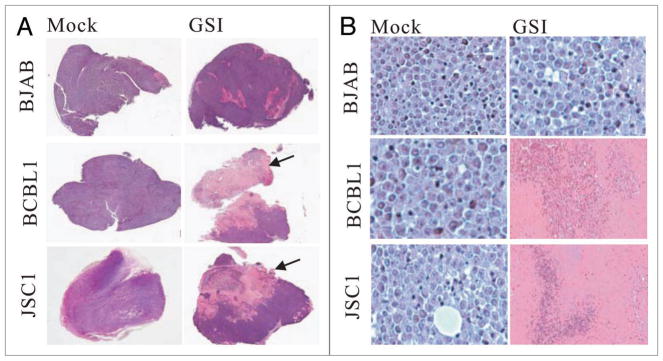
GSI treatment resulted in prominent necrosis of KSHV-positive lymphomas in vivo
The solid tumors formed in the abdominal cavities of the mice were removed for further analysis. Interestingly, under low magnification, apparent necrosis was observed in the tumor tissues from the GSI treated BCBL1/SCID as well as JSC1/SCID mice (Fig. 2A, middle and bottom, right, arrowed). In contrast, no significant necrosis was seen in the tumor tissues from mock treated BCBL1/SCID as well as JSC1/SCID mice (Fig. 2A, middle and bottom, left). We further performed histologic analysis using H&E staining and examined the tumor tissues at high magnification. The cells from the mock treated BCBL1/SCID as well as JSC1/SCID mice was healthy with no apparent necrosis, and presented a typical malignant phenotype such as abnormal big nuclear, actively dividing and uniform staining tissue (Fig. 2B, middle and bottom left). However, severe necrosis was observed in the tissues from the GSI treated BCBL1/SCID as well as JSC1/SCID mice (Fig. 2B, middle and bottom right). Remarkably, intact cellular structures were not observed within the region of necrosis with typical cell debris seen throughout the necrosis region (Fig. 2B, middle and bottom, right). Our previous study demonstrated that GSI dramatically slows the proliferation of KSHV infected PEL cells in vitro.33 This may also occur in an in vivo system and partially provides the explanation for the delayed onset of tumorigenesis and the prolonged life span in GSI treated BCBL1/SCID as well as JSC1/SCID mice. Here, necrosis of the tumor tissues as a result of GSI treatment may also be a factor which contributes to the prolonged life span of GSI treated BCBL1/SCID as well as JSC1/SCID mice. As for the control, tissues from either mock or GSI treated BJAB/SCID mice expectedly was rather healthy with little or no observed necrosis (Fig. 2A and B, top). This maybe linked to the low basal level of ICN observed in this cell line which was barely detectable. Therefore GSI was unlikely to have an effect on enhanced Notch signaling not seen in this KSHV negative cell line.
Figure 2.
GSI treatment resulted in prominent necrosis of KSHV-positive lymphomas. (A) Tumor growth post BJAB, BCBL1, JSC1 cell transplantation in PEL/SCID mice. Tumor size of BJAB cell transplantation between GSItreated and mock-treated groups had no difference, and exhibited no cell necrosis in tumor tissues; however, tumor tissues of KSHV-positive both BCBL1 and JSC1 cell transplantation with GSI-treated group showed prominent necrosis (arrowed) (HE staining, low magnification). (B) Tumor cell growth of BJAB cell transplantation with GSI-treated and mock-treated groups were good, whereas tumors of BCBL1, JSC1 cell transplantation with GSI treatment group had prominent necrosis (HE stain ×400).
