Abstract
Background
Associations between manganese (Mn) and neurodevelopment may depend on dose and exposure timing, but most studies cannot measure exposure variability over time well.
Objective
We apply temporally informative tooth-matrix biomarkers to uncover windows of susceptibility in early life when Mn is associated with visual motor ability in childhood. We also explore effect modification by lead (Pb) and child sex.
Methods
Participants were drawn from the ELEMENT (Early Life Exposures in MExico and NeuroToxicology) longitudinal birth cohort studies. We reconstructed dose and timing of prenatal and early postnatal Mn and Pb exposures for 138 children by analyzing deciduous teeth using laser ablation-inductively coupled plasma-mass spectrometry. Neurodevelopment was assessed between 6–16 years of age using the Wide Range Assessment of Visual Motor Abilities (WRAVMA). Mn associations with total WRAVMA scores and subscales were estimated with multivariable generalized additive mixed models. We examined Mn interactions with Pb and child sex in stratified models.
Results
Levels of dentine Mn were highest in the second trimester and declined steeply over the prenatal period, with a slower rate of decline after birth. Mn was positively associated with visual spatial and total WRAVMA scores in the second trimester, among children with lower (<median) tooth Pb levels: one standard deviation (SD) increase in ln-transformed dentine Mn at 150 days before birth was associated with a 0.15 [95% CI: 0.04, 0.26] SD increase in total score. This positive association was not observed at high Pb levels. In contrast to the prenatal period, significant negative associations were found in the postnatal period from ~6 to 12 months of age, among boys only: one SD increase in ln-transformed dentine Mn was associated with a 0.11 [95% CI: −0.001, −0.22] to 0.16 [95% CI: −0.04, −0.28] SD decrease in visual spatial score.
Conclusions
Using tooth-matrix biomarkers with fine scale temporal profiles of exposure, we found discrete developmental windows in which Mn was associated with visual-spatial abilities. Our results suggest that Mn associations are driven in large part by exposure timing, with beneficial effects found for prenatal levels and toxic effects found for postnatal levels.
Keywords: children, windows of susceptibility, neurodevelopment, visual motor ability, teeth, tooth matrix biomarkers, manganese, lead
1. INTRODUCTION
Manganese (Mn) is an essential nutrient necessary for normal brain development (Prohaska 1987; Takeda 2003; Wedler 1993). Maintaining Mn in the correct metabolic balance is important, as excess Mn exposure critically affects brain function (Aschner 2000). In children, both excessive intake and low blood Mn levels have been associated with adverse neurodevelopmental outcomes (Chung et al. 2015; Claus Henn et al. 2010; Menezes-Filho et al. 2011; Rink et al. 2014; Takser et al. 2003; Zoni and Lucchini 2013). Active transport of Mn across the placenta suggests that it plays a critical nutrient role in fetal development, but active transport during pregnancy also occurs in the context of an incompletely formed blood-brain barrier, which may make the fetus more vulnerable to relatively small disturbances in Mn homeostasis in pregnancy (Aschner and Aschner 2005; Ballatori et al. 1987; Yoon et al. 2011). Differences in Mn homeostatic mechanisms in young children, who absorb and retain a larger fraction of ingested Mn than adults, may create additional windows of vulnerability. Interestingly, blood Mn levels in children are consistently higher than blood levels in adults, suggesting that Mn continues to play an important developmental role in the postnatal period. On the other hand, dietary Mn deficiency can adversely affect neuronal activity and energy metabolism in the brain (Prohaska 1987; Takeda 2003; Wedler 1993). The effect of Mn on neurodevelopment may depend as much on the developmental stage when higher exposure is experienced as on dose, as the body’s need for Mn appears to vary over time. This may explain why exposures prenatally have markedly different effects than those observed with postnatal exposure (Ericson et al. 2007; Gunier et al. 2015; Mora et al. 2015). There is also evidence that Mn may interact with other metals, including lead (Pb), to enhance their toxicity (Claus Henn et al. 2012; Wright et al. 2006). These findings are supported by recent animal studies where co-exposures with Pb and Mn produced effects distinct from those observed under single exposure scenarios (Betharia and Maher 2012). Furthermore, in animals, Mn exposure exerts a variable effect on visual, motor and cognitive domains of intellectual function measures, necessitating the study of domain-specific associations in humans rather than only global scores of intellectual performance (Betharia and Maher 2012; Bonilla 1984; Cordova et al. 2013; Kern et al. 2010).
A major barrier to epidemiologic studies of the neurodevelopmental effects of prenatal Mn exposure is the lack of suitable biomarkers to measure fetal uptake. Maternal blood is a strong surrogate when passive transport occurs across the placenta and when there is a high correlation between maternal blood and cord blood levels, as is the case for blood lead. Mn, however, is actively transported, and it is unknown when in pregnancy the active transport mechanism is most active, as there are no methods to safely measure both maternal blood Mn and fetal blood Mn except at birth. Given all these factors, prenatal susceptibility windows when Mn exposure may be most strongly linked to neurodevelopmental outcomes have not been identified. Prior studies have utilized primarily cord blood or serum (Claus Henn et al. 2017; Lin et al. 2013; Yu et al. 2014), maternal blood (Chung et al. 2015; Claus Henn et al. 2017; Takser et al. 2003), and maternal or infant hair (Takser et al. 2003) to characterize Mn levels during the prenatal period. However, these spot measures are prone to measurement error, capturing only a short time frame of exposure, and cannot factor in the role of active transport, which may vary by pregnancy stage, genetics, co-exposures or disease states. A tooth biomarker, however, would overcome many of these barriers because deciduous ‘baby’ teeth are formed in pregnancy and levels in teeth represent fetal exposure downstream from active placental transport. Several studies using tooth-matrix biomarkers have distinguished trimester-specific Mn levels, and reported time-specific associations with neurodevelopmental outcomes (Gunier et al. 2015; Mora et al. 2015). Recently, we have improved upon this method by developing and validating tooth Mn biomarkers that can provide fine-scale temporal profiles of exposure on a nearly weekly basis over the second and third trimesters and during early childhood (Arora et al. 2011; Arora et al. 2012; Arora and Austin 2013). These tooth-matrix methods are based on the incremental nature of tooth development that commences prenatally and proceeds into childhood (akin to growth rings in a tree). Our more recent methodology allows for identification of narrower time frames, during which Mn may influence neurodevelopment (Arora and Austin 2013; Austin et al. 2013). Given the rapid, complex, and dynamic nature of brain development, it is paramount to characterize time-specific associations in this type of detailed manner.
Importantly, analytical methods for measuring Mn and other elements in the tooth biomarker have been extensively validated (Arora et al. 2006; Arora et al. 2011; Arora et al. 2012; Arora and Austin 2013; Arora et al. 2014; Hare et al. 2011). Validation studies in animals and humans demonstrate good correlations of tooth levels with levels in the environment and in other biospecimens. For example, in rodents with lifelong Mn exposure, tooth Mn levels were significantly, positively associated with levels in blood, brain, and bone (Austin et al. 2017). In a cohort of children living in an agricultural area where Mn-containing pesticides are used, Mn levels in dentine formed during the second trimester were significantly associated with Mn levels in house dust, and levels in dentine formed close to time of birth were significantly associated with cord blood Mn levels (Arora et al. 2012). In the same cohort, dentine Mn was also significantly associated with other exposure factors such as proximity to pesticide use and having a parent who was employed in pesticide spraying (Gunier et al. 2013). Levels of Pb in teeth measured using the same approach were associated with levels in umbilical cord blood and childhood blood, demonstrating that tooth Pb levels also reflect both timing and intensity of exposure (Arora et al. 2014).
Here, we apply tooth-matrix biomarkers in a prospective pregnancy cohort study in Mexico City to uncover potential prenatal and early postnatal developmental windows of susceptibility to Mn. We estimate time-specific associations between early life Mn levels and visual-motor, visual-spatial and fine-motor ability measured by the Wide Range Assessment of Visual Motor Ability (WRAVMA). Given prior evidence (Claus Henn et al. 2012; Gunier et al. 2015; Kim et al. 2009), we also explore effect modification by sex and Pb.
