Abstract
A recent report from the Center for Disease Control identified melanoma as being among the highest causes of cancer-related mortalities in the USA. While interventions such as checkpoint blockade have made substantial impact in terms of improving response rates and overall survival, a significant number of patients fail to respond to treatment or become resistant to therapy. A better understanding of the tumor microenvironment in these patients becomes imperative for identifying immune suppressive mechanisms that impact the development of effective anti-tumor immune responses. We have investigated innate immune cells (dendritic cells, NK cells) in the tumor microenvironment (TME) in order to devise effective targeted anticancer immune therapies. We find that matrix metalloproteinase-2 (MMP-2), secreted from melanoma cells and stromal cells, cleaves IFNAR1 and stimulates TLR-2 on dendritic cells (DC) within the TME. Both these events independently culminate in programing the DCs to promote pro-tumorigenic TH2 T cell differentiation. In addition, we have shown that NK cells become functionally exhausted in melanoma patients. We identified the expression of Tim-3 as one of the factors responsible for NK cell exhaustion and showed that anti-Tim3 antibodies partially reversed this exhaustion. We have initiated local intervention strategies such as intra-tumoral administration of DC activating Poly-ICLC and compared the efficacy of different TLR agonists and melanoma antigens for use as combination tumor vaccine in clinical trials. Such approaches will provide a unique insight into tumor biology and will facilitate in development of highly effective and cell type-specific immune therapies.
Keywords: CIMT 2015, Dendritic cell, Matrix metalloproteinase-2, NK cell exhaustion, Tim-3, Immunotherapy
Introduction
Melanoma, an aggressive form of skin cancer, has been defined as a heterogenous disease. Melanoma begins in the lowest layer of the epidermis and is a highly metastatic tumor where dissemination is a multistage process. Melanoma compromises of malignant cells, mainly melanocytes, but also the supporting stroma. These tumor cells actively interact with their microenvironment and modulate the cells in this niche to promote tumor growth and metastasis. The tumor microenvironment (TME) is described as a complex arrangement of stromal cells, mutated cancer cells and immune cells such as macrophages, dendritic cells (DCs), natural killer (NK) cells and T cells. Herein we will discuss the recent advances made by our group toward understanding the molecular switches that dictate the construction of a TME, specifically focusing on innate immune cells (DCs, NK cells), and how intra-tumoral administration of DC activating agents could potentially reprogram the TME and promote tumor regression. In addition, the effects of clinical vaccine trials performed using different combinations of tumor antigens and adjuvants that target innate immune cells will be described.
Dendritic cell driven TH2 cell differentiation in melanoma: role of MMP-2
The immune cell component of the TME largely dictates the inflammatory milieu and can regulate cancer growth. A higher ratio of TH1 vs TH2 inflammatory phenotype is associated with tumor regression and a more promising prognosis. Inversely, a TH2 heavy TME has been correlated with metastasis and higher patient mortality [1]. One of the goals of our laboratory is to characterize elements in the TME that promote TH2 skewing, and to identify approaches to reverse or block their differentiation. In this context, we have explored the role of matrix metalloproteinase-2 (MMP-2) in conditioning DCs to promote TH2 polarization. MMP-2 is a secreted proteinase that degrades the extracellular matrix (ECM) [2] and is basally expressed in several cells including lymphocytes, endothelial cells, glandular cells, stromal cells, supportive tissue cells, smooth muscle cells and skeletal muscle cells [3, 4]. Interestingly, increased expression of active MMP-2 has been directly correlated with increased metastasis and poorer prognosis in both human and murine models of cancer [5, 6]. Besides promoting metastasis, MMP-2 has been known to enhance tumor growth by cleaving death ligands on tumor cells, enhancing angiogenesis [6], increasing bioavailability of growth factors and aiding in immune evasion [7].
Surprisingly, despite the large number of pro-tumorigenic functions of MMPs, broad inhibitors of MMP activity have yielded disappointing results in clinical trials [8], suggesting that a deeper exploration of MMP functions is required before it can be targeted for cancer therapy. MMP-2-specific, tumor-infiltrating CD8+ T cells have been identified in the tumor-infiltrating lymphocytes (TILs) of melanoma patients. These CD8+ T cells were specific for HLA-A*0201–restricted antigen derived from secreted MMP-2 and cross-presented on αvβ3-positive melanoma cells. However, the anti-tumorigenic contribution of these cells remains unknown [9, 10]. Our investigations into the role of MMP-2 as a tumor antigen for activation of CD4+ T cells led us to discover MMP-2-specific CD4+ tumor-infiltrating lymphocytes in 13 out of 31 melanoma patients. Noticeably, 40 % of these TILs secreted TNF-α and IL-4 upon stimulation, indicating a TH2 phenotype. In concert with the pro-tumorigenic role of TH2 cytokines, we were able to depict a trend between MMP-2-specific CD4+ T cell responders and poorer clinical outcome. Significantly, DCs pre-loaded with MMP-2-differentiated naïve T cells into a TH2-type cell characterized by secretion of TNF-α, IL-4 and IL-13 with concomitant expression of GATA-3 [11]. As IL-12 secretion is a widely known inducer of TH1 polarization, we investigated whether exposure to exogenous MMP-2 could affect IL-12 production in DCs. We confirmed the significance of DC-derived IL-12 in TH1-T cell polarization and observed a marked decline in IL-12 secretion from DCs exposed to active MMP-2. Further investigation revealed that active MMP-2 degraded the type I IFN receptor1 (IFNAR1) on DCs, thus inhibiting Stat1 phosphorylation and subsequent IL-12 p35 transcription in response to stimulation with the TLR-3 agonist Poly-ICLC [11].