GSI treatment induced apoptosis in KSHV-positive lymphomas in vivo
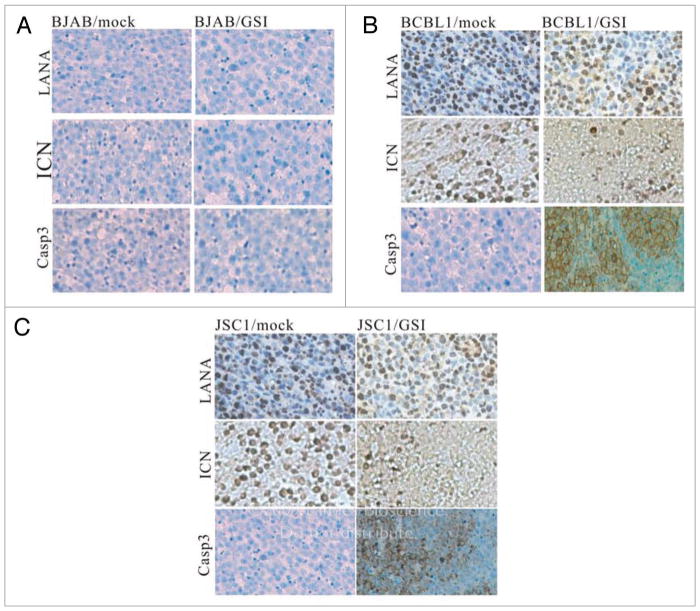
Immunohistochemistry assay (IFA) was carried out to further characterize the tumor tissues collected from the mock as well as GSI treated mice. As expected, KSHV LANA staining was negative in BJAB derived tissue but shows almost 100% strong positive in the BCBL1 as well as JSC1 derived tissue (Fig. 3A–C, top). The status of ICN expression was also determined using the ICN specific antibody Val1744 which recognizes the cleaved ICN product.33 As shown here, there is no detectable positive signal in BJAB tissue (Fig. 3A, middle). In contrast, strong immuno-staining was observed in tissues from mock treated BCBL1/SCID as well as JSC1/SCID mice (Fig. 3B and C, middle, left). More importantly, ICN staining was only observed in the section of tissue which contains relatively intact cells in the tissue removed from GSI treated BCBL1/SCID and JSC1/SCID mice (Fig. 3B and C, middle, right). However, there are little or no positive signals for ICN in the adjacent tissue which had undergone cell death or necrosis. This observation strongly suggests that blockage of ICN (i.e., inhibition of the activation of Notch signaling) not only slows down the proliferation of the KSHV infected cells in GSI treated BCBL1/SCID as well as JSC1/SCID mice but also induced cell death in vivo which was not previously seen in the in vitro cell culture.33 Clearly, this is linked to the delayed onset of tumorigenesis in these animals. Furthermore, Caspase 3 signals were also observed in tissues from GSI treated BCBL1/SCID and JSC1/SCID mice (Fig. 3B and C, bottom, right) strongly supporting our hypothesis that there were enhanced levels of apoptosis in the GSI treated KSHV positive cells.
Figure 3.
Immunohistochemical staining of tumor cells. (A) All the signals for LANA, ICN and Caspase 3 proteins in BJAB cells with GSI-treated and mock treated groups were negative. (B) LANA protein in BCBL1 cells with GSI-treated and mock-treated groups were positive. ICN protein in BCBL1 cells with mock-treated groups showed strong positivity, whereas a weak positivity was shown with the GSI-treated group. Caspase3 protein in BCBL1 cells with mock-treated group showed negative stain and was positive in BCBL1 cells from the GSI-treated group. (C) LANA protein in JSC1 cells with GSI-treated and mock-treated groups were positive. ICN protein in JSC1 cells with mock-treated groups showed strong positivity, whereas weak signals positive for ICN was seen in the GSI-treated group. Caspase3 protein in JSC1 cells with mock-treated group showed negative staining and was positive in JSC1 cells with GSI-treated group. (SP ×250).
Discussion
Notch signaling pathway is highly conserved from worm to human and plays a fundamental role in cell fate decisions including proliferation, differentiation and apoptosis.29 Numerous studies have also established a link between aberrant Notch signaling and oncogenesis.30–32,36 Previously we showed that KSHV can play a direct role in terms of inducing ICN and also usurp this signaling pathway to maintain the oncogenic phenotype of KSHV infected B lymphoma cells.33 We also demonstrated that elevated ICN functionally affect the proliferation of KSHV positive B cells in vitro.33 Moreover, we observed that ICN is critical for survival of KSHV infected primary B cells since the treatment of γ-secretase inhibitor can cause cell death for KSHV infected primary B cells in vitro.27,33 Interestingly, studies by Foreman et al. showed that the γ-secretase inhibitor can induce apoptosis in KSHV infected endothelial KS cells.37 In addition, inhibition of Notch signaling in KS tumor cells also resulted in micronucleation and mitotic arrest.38 Interestingly, a number of studies have shown that inhibition of Notch signaling by an inhibitor of γ-secretase can reduce proliferation or trigger apoptosis in a range of different cancer cells.39–42 These data suggested a potential role for ICN in KSHV mediated B cell proliferation and therefore may have a therapeutic potential for management of the Notch signaling pathway for KSHV induced B cell lymphomas.