2. METHODS
2.1. Study participants
Mother–child pairs in this study were drawn from four successively enrolled longitudinal birth cohort studies in Mexico City that comprise the Early Life Exposures in MExico and NeuroToxicology (ELEMENT) project. These cohorts are uniquely poised to examine the effects of Mn because (1) air pollution, of which Mn and other metals are key components, is severe in Mexico City (Calderon-Garciduenas et al. 2013); (2) rich sources of Mn in the diet, such as beans, are commonly consumed in Mexican diets; and (3) higher Mn exposure, measured in biological and environmental samples, has been reported in Mexico than in the U.S. and Canada (Santos-Burgoa et al. 2001). Detailed information on the study design and data collection procedures has been published previously (Braun et al. 2012; Ettinger et al. 2009; Gonzalez-Cossio et al. 1997; Hernandez-Avila et al. 2002). Briefly, mothers were originally recruited during pregnancy or at delivery between 1994 and 2004 to investigate the long-term consequences of prenatal environmental factors on child development (Ettinger et al. 2009; Gonzalez-Cossio et al. 1997; Tellez-Rojo et al. 2004). All four cohorts were recruited at public maternity hospitals, which serve low to moderate income populations in Mexico City. Exposure, outcome, and covariate data from all cohorts were collected in a standard manner by the same study staff, allowing us to pool data across cohorts (see Supplemental Table 1 for comparison of characteristics across cohorts). Exclusion criteria included an Apgar score of ≤6 at 5 minutes, a condition requiring treatment in a neonatal intensive care unit; serious birth defects; and maternal factors including psychiatric illness, seizures, or kidney or cardiac disease; preeclampsia (systolic BP > 140 mmHg or diastolic BP > 90 mmHg); gestational diabetes; daily consumption of alcoholic beverages; addiction to illegal drugs; continuous use of corticosteroids; and factors that could interfere with calcium metabolism. Only one child for each mother was included in this study, regardless of birth order. Of the 1,079 children born into the cohort and followed until 5 years of age, 826 (77%) returned for an additional follow-up assessment between 2008 and 2010 at 6–16 years of age. Tooth collection was incorporated into this visit. Participants were asked to bring or mail in teeth as they were naturally shed. Deciduous teeth were available for metals analysis on 138 of these children.
All participating mothers signed a written consent form and received a detailed explanation of the study intent and research procedures. In addition, at the 6- to 16-year follow-up assessment, the children signed a written assent of minor form and received a detailed explanation of the study. All participants were encouraged to ask questions about the study in order to ensure their understanding. The research protocol was approved by the Research, Ethics, and Biosafety committees of the National Institute of Public Health of Mexico and National Institute of Perinatology, and by the human subjects committees of the Harvard T.H. Chan School of Public Health, Michigan School of Public Health, and the American British Cowdray Medical Center.
2.2. Measurement of manganese in teeth by LA-ICP-MS
We analyzed teeth that were free of obvious defects such as caries and extensive tooth wear. Our approach to measuring Mn and other metals in teeth using laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) and assigning developmental times has been detailed elsewhere (Arora and Austin 2013; Austin et al. 2013; Hare et al. 2011). Briefly, we used the neonatal line (a histological feature formed in enamel and dentine at birth) and daily growth incremental markings to assign temporal information to sampling points. Dentin of incisors, the first teeth to mineralize, begins to form at approximately 3 months gestation and continues until the tooth is shed (Ash and Nelson 2003).
The laser ablation unit used was a New Wave Research NWR-193 system (Kennelec Technologies, Mitcham, Victoria, Australia) equipped with an excimer argon-fluoride laser emitting a nanosecond laser pulse with a wavelength of 193 nm, and a two volume ablation chamber. An approximately 40 cm length of Tygon® tubing (i.d. 3 mm) connected the laser ablation unit to an Agilent Technologies 8800 (Agilent Technologies Australia, Forrest Hill, Victoria, Australia) ICP-MS. The instrument was fitted with a ‘cs’ lens system for enhanced sensitivity. The system was tuned daily for sensitivity using NIST SRM 612 (trace elements in glass). Polyatomic oxide interference was evaluated and minimized by monitoring the Th+/ThO+ (m/z 232/248) ratio. Typical oxide formation was consistently under 0.3%. Using the laser we sampled 50 sampling points of 35 μm diameter in enamel and primary dentine adjacent to the enamel-dentine junction. Depending on tooth type, these 50 sampling points per tooth covered a timeline from the start of the second trimester (14 weeks gestation) through one year of age, thereby representing a sampling frequency of approximately every 10 days. The detection limit was 0.05 μg/g for each metal. Metal intensities were normalized to calcium (Ca) to control for any variations in mineral content within a tooth and between tooth type and individuals, and because there are no matrix-matched certified reference materials for dentine-Mn. Data were analyzed as 55Mn:43Ca and 208Pb:43Ca ratios.
2.3. Measurement of child neurodevelopment and potential confounders
Child neurodevelopment was assessed between 6 and 16 years of age using the Wide Range Assessment of Visual Motor Abilities (WRAVMA). The WRAVMA is an age-normed test of visual motor development that is moderately correlated with IQ (r~0.60) (Adams and Sheslow 1995). Trained study staff (three testers) administered the WRAVMA pegboard (fine motor), matching (visual spatial), and drawing (visual motor) subtests. On the pegboard task, children insert as many round pegs on a wooden board as possible within 90 seconds, using the dominant hand. On the matching task, children choose a picture that “goes best” with a stimulus picture. On the drawing task, children copy a series of line drawings arranged in order of increasing difficulty. Scores on individual subtests are reported and then combined to yield a total score of overall visual motor development. Each subtest and total scores have an expected mean of 100 and standard deviation of 15. The subscale and total scores were used as primary outcomes in our analysis. Study staff administering the WRAVMA were blinded to children’s exposure levels and were overseen by an expert neuropsychologist (LS).
Information on sociodemographic and other characteristics that could confound the relationship between Mn exposure and child neurodevelopment were collected by questionnaire at enrollment, delivery, 1 month postpartum, and/or during subsequent study visits. These characteristics included child sex, maternal characteristics (age at time of delivery, marital status, smoking during pregnancy), maternal and paternal education, and characteristics of the household. Socioeconomic status (SES) index was calculated based on the algorithm AMAI rule 13x6 from 1994, in which families are classified into six levels based on 13 questions about characteristics of the household: (1) education of the head of household; (2) number of rooms; (3) number of bathrooms with showers; (4) type of floor; (5) number of light bulbs; ownership of: (6) car; (7) hot water heater; (8) automatic washing machine; (9) videocassette recorder; (10) toaster; (11) vacuum cleaner; (12) microwave oven; and (13) personal computer (Carrasco 2002). The six levels were then collapsed into low, medium and higher SES. Maternal IQ was assessed with the Information, Comprehension, Similarities, and Block Design subscales of the Wechsler Adult Intelligence Scale, Spanish version (Wechsler 1968). Anthropometric data from the mother and newborn were gathered within 12 hr of delivery. Information on estimated gestational age at birth, based on the date of last menstrual period, and characteristics of the birth and newborn period, including birth weight and head circumference, were abstracted from medical records. Hemoglobin was measured in whole blood samples collected from children at 1- and/or 2-years of age. Following WHO guidance, we adjusted hemoglobin values for altitude by −0.8 g/dl in all participants, because they reside at ~2000 m elevation in Mexico City (WHO 2011). Anemia status (ever anemic during early childhood), was defined as having hemoglobin <11.0 g/dl, after altitude adjustment, at either 1- or 2-years of age (WHO 2011).
2.4. Statistical Analysis
We conducted standard univariate and bivariate explorations of the data and examined distributional plots for all variables. Neurodevelopment (WRAVMA) scores were approximately normally distributed and were modeled as continuous outcomes. Mn and Pb concentrations were right-skewed; therefore the data were natural log (ln)-transformed to satisfy model assumptions.
We used two approaches to uncover discrete developmental periods when tooth Mn levels were most strongly associated with WRAVMA total and subtest (visual motor, visual spatial, fine motor) scores. Initially, we averaged the fine-scale resolution tooth Mn within each subject for each of four time periods: 150 to 90 days before birth (approximately second trimester), 89 days to 1 day before birth (approximately third trimester), birth to 180 days after birth (0 to 6 months postnatal), and 181 to 365 days after birth (>6 to 12 months postnatal). We fit separate multivariable generalized additive models (GAMs) for each of the four time periods and modeled average Mn levels with penalized splines. When associations appeared linear based on visual examination, average Mn levels were also modeled as linear terms. This initial approach using Mn levels averaged over four time periods has two limitations: (1) it treats each time period separately, which fails to account for associations at other time points, and (2) it is subject to exposure misclassification given that tooth metals data are averaged over periods of time when Mn levels are changing. Relatively high correlations of average Mn levels between time windows (e.g., Spearman r = 0.7 for Mnsecond trimester and Mnthird trimester; r = 0.6 for Mn0–6months and Mn>6–12months) precluded us from fitting models for each time window adjusted for Mn levels from previous time windows (e.g., models for Mn0–6months including Mnsecond trimester and Mnthird trimester).