KSHV possesses a complex series of molecular strategies to regulate cell proliferation, induce cell transformation, and to prevent cell apoptosis. Deregulation of the Notch signaling pathway is one of these strategies to promote oncogenesis. The initiative of this study is to explore the possibility that downregulation of the Notch signaling pathway can affect the growth of KSHV positive B cell lymphoma cells in vivo. In this study, we constructed PEL-engrafted NOD/SCID mice (PEL/SCID) model and tested the potential of the γ-secretase inhibitor DAPT for treatment of KSHV positive B cell lymphomas. Indeed, we demonstrated a significant improvement in the mean survival time (MST) in GSI treated mice. In addition, γ-secretase inhibitor treatment of these mice resulted in necrosis as well as apoptosis in the tissues formed by KSHV positive PEL cells. The data obtained here maybe also explained by the fact that GSI may slow the proliferation of KSHV infected B lymphoma cells in vivo, therefore prolonging the life span of treated mice. In addition, the necrosis as well as apoptosis caused by GSI may also contribute to the survival of the treated mice. These data coincides with the other results which showed that the γ-secretase inhibitor dramatically reduced proliferation of KSHV positive cells in vitro.33 However, we also noticed that GSI at the time introduced post-inoculation did not prevent the treated mice from death. This suggests that oncogenesis driven by KSHV is likely to be driven by multiple complex mechanisms which involves multiple signaling pathways. As evident from these studies manipulation of the Notch signaling pathway is not sufficient to completely eliminate KSHV induced B cell lymphomas. However, the improved survival time resulting from GSI treatment should be carefully considered as the Notch signaling pathway is clearly a potential and valuable target for therapy of KSHV related B cell lymphomas. The targeting of multiple pathways usurped by KSHV for therapeutic value may eventually lead to a successful approach in treatment of KSHV associated cancers.
In summary, this study provides strong evidence that inactivation of the Notch signaling pathway can result in the delayed onset of tumorigenesis induced by KSHV infected B cells in vivo. This delayed onset of tumorigenesis prolonged the life span of the treated mice. The observed necrosis as well as apoptosis induced by GSI may be directly related to the observed increase in survivability in the treated animals. Importantly, introduction of GSI at an earlier time post inoculation may also be an approach that may lead to further enhancement in survival. The possibility of combination approaches where multiple signaling targets (for example, the Notch and mTOR pathways) are inhibited may therefore be important to consider in developing therapeutics for KSHV associated tumors. Therefore, inhibiting the Notch signaling pathway, which is greatly enhanced in KSHV, infected cells can have a definite positive effect on the long term survival of the animals (Fig. 4). The detailed molecular mechanisms by which GSI results in necrosis as well as apoptosis of the KSHV infected PEL cells in vivo will be further explored. This in vivo study strongly supports the strong therapeutic potential of GSI in management of KSHV infected PEL and also provides a critical basis for further preclinical trials.
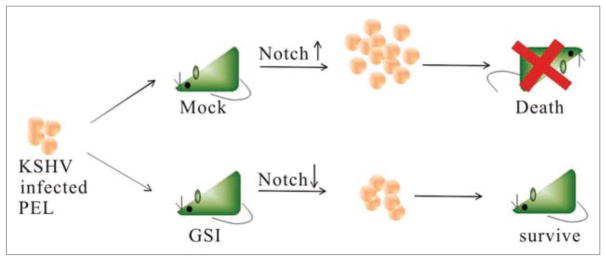
Figure 4.
Model showing GSI inhibition of PEL cells outgrowth in vivo. GSI slows down the proliferation of KSHV positive PEL cells in mice, therefore prolongs the life span of the treated mice. Mice transplanted with KSHV positive PEL cells showed an increased level of expansion of these cells in vivo resulting in death of the mice. However, mice treated with GSI which inhibits the production of ICN showed an increased survival compared to untreated mice.
Materials and Methods
Cell lines
BJAB cell line is KSHV negative and was isolated from a Burkitt’s lymphoma patient and was provided by Elliott Kieff (Harvard Medical School, Boston, MA). BCBL1 is a PEL cell line positive for KSHV infection and was obtained from Dr. Don Ganem (University of California School of Medicine, San Francisco, CA). JSC1, a kind gift from Richard F. Ambinder (Johns Hopkins University School of Medicine, Baltimore, MD), is also a PEL cell line dually infected with KSHV as well as EBV in which KSHV gene expression is predominant.
SCID mice
NOD.CB17-PrkdcScid/J (NOD/SCID) mice (Jackson Laboratory, Bar Harbor, ME) were kept under specific pathogen-free conditions at the SCID Mouse Core Facility at University of Pennsylvania, Philadelphia, PA. NOD/SCID mice were housed in micro-isolator cages, and all food, water and bedding were autoclaved prior to use. All experiments were approved by the Institutional Animal Care and Use Committee of University of Pennsylvania (IACUC protocol No.801204). Mice were weighed and monitored, and weight was used as a criterion for ascites or tumorigenesis as well as a marker for γ-secretase inhibitor efficacy studies. Animals were monitored and euthanized when signs for distress were clearly visible according to our protocol.