As a second approach, to address the limitations of the first approach, we used an adapted distributed lag-type approach that treats X (tooth metals data) as a longitudinal dependent variable and Y (WRAVMA score) as a time-invariant independent variable. The approach is valid because an estimate of association between two variables is sound no matter which is specified as response or predictor, and has the advantage of using the measured exposure concentrations directly with no further assumption about the temporal trend. Specifically, we used the following generalized additive mixed model (GAMM) to characterize associations between the fine-scale resolution tooth Mn data (not averaged) and neurodevelopment: Xi(t) = β0 + b0i + f1(t) + f2(t)Yi + f3(t)Pbi + βZZi + εit, where Xi(t) is the continuous tooth Mn measurement for subject i at time t, b0i is the random intercept for subject i, Yi is the continuous WRAVMA score for subject i, Pbi is the continuous tooth Pb measurement for subject i, Zi is the vector of covariates, and f1(.), f2(.), and f3(.) are smooth functions of the data, e.g., f3(.) is a smooth function of the fine-scale resolution tooth Pb levels (Chen et al. 2015). The model allows for subject-specific random intercepts to account for non-independence of the repeated tooth Mn measurements for each subject, and to allow for subject-specific baseline Mn levels. Additional details are available in previously reported applications for identifying time windows of susceptibility (Chen et al. 2015; Sanchez et al. 2011). For these models, we standardized neurodevelopment scores as well as (natural log-transformed) tooth metal concentrations by dividing by their standard deviations to obtain a common variance. We report results as standardized coefficients; therefore, output of this model can be interpreted as a correlation between tooth Mn levels and neurodevelopment. Smoothly-varying standardized coefficients (f2(t)) and 95% confidence intervals over time are plotted, and a sensitive window is identified when the estimated confidence intervals on the correlation between exposure and outcome do not include zero.
Covariates that are known predictors of neurodevelopment or strong potential confounders of the Mn-neurodevelopment association were included a priori in regression models, based on previous literature (Claus Henn et al. 2010; Mora et al. 2015) and on biologic plausibility. In all models, we included child sex, tooth Pb levels (allowed to vary smoothly over time), maternal IQ, maternal education (years of school), and study cohort. WRAVMA scores are age-normed; therefore, child age at time of WRAVMA assessment was not included as a covariate in the models. We controlled for study cohort because cognitive test examiners and childhood blood Pb levels varied across cohorts (Schnaas et al. 2004). We additionally considered the following potential confounders in sensitivity analyses, but did not include them in main models because they may lie on the causal pathway between prenatal Mn and childhood neurodevelopment (e.g. gestational age, birth weight) and/or because they did not materially change the Mn associations: maternal age at delivery and anemia status (ever anemic during early childhood).
Given previous evidence of Mn-Pb interactions (Betharia and Maher 2012; Claus Henn et al. 2012) and the potential for Pb to modify Mn associations, we stratified analyses by Pb at the median of the distribution of average tooth Pb levels (i.e., median of mean values for each subject over entire prenatal and postnatal assessment periods). Recent literature also suggests possible sex-specific Mn associations with neurodevelopment (Bauer et al. 2017; Chiu et al. 2017; Gunier et al. 2015; Menezes-Filho et al. 2011). Therefore, separately, we explored whether Mn associations differed for girls and boys in our cohort using sex-stratified models.
Some participants were missing data on key potential confounders (e.g., maternal IQ, maternal education). In sensitivity analyses, we therefore multiply imputed missing values using chained equations with the MI procedure in SAS (SAS Institute, Inc., Cary, NC, USA) (van Buuren 2007; White et al. 2011). We assumed data were missing at random and generated 20 imputed datasets. We included all exposure, outcome, and potential confounder data thought to be related to the process causing missing data (see Supplemental Table 2). We combined results from models fit with the multiply imputed datasets averaging the results of the 20 imputations to give the final effect estimates for sensitivity analyses. We calculated standard errors using methods that combine the within- and between-imputation uncertainty (Rubin 2004).
We identified outliers of log-dentine Mn (8 measurements on n=6 participants) using the generalized Extreme Studentized Deviate Many-Outlier procedure (Rosner 1983). Results with and without these participants were compared in sensitivity analyses. We also conducted a sensitivity analysis excluding premature (<37 weeks gestation) or low weight (<2500 g) births (n=10).
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA) and R version 3.3.2 (The R Foundation for Statistical Computing, www.r-project.org).
3. RESULTS
3.1. Sociodemographic characteristics, neurodevelopment scores, and tooth metals levels
A total of 138 children had available neurodevelopment scores and tooth metals data and were included in analyses. Mothers who participated in this study were mostly under 30 years of age (65.6%), had completed high school (55.4%), and reported not smoking during pregnancy (98.3%) (Table 1). A majority of the children were girls (53.6%), and few were born preterm (less than 37 weeks gestation, 5.9%) or had low birth weight (less than 2500 g, 3.0%). A substantial portion of the children had experienced iron deficiency anemia in the first two years of life (57.5%), based on their hemoglobin levels. The median age at WRAVMA testing was 8 years, with a majority (81.2%) completing the test between 7 and 9 years of age. The mean (±SD) WRAVMA total score was 98.0 (±10.6); visual motor, 98.0 (±11.3); visual spatial, 94.9 (±13.4) and fine motor, 104.0 (±13.7). WRAVMA total scores were significantly correlated with visual motor, visual spatial, and fine motor scores (Spearman r=0.6, p<0.001 for each pair), but subtest scores were not highly correlated with each other (e.g., visual motor and fine motor, r=0.2, p=0.05; visual motor and visual spatial, r=0.01, p=0.9). For most characteristics, children included in analyses were similar to those excluded due to missing tooth metals data (Table 1).
Table 1.
Sociodemographic characteristics of participants included in analysis (n=138)a and excluded from analysis due to missing tooth metals data (n=688)
| Included in analysis | Excluded from analysis | |||
|---|---|---|---|---|
|
| ||||
| N | Mean ± SD or % | N | Mean ± SD or % | |
| Child sex: | ||||
| Male | 64 | 46.4% | 355 | 51.6% |
| Female | 74 | 53.6% | 333 | 48.4% |
| Gestational age at birth (weeks) | 135 | 38.9 ± 1.6 | 685 | 38.9 ± 1.5 |
| Preterm birth (<37 weeks gestation) | 8 | 5.9% | 42 | 6.1% |
| Birth weight (kg) | 135 | 3.2 ± 0.4 | 687 | 3.1 ± 0.4 |
| Low birth weight (<2500 g) | 4 | 3.0% | 44 | 6.4% |
| First child: | ||||
| Yes | 19 | 17.9% | 3 | 0.5% |
| No | 87 | 82.1% | 639 | 99.5% |
| Maternal age at delivery (yrs) | 131 | 28.6 ± 5.8 | 686 | 28.9 ± 5.9 |
| <30 yrs old | 86 | 65.6% | 372 | 54.2% |
| Maternal education (yrs school) | 130 | 11.2 ± 3.0 | 685 | 9.0 ± 3.9 |
| ≥12 yrs school | 72 | 55.4% | 218 | 31.8% |
| Maternal IQ score | 120 | 93.6 ± 20.0 | 628 | 88.1 ± 22.2 |
| Family SES: | ||||
| Low | 65 | 55.1% | 188 | 54.0% |
| Medium | 50 | 42.4% | 151 | 43.4% |
| High | 3 | 2.5% | 9 | 2.6% |
| Maternal smoking during pregnancy: | ||||
| Yes | 2 | 1.7% | 15 | 4.5% |
| No | 115 | 98.3% | 320 | 95.5% |
| Hemoglobin at 1 yr of age | 95 | 12.4 ± 1.4 | 380 | 12.0 ± 1.3 |
| Hemoglobin at 2 yrs of age | 111 | 13.2 ± 1.1 | 537 | 12.7 ± 1.2 |
| Anemic at 1 or 2 years of ageb: | ||||
| Yes (hemoglobin ever <11.0 g/dl) | 73 | 57.5% | 429 | 73.0% |
| No (hemoglobin never <11.0 g/dl) | 54 | 42.5% | 159 | 27.0% |
| Study cohort (years of child birth): | ||||
| 1 (1994–1995) | 2 | 1.4% | 202 | 29.4% |
| 2A (1997–2002) | 19 | 13.8% | 243 | 35.3% |
| 2B (1998–2001) | 33 | 23.9% | 111 | 16.1% |
| 3 (2001–2006) | 84 | 60.9% | 132 | 19.2% |
| Age at WRAVMA testing (yrs) | 138 | 8.0 ± 1.4 | 688 | 10.6 ± 2.6 |
| WRAVMA score: Total | 138 | 98.0 ± 10.6 | 688 | 98.1 ± 13.1 |
| WRAVMA score: Visual motor | 138 | 98.0 ± 11.3 | 688 | 96.8 ± 12.9 |
| WRAVMA score: Visual spatial | 138 | 94.9 ± 13.4 | 688 | 94.2 ± 14.7 |
| WRAVMA score: Fine motor | 138 | 104.0 ± 13.7 | 688 | 103.0 ± 13.0 |
Missing data for some covariates: birth weight (n=3), gestational age (n=3), first child (n=32), mother’s age at delivery (n=7), mother’s education (n=8), mother’s IQ (n=18), SES (n=20), mother smoked during pregnancy (n=21), hemoglobin at 1 yr (n=43), hemoglobin at 2 yrs (n=27), anemic (n=11).
Anemic defined by WHO for children 6–59 months of age as hemoglobin <11.0 g/dl, after altitude adjustment. Hemoglobin levels in participants were adjusted for altitude by −0.8 g/dl (participants reside at ~2000 m elevation in Mexico City) to define anemia status.
Ref: http://www.who.int/vmnis/indicators/haemoglobin.pdf, accessed May 2016
The overall median (25th to 75th percentile) Mn level (as 55Mn:43Ca) across all time points was 1.2E-3 (6.8E-4 to 2.0E-3). Levels of Mn (as ln 55Mn:43Ca) were highest in the second trimester and declined steeply over the prenatal period. Levels continued to decline after birth but at a slower rate (Figure 1A). The median Mn level (original scale) at 120 (± 7) days before birth was 3.2E-3 55Mn:43Ca (range: 4.6E-4 to 8.1E-3); at birth (± 3 days) was 1.1E-3 (range: 1.7E-4 to 5.1E-3); at 6 months (i.e., 180 ± 7 days) postnatally was 6.9E-4 (range: 2.0E-4 to 2.0E-3); and at 1 year (i.e., 365 ± 7 days) was 6.4E-4 (range: 1.1E-5 to 1.3E-3). In contrast, tooth-Pb levels (as 208Pb:43Ca) were steadier over time, with slightly increasing levels after birth (Figure 1B). Ln-Mn levels at birth (± 3 days) were approximately 60% and 22% lower among children in cohorts 1 and 2B, respectively, than children in cohort 3 (% change calculated as: (eβ−1)*100; cohort 1 vs. 3: β = −0.92 [95% CI: −1.70, −0.14]; cohort 2B vs. 3: β = −0.25 [95% CI: −0.48, −0.03]) (Table 2), although these estimates are based on limited numbers of observations (n=2, n=33, respectively). Tooth-Mn was not associated with sex, gestational age, birth weight, anemia status, maternal age at delivery, maternal education, or maternal IQ. Tooth-Pb levels at birth (± 3 days) were positively associated with maternal age at delivery (β = 0.03 [95% CI: 0.001, 0.05]), and were approximately 50% higher among children in cohort 2B compared to cohort 3 (β = 0.41 [95% CI: 0.10, 0.73]) (Table 3).
Figure 1.
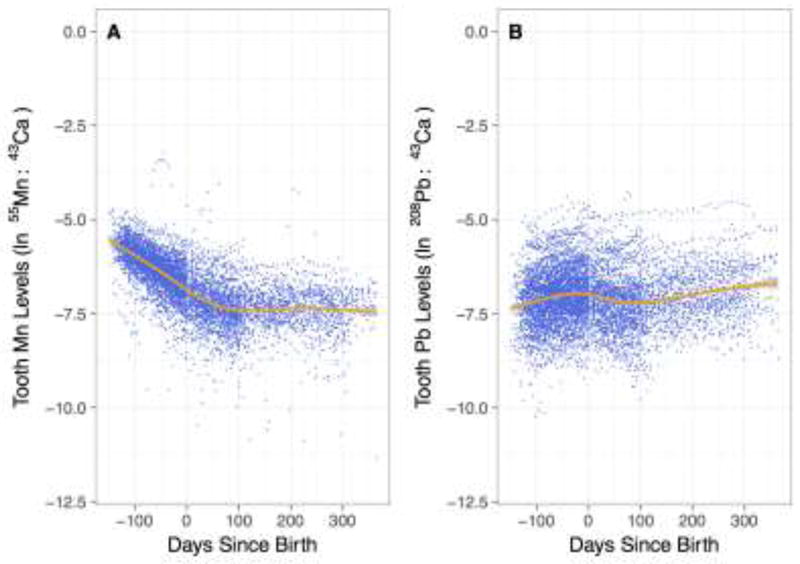
Tooth metal levels from early second trimester (150 days before birth) to one year of age (365 days after birth) for (A) Mn, as ln 55Mn:43Ca ratio, and (B) Pb, as ln 208Pb:43Ca ratio. Dots represent individual tooth metal measurements for 138 participants, with approximately 50 measurements per participant. Line represents Loess smoother.
Table 2.
Unadjusted associations between covariates and tooth Mn (ln 55Mn:43Ca) around time of birtha
| N | Beta (95% CI) | |
|---|---|---|
| Child sex, female vs. male | 138 | 0.08 (−0.11, 0.27) |
| Gestational age at birth (wks) | 135 | −0.01 (−0.07, 0.05) |
| Preterm birth (<37 wks gestation) vs. normal | 135 | 0.07 (−0.34, 0.47) |
| Birth weight (g) | 135 | 0.16 (−0.07, 0.38) |
| Mother’s age at delivery (yrs) | 131 | 0.01 (−0.01, 0.03) |
| ≥30 yrs old vs. <30 | 131 | 0.02 (−0.19, 0.23) |
| Mother’s education, <12 yrs school vs. ≥12 yrs | 130 | 0.09 (−0.10, 0.28) |
| Mother’s IQ score | 120 | −0.001 (−0.007, 0.004) |
| Mother smoked during pregnancy, yes vs. no | 117 | −0.77 (−1.58, 0.04) |
| Hemoglobin at 1 yr of age (g/dl) | 95 | −0.05 (−0.13, 0.03) |
| Hemoglobin at 2 yrs of age (g/dl) | 111 | 0.001 (−0.09, 0.10) |
| Anemic at 1 or 2 years of age, yes vs. no | 127 | 0.14 (−0.06, 0.34) |
| Study cohort (years of child birth) | ||
| 1 (1994–1995) vs. 3 (2001–2006) | 2 | −0.92 (−1.70, −0.14) |
| 2A (1997–2002) vs. 3 (2001–2006) | 19 | −0.17 (−0.45, 0.11) |
| 2B (1998–2001) vs. 3 (2001–2006) | 33 | −0.25 (−0.48, −0.03) |
Around time of birth defined as birth ± 3 days
Table 3.