Engraftment of BJAB, BCBL-1 and JSC1 cells in NOD/SCID mice
BJAB, BCBL-1 or JSC1 cells were inoculated intraperitoneally (i.p.) into NOD/SCID mice. In each group, 16 mice were injected with 1 × 107 cells per mouse. Among these mice, half of them were mock treated with DMSO, another half of them were treated with the γ-secretase inhibitor (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester, DAPT, purchased from Calbiochem, Catalog No. 565770) daily. The mice were closely watched and the tissue samples were excised and fixed in 10% buffered neutral formalin, sectioned and stained with hematoxylin and eosin (H&E) for histological analysis as well as immunohistochemistry analysis as described previously43 when the animals were euthanized due to stress or death due to a dramatic increase in tumor load.
GSI treatment of PEL-engrafted NOD/SCID mice
BJAB, BCBL-1 or JSC1 cells were inoculated intraperitoneally (i.p.) into NOD/SCID mice at a dose ensuring lymphomagenesis in the animals. Daily i.p. injections of GSI/DAPT34 (1.1 mg/mice) were performed in 0.1 ml of PBS at 3 days post cell inoculation. Mock-treated mice were given daily i.p. injections of 0.5 ml PBS. Upon the death of the mice, the tumor tissue samples were collected as described as above. Individual survival times of GSI-treated and mock treated mice were averaged to calculate mean survival time (MST) of treated and mock mice for each cell line used. Percent MST increase of GSI-treated mice was calculated by using the following formula:
Significant difference between the survival times of GSI-treated and mock-treated animals was determined using single-tail Student’s t-test. Statistical significance was assumed at a p value of less than 0.05.
Acknowledgments
This work was supported by grants from the Leukemia and Lymphoma Society of America and public health service grants from the NCI CA108461, CA091792, A1067037 from the NIAID and DE017338 from the NIDCR (to ESR) and grants from the 100 Talent Program of the Chinese Academy of Sciences and Natural Science Foundation of China (30770098) to (K.L.). K.L. is a special fellow and E.S.R. is a scholar of the Leukemia and Lymphoma Society of America.
References
- 1.Boshoff C, Weiss RA. Epidemiology and pathogenesis of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:517–34. doi: 10.1098/rstb.2000.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshoff C, Whitby D, Talbot S, Weiss RA. Etiology of AIDS-related Kaposi’s sarcoma and lymphoma. Oral Dis. 1997;3:129–32. doi: 10.1111/j.1601-0825.1997.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 3.Petre CE, Dittmer DP. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int J Nanomedicine. 2007;2:277–88. [PMC free article] [PubMed] [Google Scholar]
- 4.Petre CE, Sin SH, Dittmer DP. Functional p53 signaling in Kaposi’s sarcoma-associated herpesvirus lymphomas: implications for therapy. J Virol. 2007;81:1912–22. doi: 10.1128/JVI.01757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff C. Kaposi virus scores cancer coup. Nat Med. 2003;9:261–2. doi: 10.1038/nm0303-261. [DOI] [PubMed] [Google Scholar]
- 7.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–8. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–6. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 9.Lan K, Kuppers DA, Verma SC, Sharma N, Murakami M, Robertson ES. Induction of Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J Virol. 2005;79:7453–65. doi: 10.1128/JVI.79.12.7453-7465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor JL, Bennett HN, Snyder BA, Moore PS, Chang Y. Transcriptional Analysis of Latent and Inducible Kaposi’s Sarcoma-Associated Herpesvirus Transcripts in the K4 to K7 Region. J Virol. 2005;79:15099–106. doi: 10.1128/JVI.79.24.15099-15106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–7. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma SC, Lan K, Robertson E. Structure and function of latency-associated nuclear antigen. Curr Top Microbiol Immunol. 2007;312:101–36. doi: 10.1007/978-3-540-34344-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter MA, 2nd, Subramanian C, Robertson ES. The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology. 2001;291:241–59. doi: 10.1006/viro.2001.1202. [DOI] [PubMed] [Google Scholar]
- 14.Ballestas ME, Kaye KM. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J Virol. 2001;75:3250–8. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–4. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 16.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–94. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 17.Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 2000;6:1121–7. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 18.Fujimuro M, Hayward SD. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J Virol. 2003;77:8019–30. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimuro M, Wu FY, ApRhys C, Kajumbula H, Young DB, Hayward GS, Hayward SD. A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpesvirus latency. Nat Med. 2003;9:300–6. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- 20.Knight JS, Cotter MA, 2nd, Robertson ES. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J Biol Chem. 2001;276:22971–8. doi: 10.1074/jbc.M101890200. [DOI] [PubMed] [Google Scholar]