Unadjusted associations between covariates and tooth Pb (ln 208Pb:43Ca) around time of birtha
| N | Beta (95% CI) | |
|---|---|---|
| Child sex, female vs. male | 138 | −0.01 (−0.28, 0.26) |
| Gestational age at birth (wks) | 135 | 0.04 (−0.04, 0.13) |
| Preterm birth (<37 wks gestation) vs. normal | 135 | −0.35 (−0.93, 0.23) |
| Birth weight (g) | 135 | 0.26 (−0.05, 0.58) |
| Mother’s age at birth (yrs) | 131 | 0.03 (0.001, 0.05) |
| ≥30 yrs old vs. <30 | 131 | 0.18 (−0.12, 0.47) |
| Mother’s education, <12 yrs school vs. ≥12 yrs | 130 | 0.08 (−0.20, 0.36) |
| Mother’s IQ score | 120 | 0.0004 (−0.007, 0.008) |
| Mother smoked during pregnancy, yes vs. no | 117 | −0.43 (−1.62, 0.75) |
| Hemoglobin at 1 yr of age (g/dl) | 95 | 0.06 (−0.06, 0.19) |
| Hemoglobin at 2 yrs of age (g/dl) | 111 | −0.06 (−0.19, 0.07) |
| Anemic at 1 or 2 years of age, yes vs. no | 127 | −0.02 (−0.31, 0.26) |
| Study cohort (years of child birth) | ||
| 1 (1994–1995) vs. 3 (2001–2006) | 2 | 0.09 (−1.02, 1.20) |
| 2A (1997–2002) vs. 3 (2001–2006) | 19 | 0.38 (−0.01, 0.77) |
| 2B (1998–2001) vs. 3 (2001–2006) | 33 | 0.41 (0.10, 0.73) |
Around time of birth defined as birth ± 3 days
3.2. Critical windows of neurodevelopmental susceptibility to Mn exposure
With our first approach, using models with Mn levels averaged over each of four time periods, we observed no significant associations for any time period with the WRAVMA total or subtest scores, after adjusting for child age, sex, tooth Pb levels, maternal IQ, maternal education, and study cohort (Figure 2 for total scores; Supplemental Figure 1 for subtests). Slopes were generally weakly positive or null for Mn measurements averaged across the second and third trimesters, and weakly negative for measurements averaged across the postnatal periods, with the exception of the fine motor subtest (associations with total score: second trimester, β = 3.5 [95% CI: −1.6, 8.7]; third trimester, β = 1.6 [95% CI: −3.2, 6.4]; 0 to 6 months, β = −0.5 [95% CI: −4.9, 3.9]; 6 to 12 months, β = −0.6 [95% CI: −6.7, 5.6]).
Figure 2.
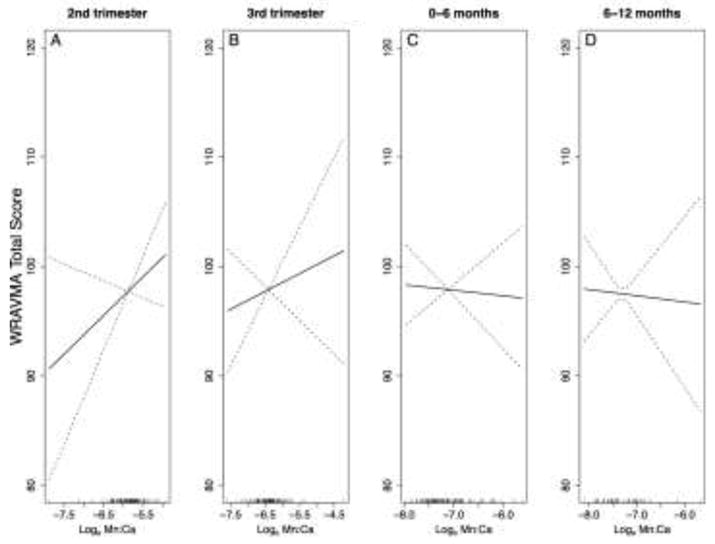
Smoothed associations between tooth Mn levels, averaged over each of four time periods, and child WRAVMA total score. Generalized additive models with penalized splines (constrained to 4 knots), adjusted for child sex, tooth Pb levels (centered at mean of distribution), maternal IQ, maternal education, and study cohort. Models use complete case method. (A) Second trimester, n=104, pGAM=0.18; (B) Third trimester, n=114, pGAM=0.50; (C) Birth to 6 months, n=114, pGAM=0.82; (D) Six to 12 months, n=56, pGAM=0.85.
With our second approach, using the adapted distributed lag-type method described above, results for Mn associations were consistent with results from our initial approach (i.e., using Mn levels averaged over each of four time periods): positive associations, indicated by standardized coefficients above zero, were estimated during the prenatal period, and negative correlations, suggesting an adverse Mn effect, were estimated mostly during the postnatal period (Figure 3). However, no statistically significant developmental window of susceptibility was identified, as confidence intervals all included zero.
Figure 3.
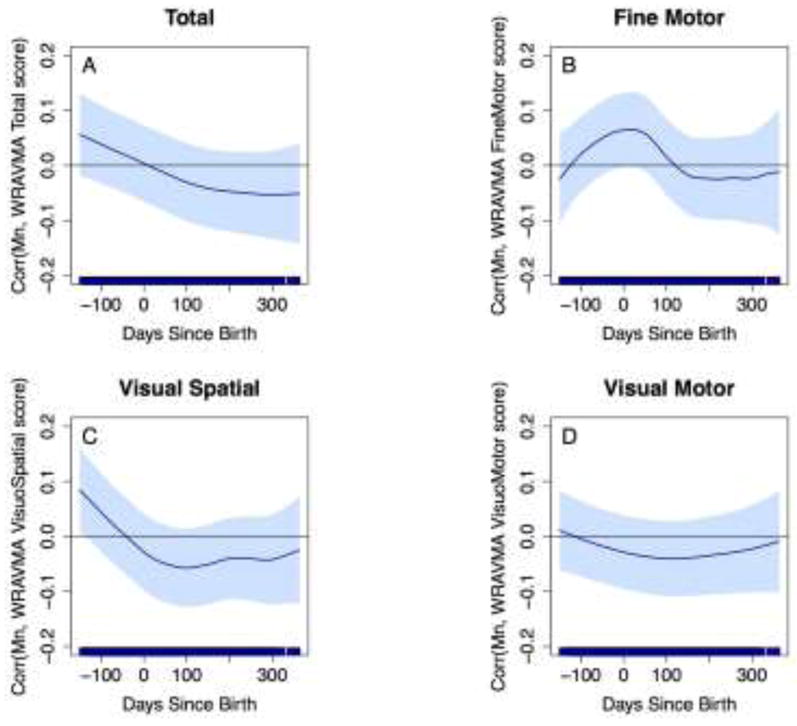
Associations between fine-scale resolution tooth Mn levels over time and WRAVMA scores. GAMMs using adapted distributed lag-type approach, adjusted for child sex, tooth Pb levels, maternal IQ, maternal education, and study cohort. Models use complete case method. Dark blue line represents association over time; light blue shading represents 95% confidence intervals. Thin black line indicates correlation = 0. (A) Total score; (B) Fine motor subtest score; (C) Visual spatial subtest score; (D) Visual motor subtest score.
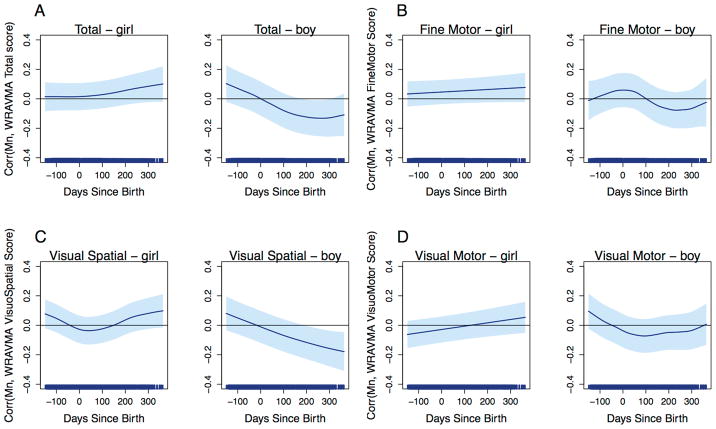
When we stratified models by tooth Pb, however, we observed significant associations of tooth Mn levels with WRAVMA total and visual spatial subtest scores, among children with low tooth Pb levels (Figures 4A, 4C). At low Pb, higher tooth Mn in the second trimester was associated with better performance on visual motor ability. Significant positive associations were observed with total WRAVMA scores during the exposure window from 150 to 79 days before birth: at 150 days before birth, a one unit increase in SD of ln-transformed dentine Mn was associated with a 0.15 (95% CI: 0.04, 0.26) increase in SD of WRAVMA total score; at 79 days before birth, a one unit increase in SD of ln-transformed dentine Mn was associated with a 0.10 (95% CI: 0.0002, 0.21) increase in SD of WRAVMA total score. For visual spatial scores, we also found positive associations during the second trimester, with significant associations from 150 to 92 days before birth (150 days before birth: β=0.17 [95% CI: 0.07, 0.28]; 92 days before birth: β=0.10 [95% CI: 0.0003, 0.19]). A similar pattern was observed with fine motor scores, where increasing prenatal Mn levels were associated with better performance in children with low Pb levels, although associations did not reach statistical significance (Figure 4B). In contrast, the pattern with visual motor scores was different from the other three outcomes, with suggestive positive associations in the postnatal period (Figure 4D). Among children with high tooth Pb levels, there were no significant Mn associations with any WRAVMA outcomes.
Figure 4.
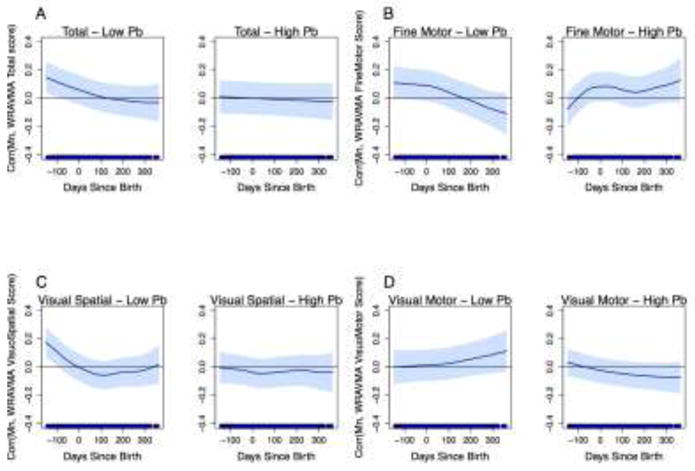
Associations between fine-scale resolution tooth Mn levels and WRAVMA scores, stratified by tooth Pb levels at median. GAMMs using adapted distributed lag-type approach, adjusted for child sex, tooth Pb levels, maternal IQ, maternal education, and study cohort. Models use complete case method. Dark blue line represents association over time; light blue shading represents 95% confidence intervals. Thin black line indicates correlation = 0. (A) Total score; (B) Fine motor subtest score; (C) Visual spatial subtest score; (D) Visual motor subtest score.
When we stratified models by child sex, we estimated weak positive correlations in the prenatal period, followed by significant negative correlations during the postnatal period among boys for both WRAVMA total and visual spatial subtest scores. A critical developmental window was identified from 187 to 365 postnatal days, corresponding to ~6 months to 1 year of age, for the association between Mn and visual spatial scores among boys (187 days after birth: β=−0.11 [95% CI: −0.0001, −0.22]; 365 days after birth: β=−0.16 [95% CI: −0.04, −0.28]). Similarly, for total scores, a critical window was identified from ~6 to 10 months of age (i.e., 176 to 309 postnatal days) (Figure 5). A unit increase in SD of ln-transformed dentine Mn was associated with a 0.13 [95% CI: −0.25, −0.01] decrease in SD of total WRAVMA score at 274 days after birth. No significant association was observed for fine motor or visual motor subtests among boys, and no significant association was observed among girls for any outcome.
Figure 5.
Associations between fine-scale resolution tooth Mn levels and WRAVMA scores, stratified by child sex. GAMMs using adapted distributed lag-type approach, adjusted for tooth Pb levels, maternal IQ, maternal education, and study cohort. Models use complete case method. Dark blue line represents association over time; light blue shading represents 95% confidence intervals. Thin black line indicates correlation = 0. (A) Total score; (B) Fine motor subtest score; (C) Visual spatial subtest score; (D) Visual motor subtest score.
In sensitivity analyses, we ran models with multiply imputed covariate data, and results were similar to models using complete data (Supplemental Figure 2). Models excluding outliers of dentine Mn (n=6) were similar to models with all data, although Mn correlations in the postnatal period among children with low Pb were more negative for all outcomes (Supplemental Figure 3). Similarly, in models excluding premature or low weight births (n=10), stronger negative correlations for Mn were estimated in the postnatal period among low Pb children, in particular for total and visual motor scores (Supplemental Figure 4).
4. DISCUSSION
Manganese is an essential nutrient that has clear neurotoxic properties with excess exposure. Increasing evidence supports that both deficiency and excess Mn levels are associated with adverse neurodevelopmental outcomes (Chung et al. 2015; Claus Henn et al. 2010; Gunier et al. 2015; Kordas et al. 2015; Lin et al. 2013; Mora et al. 2015; Ode et al. 2015; Rodriguez-Barranco et al. 2013; Yu et al. 2014). In particular, both higher and insufficiently low blood Mn levels, suggestive of an imbalance in Mn homeostasis, are being associated with poorer developmental test scores (Chung et al. 2015; Claus Henn et al. 2010; Lin et al. 2013; Ode et al. 2015; Yu et al. 2014). The weight of the evidence suggests that the biological role of Mn in brain development is highly complex and that maintaining homeostasis is crucial.
Emerging evidence suggests that effects of Mn are dependent on the developmental age at which exposures occur (Claus Henn et al. 2010; Gunier et al. 2015; Lin et al. 2013; Mora et al. 2015). Proper homeostatic regulation can be as time dependent as environmental exposures, as blood Mn has well-known life stage dependencies, with higher levels occurring in infancy/childhood than in adulthood. Because of the complex interaction of Mn with brain function, it has been challenging to disentangle the dose and time periods when Mn is beneficial for neurodevelopment versus when Mn exposure may lead to neurodevelopmental decrements. Furthermore, the effects of Mn may be modified by co-exposure to other metals, with both human and animal studies showing effect modification between concurrent Pb and Mn levels on neurobehavioral outcomes (Betharia and Maher 2012; Claus Henn et al. 2012). Our tooth biomarker enables us to examine time-dependent associations of prenatal Mn with health outcomes, and has the advantage of being a direct measure of fetal levels, rather than a surrogate like maternal blood. Furthermore, because the tooth biomarker measures Mn levels repeatedly over time, the shifts in homeostasis (i.e. decreases or increases in levels due to developmental life stage) are captured, allowing us to determine the role of Mn levels in predicting subsequent neurodevelopment. Here, we combined tooth-matrix biomarkers, which provide fine scale temporal profiles of Mn levels over the prenatal and early childhood periods, with recently developed statistical methods (adapted distributed lag-type approach) to study developmental stages when Mn levels are significantly associated with fine-motor, visual-motor and visual-spatial ability (as measured by the WRAVMA test) in Mexican children.
We observed a complex relationship between Mn exposure in early life and neurodevelopment, which was dependent on developmental stage, level of Pb exposure, and child sex. Small but significant associations were estimated for narrow time windows, and the findings overall suggest a potential shift over time in the direction of the Mn association with visual motor ability scores, from positive associations during the second and third trimesters of pregnancy to weak negative associations during the early postnatal period. The positive association of prenatal tooth Mn levels with visual motor ability scores was most evident in the visual spatial subtest among low Pb children, over a narrow developmental time window from 150 days before birth (approximately gestational week 17) to birth. This significant positive correlation in the prenatal period was not observed among children with high Pb, suggesting that in the presence of higher Pb levels, the benefit of prenatal Mn is dampened. Finally, negative associations estimated in the postnatal period were significant in boys only, suggesting a sexually dimorphic Mn association with visual motor ability.
Our finding of a positive Mn association in the prenatal period with visual motor ability, particularly visual spatial ability, is consistent with Mn being an essential element critical for healthy brain development. Mn may be in greatest demand during the prenatal period when developmental processes of neuronal proliferation and migration are at their peak (Tau and Peterson 2010). The positive Mn correlation we observed in the prenatal period among only low Pb exposed children suggests that higher Pb levels diminish the beneficial Mn effects, thereby modifying the Mn association. Interactions between Mn and Pb have been reported previously (Betharia and Maher 2012; Claus Henn et al. 2012; Kim et al. 2009) and may be related to competitive binding to receptors (e.g., divalent metal transporter [DMT1], transferrin receptor [TfR]) or altered distribution of metals in the body including in target organs such as the brain (Betharia and Maher 2012; Shukla and Chandra 1987). While prenatal Mn levels appear to be positively associated with visual motor ability, Mn benefits could, in the presence of neurotoxic co-exposures such as Pb, be overwhelmed or masked.