- 21.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, et al. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae. J Virol. 1996;70:549–58. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 23.Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus- like (KSHV) DNA sequences. Blood. 1995;86:2708–14. [PubMed] [Google Scholar]
- 24.Groves AK, Cotter MA, Subramanian C, Robertson ES. The latency-associated nuclear antigen encoded by Kaposi’s sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters. J Virol. 2001;75:9446–57. doi: 10.1128/JVI.75.19.9446-9457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyun TS, Subramanian C, Cotter MA, 2nd, Thomas RA, Robertson ES. Latency-associated nuclear antigen encoded by Kaposi’s sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J Virol. 2001;75:8761–71. doi: 10.1128/JVI.75.18.8761-8771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renne R, Barry C, Dittmer D, Compitello N, Brown PO, Ganem D. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2001;75:458–68. doi: 10.1128/JVI.75.1.458-468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan K, Verma SC, Murakami M, Bajaj B, Kaul R, Robertson ES. Kaposi’s sarcoma herpesvirus-encoded latency-associated nuclear antigen stabilizes intracellular activated Notch by targeting the Sel10 protein. Proc Natl Acad Sci USA. 2007;104:16287–92. doi: 10.1073/pnas.0703508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–62. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 29.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 30.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–61. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 31.Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, Merlino G, Callahan R. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–55. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- 32.Girard L, Hanna Z, Beaulieu N, Hoemann CD, Simard C, Kozak CA, Jolicoeur P. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 1996;10:1930–44. doi: 10.1101/gad.10.15.1930. [DOI] [PubMed] [Google Scholar]
- 33.Lan K, Choudhuri T, Murakami M, Kuppers DA, Robertson ES. Intracellular activated Notch1 is critical for proliferation of Kaposi’s sarcoma-associated herpesvirus-associated B-lymphoma cell lines in vitro. J Virol. 2006;80:6411–9. doi: 10.1128/JVI.00239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezovska O, Jack C, McLean P, Aster JC, Hicks C, Xia W, et al. Aspartate mutations in presenilin and gamma-secretase inhibitors both impair notch1 proteolysis and nuclear translocation with relative preservation of notch1 signaling. J Neurochem. 2000;75:583–93. doi: 10.1046/j.1471-4159.2000.0750583.x. [DOI] [PubMed] [Google Scholar]
- 35.Picchio GR, Sabbe RE, Gulizia RJ, McGrath M, Herndier BG, Mosier DE. The KSHV/HHV8-infected BCBL-1 lymphoma line causes tumors in SCID mice but fails to transmit virus to a human peripheral blood mononuclear cell graft. Virology. 1997;238:22–9. doi: 10.1006/viro.1997.8822. [DOI] [PubMed] [Google Scholar]
- 36.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 37.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene. 2005;24:6333–44. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 38.Curry CL, Reed LL, Broude E, Golde TE, Miele L, Foreman KE. Notch inhibition in Kaposi’s sarcoma tumor cells leads to mitotic catastrophe through nuclear factor-kappaB signaling. Mol Cancer Ther. 2007;6:1983–92. doi: 10.1158/1535-7163.MCT-07-0093. [DOI] [PubMed] [Google Scholar]
- 39.Rasul S, Balasubramanian R, Filipovic A, Slade MJ, Yague E, Coombes RC. Inhibition of gamma-secretase induces G2/M arrest and triggers apoptosis in breast cancer cells. Br J Cancer. 2009;100:1879–88. doi: 10.1038/sj.bjc.6605034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Keersmaecker K, Lahortiga I, Mentens N, Folens C, Van Neste L, Bekaert S, et al. In vitro validation of gamma-secretase inhibitors alone or in combination with other anti-cancer drugs for the treatment of T-cell acute lymphoblastic leukemia. Haematologica. 2008;93:533–42. doi: 10.3324/haematol.11894. [DOI] [PubMed] [Google Scholar]
- 41.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–9. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 42.Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–7. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 43.Kaul R, Murakami M, Choudhuri T, Robertson ES. Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. J Virol. 2007;81:10352–61. doi: 10.1128/JVI.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]