In contrast to the in utero period, early postnatal Mn levels were associated with poorer visual spatial ability, particularly among boys. In the postnatal period, the placenta is no longer controlling or regulating Mn levels that are transported to the fetus. Instead, direct exposure to the young child can occur, and with potentially underdeveloped homeostatic mechanisms and a lower need for Mn than during the prenatal period, the developing central nervous system in early postnatal life may be more sensitive to excess Mn levels. Evidence from our study suggests that visual spatial ability, in particular, may be more sensitive to increasing Mn levels than fine motor function, among boys. Mn appears to target the basal ganglia, a brain region whose primary functions are to control voluntary and fine motor movements as well as cognition and emotion, but basal ganglia dysfunction has also been associated with altered visual perception (Middleton and Strick 1996).
Sex-specific Mn associations have also been reported previously in epidemiologic studies, but results are inconsistent. Sexual dimorphism in the association between Mn and neurodevelopment may be related to biological differences in neurochemistry and hormone activity (Ngun et al. 2011), such as testosterone, which has been shown to improve visuospatial performance in animals (Lund and Lephart 2001) and humans (Mueller et al. 2008). Animal studies have demonstrated sex differences in the accumulation of Mn in body tissues (Dorman et al. 2004; Yamagata et al. 2017), neuron morphology (Madison et al. 2011), and performance on tests of behavior and motor function (Yamagata et al. 2017).
Our findings have some consistencies with prior studies. A similar shift in the direction of the Mn association with neurodevelopmental outcomes between the prenatal and postnatal periods has been reported previously (Gunier et al. 2015). Among 197 children residing in an agricultural area of California, significant negative associations were reported between postnatal, but not prenatal, Mn levels in tooth dentine with mental and psychomotor development scores on the Bayley Scales of Infant Development-Second Edition (BSID) at 6 and 12 months of age, controlling for prenatal Mn (Gunier et al. 2015). Although this study did not observe significant beneficial associations of prenatal Mn, as we did in our study, findings are generally consistent with ours: results suggest that Mn in the postnatal period adversely impacts brain development, but that in utero Mn may play a different, potentially beneficial, role during fetal development. This is congruous with prior evidence of up-regulated maternal Mn absorption as a response to high fetal demand for Mn to support healthy and rapid growth and development in the prenatal period (Mistry and Williams 2011; NAS 2001).
In the same cohort as Gunier et al. (2015) but including 248 school-age children, positive associations were estimated for prenatal dentine Mn with scores on tests of cognition, motor function, and visuospatial memory (Mora et al. 2015). These results are consistent with our findings, although associations in the California study were observed among boys only. In contrast to our postnatal results, however, Mora et al. reported that higher postnatal dentine Mn was associated with better WRAVMA fine motor scores, the only WRAVMA subtest administered, among boys, although this was only for the non-dominant hand. No significant associations were observed for the dominant hand, which is the hand used in our study. In the California study, results from Pb-stratified analyses varied by outcome, but some Mn associations (e.g., with behavior) were only apparent in children exposed to lower prenatal Pb levels. The primary source of Mn exposure in the California studies was Mn-containing fungicides applied to agriculture, while in our Mexico City cohort, primary sources have not been identified; exposures likely occur from a combination of dietary and airborne sources. The only other study of neurodevelopment that measured Mn in the prenatal and postnatal periods used enamel to obtain an exposure estimate at approximately gestational week 20 and at 7 months postnatal (Ericson et al. 2007). At both time points, higher Mn levels were associated with more behavioral problems (impulsivity, externalizing and attention problems) among 27 U.S. school-age children. Of note, the aforementioned studies employed different approaches for estimating Mn levels in teeth: Ericson et al. (2007) used enamel, capturing only snapshots at two distinct times; Gunier et al. (2015) and Mora et al. (2015), similar to our study, sampled dentine, but used an earlier technology to measure Mn in the tooth-matrix biomarker that was integrated over longer time periods to derive a single prenatal or postnatal measurement, and thus may not have captured time dependent effects with the high resolution that we used here.
Associations of Mn with neurobehavior have also been reported in longitudinal studies using umbilical cord blood and childhood blood Mn measures (Claus Henn et al. 2012; Lin et al. 2013). As previously noted, it is important to consider that associations of maternal pregnancy or cord blood Mn with children’s neurodevelopment may not be directly comparable to associations observed with prenatal dentine Mn biomarkers, as Mn is actively transported across the placenta. The degree of active transport may vary by pregnancy stage, disease state, genetics, or co-exposures. Thus, maternal blood Mn likely has considerably more error than teeth when used as a surrogate for fetal Mn levels. The decline in dentine Mn from the second trimester to birth, as we have shown here, is in contrast to the rise in maternal blood Mn during pregnancy (Takser et al. 2003). While the physiological mechanisms that result in this discrepancy between maternal and fetal Mn uptake during pregnancy are not completely understood, it is possible that mothers with high levels of Mn during pregnancy do not necessarily reflect higher fetal exposure; in fact, the converse might be true. For example, in a study that collected umbilical cord and maternal blood at delivery, Mn levels in cord blood increased linearly with increases in maternal blood to a level of 40 μg/L Mn in maternal blood, but then leveled off or even declined with increasing maternal Mn levels (Claus Henn et al. 2017).
Studies that have dosed animals with Mn alone have shown that higher exposure to Mn during early life, including the postnatal period, results in motor coordination dysfunction (Cordova et al. 2013), hyperactivity and spatial learning deficits (Kern et al. 2010). However, the picture emerging from experiments where animals are co-exposed to both Mn and Pb is more complex, revealing that mixture-exposed rats perform better on some tasks (e.g. learning and memory) but worse on others (e.g., swimming velocity) (Betharia and Maher 2012). As the epidemiologic literature on Mn and neurobehavior expands, and more nuanced analyses are conducted, more of this potential complexity in the Mn associations becomes apparent.
There are several limitations to our study. The relatively small sample size limited the statistical power to detect associations, particularly in the stratified analyses, and precluded us from examining analyses stratified jointly by Pb and sex. Our findings should be confirmed in a larger sample. The distributed lag-type approach we used models the correlation between visual motor ability score and Mn levels while assuming a linear relationship of the outcome with Mn at each time point. Prior evidence from studies using blood Mn as a biomarker suggests that Mn may follow a nonlinear or inverted U-shaped dose-response with neurodevelopment (Chung et al. 2015; Claus Henn et al. 2010; Yu et al. 2014). Therefore, there may be model misspecification if tooth Mn associations are nonlinear at some time points. Our initial analyses using GAMs with average Mn levels in four time windows suggest that most estimated relationships visually appear to be approximately linear. We cannot rule out the possibility of unmeasured confounding by secondhand smoke exposure or by other co-occurring neurotoxicants, such as arsenic. In secondary analyses, we explored models adjusted for secondhand smoke exposure (i.e., time spent in room with smoker on typical day during pregnancy) and overall patterns remained (data not shown). However, only limited data were available on this variable (40% of participants with available data). Effect modification by other unmeasured exposures is also possible, which could enhance or reduce the estimated Mn associations. Higher-order interactions were not assessed in this study but are plausible and will be examined in future analyses with a larger sample size. Last, because deciduous teeth begin developing only in the second trimester, we were unable to examine the effects of first trimester metal exposure on neurodevelopmental outcomes.
Despite these limitations, there are also several unique strengths to our study. This is among the first studies to characterize internal levels of prenatal and early postnatal metals over time in a nearly continuous manner. By linking levels of metals in deciduous teeth to developmental timing of exposure, we were able to capture distinct developmental windows that are more sensitive to the beneficial and/or harmful effects of metals. This biomarker has been validated against other Mn and Pb biomarkers (Arora et al. 2012; Arora et al. 2014; Austin et al. 2017). In addition to this highly innovative exposure measurement, we implemented a novel statistical technique to incorporate the high-resolution exposure data. We adjusted for many important confounders and imputed missing data to maximize our sample size in sensitivity analyses. Finally, the prospective study design combined with a retrospective exposure biomarker allowed us to examine neurodevelopmental outcomes in mid-childhood when assessments are more sensitive and reliably related to cognitive abilities than outcomes measured at younger ages (White et al. 2009).
5. CONCLUSIONS
Overall, in this study of Mexican children, we found discrete developmental windows when exposure to Mn was associated with visual-spatial abilities in childhood. The associations we observed were modest, but suggest that they may be dependent on the developmental age, sex, and level of concurrent exposure to Pb. This is the first study of metals and children’s neurodevelopment to characterize prenatal and early postnatal Mn levels in a nearly continuous manner. These results add to the growing evidence that the relationship of Mn exposure with neurodevelopment is complex and multifactorial.
Supplementary Material
Highlights.
Tooth-matrix biomarkers were used to identify windows of susceptibility to Mn
Dentine Mn levels were highest in the second trimester, then declined
Prenatal tooth Mn was associated with better visual-spatial scores at low Pb levels
Postnatal tooth Mn was associated with worse visual-spatial scores among boys
Acknowledgments
We are grateful to all the participants and their families for taking part in this study. We thank the staff of the ELEMENT cohort studies, as well as the American British Cowdray Medical Center for providing facilities during the study period.
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIH/NIEHS) grants R00ES022986, R00ES019597, DP2ES025453, R01ES026033, P30ES023515, and U2CES026555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study was also supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico.
Abbreviations
- Ca
Calcium
- Mn
Manganese
- Pb
Lead
- WRAVMA
Wide Range Assessment of Visual Motor Abilities
Footnotes
Competing Interests Declaration
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams W, Sheslow D. Wide range assessment of visual motor abilities. Wilmington, DE: Wide Range Inc; 1995. [Google Scholar]
- Arora M, Kennedy BJ, Elhlou S, Pearson NJ, Walker DM, Bayl P, et al. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci Total Environ. 2006;371:55–62. doi: 10.1016/j.scitotenv.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46:5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Austin C. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr. 2013;25:261–267. doi: 10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- Arora M, Austin C, Sarrafpour B, Hernandez-Avila M, Hu H, Wright RO, et al. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9:e97805. doi: 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash M, Nelson S. Wheeler’s dental anatomy, physiology, and occlusion. Philadelphia, PA: W.B. Saunders; 2003. [Google Scholar]
- Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, Bishop D, et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498:216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C, Richardson C, Smith D, Arora M. Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res. 2017;155:373–379. doi: 10.1016/j.envres.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N, Miles E, Clarkson TW. Homeostatic control of manganese excretion in the neonatal rat. Am J Physiol. 1987;252:R842–847. doi: 10.1152/ajpregu.1987.252.5.R842. [DOI] [PubMed] [Google Scholar]
- Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environ Int. 2017;108:299–308. doi: 10.1016/j.envint.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betharia S, Maher TJ. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Bonilla E. Chronic manganese intake induces changes in the motor activity of rats. Exp Neurol. 1984;84:696–700. doi: 10.1016/0014-4886(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Braun JM, Hoffman E, Schwartz J, Sanchez B, Schnaas L, Mercado-Garcia A, et al. Assessing windows of susceptibility to lead-induced cognitive deficits in mexican children. Neurotoxicology. 2012;33:1040–1047. doi: 10.1016/j.neuro.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Serrano-Sierra A, Torres-Jardon R, Zhu H, Yuan Y, Smith D, et al. The impact of environmental metals in young urbanites’ brains. Exp Toxicol Pathol. 2013;65:503–511. doi: 10.1016/j.etp.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco AV. The amai system of classifying households by socio-economic level. 2002 http://Www.Esomar.Org.
- Chen YH, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health. 2015;14:9. doi: 10.1186/1476-069X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Claus Henn B, Hsu HL, Pendo MP, Coull BA, Austin C, et al. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res. 2017;159:458–465. doi: 10.1016/j.envres.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SE, Cheong HK, Ha EH, Kim BN, Ha M, Kim Y, et al. Maternal blood manganese and early neurodevelopment: The mothers and children’s environmental health (moceh) study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernandez-Avila M, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012;120:126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Bellinger DC, Hopkins MR, Coull BA, Ettinger AS, Jim R, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ Health Perspect. 2017;125:067020. doi: 10.1289/EHP925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FM, Aguiar AS, Jr, Peres TV, Lopes MW, Goncalves FM, Pedro DZ, et al. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by trolox. Arch Toxicol. 2013;87:1231–1244. doi: 10.1007/s00204-013-1017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Marshall MW, James RA, Struve MF. Old age and gender influence the pharmacokinetics of inhaled manganese sulfate and manganese phosphate in rats. Toxicol Appl Pharmacol. 2004;197:113–124. doi: 10.1016/j.taap.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicology and teratology. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, et al. Effect of calcium supplementation on blood lead levels in pregnancy: A randomized placebo-controlled trial. Environ Health Perspect. 2009;117:26–31. doi: 10.1289/ehp.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cossio T, Peterson KE, Sanin LH, Fishbein E, Palazuelos E, Aro A, et al. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, et al. Determinants of manganese in prenatal dentin of shed teeth from chamacos children living in an agricultural community. Environ Sci Technol. 2013;47:11249–11257. doi: 10.1021/es4018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. Manganese in teeth and neurodevelopment in young mexican-american children. Environ Res. 2015;142:688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D, Austin C, Doble P, Arora M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J Dent. 2011;39:397–403. doi: 10.1016/j.jdent.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57:482–488. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30:564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Kordas K, Ardoino G, Coffman DL, Queirolo EI, Ciccariello D, Manay N, et al. Patterns of exposure to multiple metals and associations with neurodevelopment of preschool children from montevideo, uruguay. J Environ Public Health. 2015;2015:493471. doi: 10.1155/2015/493471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, de Novaes CO, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci U S A. 1996;93:8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:841749. doi: 10.1155/2011/841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernandez-Bonilla D, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the chamacos cohort. Environ Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (cah) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS. Dietary reference intakes for vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. [accessed November 27 2016];2001 Available: http://www.nap.edu/catalog/10026. [PubMed]
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 2011;32:227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode A, Rylander L, Gustafsson P, Lundh T, Kallen K, Olofsson P, et al. Manganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhood. Environ Res. 2015;137:373–381. doi: 10.1016/j.envres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987;67:858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- Rink SM, Ardoino G, Queirolo EI, Cicariello D, Manay N, Kordas K. Associations between hair manganese levels and cognitive, language, and motor development in preschool children from montevideo, uruguay. Arch Environ Occup Health. 2014;69:46–54. doi: 10.1080/19338244.2012.725229. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci Total Environ. 2013;454–455:562–577. doi: 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Rosner B. Percentage points for a generalized esd many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119:409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, et al. Exposure to manganese: Health effects on the general population, a pilot study in central mexico. Environ Res. 2001;85:90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Flores MF, Martinez S, Hernandez C, Osorio E, et al. Blood lead secular trend in a cohort of children in mexico city (1987–2002) Environ Health Perspect. 2004;112:1110–1115. doi: 10.1289/ehp.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla GS, Chandra SV. Concurrent exposure to lead, manganese, and cadmium and their distribution to various brain regions, liver, kidney, and testis of growing rats. Arch Environ Contam Toxicol. 1987;16:303–310. doi: 10.1007/BF01054947. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Hernandez-Avila M, Lamadrid-Figueroa H, Smith D, Hernandez-Cadena L, Mercado A, et al. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol. 2004;160:668–678. doi: 10.1093/aje/kwh271. [DOI] [PubMed] [Google Scholar]
- van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- Wechsler H. Wechsler adult intelligence scale. Spanish version. San Antonio, Texas: The Psychological Corporation; 1968. [Google Scholar]
- Wedler FC. Biological significance of manganese in mammalian systems. Prog Med Chem. 1993;30:89–133. doi: 10.1016/s0079-6468(08)70376-x. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- White RF, Campbell R, Echeverria D, Knox SS, Janulewicz P. Assessment of neuropsychological trajectories in longitudinal population-based studies of children. J Epidemiol Community Health. 2009;63(Suppl 1):i15–26. doi: 10.1136/jech.2007.071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. [accessed may 2016];Hemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011 http://www.Who.Int/vmnis/indicators/haemoglobin.En.
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Yamagata AT, Guimaraes NC, Santana DF, Goncalves MR, Souza VC, Barbosa F, Junior, et al. Gender influence on manganese induced depression-like behavior and mn and fe deposition in different regions of cns and excretory organs in intraperitoneally exposed rats. Toxicology. 2017;376:137–145. doi: 10.1016/j.tox.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC, Andersen ME, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: Describing manganese homeostasis during development. Toxicol Sci. 2011;122:297–316. doi: 10.1093/toxsci/kfr141. [DOI] [PubMed] [Google Scholar]
- Yu XD, Zhang J, Yan CH, Shen XM. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–238. doi: 10.1016/j.envres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Zoni S, Lucchini RG. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr Opin Pediatr. 2013;25:255–260. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.