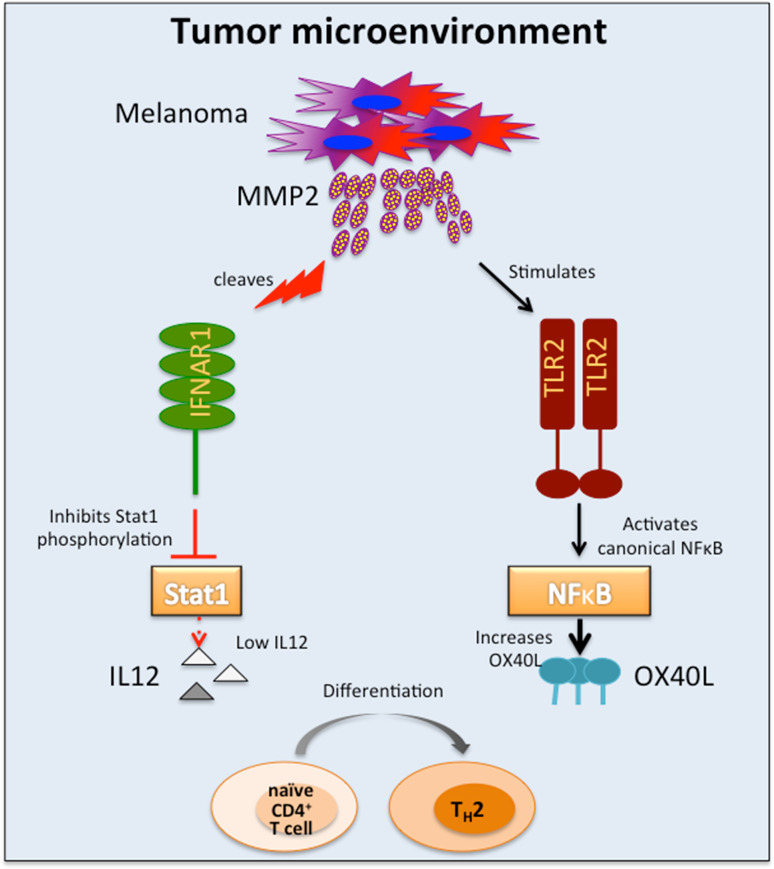
OX40L is a co-stimulatory molecule that has been implicated in T cell differentiation and tumor progression. Engagement of thymic stromal lymphopoietin (TSLP)-induced OX40L on DCs with cognate binding partners, such as OX40 on activated T cells, has been shown to enhance TH2 differentiation in the absence of IL-I2. Such TH2-differentiated CD4+ T cells were characterized by secretion of TH2 cytokines IL-4, IL-5, IL-13 and high amounts of pathogenic TNF-α but no IL-10. Furthermore, OX40L-mediated TH2 differentiation was reversed by introduction of exogenous IL-12, indicating that the mechanism of DC-mediated T cell differentiation is amenable to manipulation by pharmacological intervention [12–14]. We found that apart from directly inhibiting IL-12 and consequent TH1 polarization, exogenous MMP-2 also enhanced TH2-polarization by upregulating OX40L expression on DCs [11, 15]. It is interesting to note that while only active MMP-2 could degrade IFNAR1 and thus inhibit IL-12 secretion, both active and inactive forms of MMP-2 were able to enhance OX40L expression and stimulate secretion of inflammatory cytokines via the canonical NFκB pathway. Screening for MMP-2-signaling partners led us to identify Toll-like receptor-2 (TLR-2) as a direct binding partner for MMP-2, independently of TLR-1 and TLR-6 [15]. These findings were corroborated in human monocyte-derived DCs, murine bone marrow-derived DCs as well as in HEK cells over expressing TLR-2. To ascertain physiological relevance for these findings, WT, myd88-/- and tlr2-/- mice were injected with MMP-2. Analysis of serum cytokines revealed a distinct inflammatory signature (TNF-α and IL-6) only in sera obtained from WT mice where as the sera from knockout mice had no inflammatory cytokines. Similarly, WT and tlr2-/- mice adoptively transferred with OT-II cells and injected with OVA + MMP-2 revealed TH2 polarization upon re-stimulation only in WT-derived T cells and not in tlr2-/- cells [15]. Taken together, these studies indicate that MMP-2 primes DCs to skew the adaptive immune response toward a pro-tumorigenic TH2 phenotype through multiple mechanisms. First, MMP-2 inhibits the production of IL-12 in response to Poly-ICLC stimulation, by degrading IFNAR1 and preventing phosphorylation of Stat1. Second, MMP-2 directly binds to and activates TLR-2, which results in the induction of OX40L expression (Fig. 1).
Fig. 1.
MMP-2 in the TME promotes DC driven TH2 differentiation. MMP-2 derived from melanoma cells initiates two independent signaling cascades in DCs that cause TH2 polarization. (1) MMP-2 cleaves IFNAR1 on DCs, thus inhibiting Stat1 phopshorylation and downregulating IL12 secretion and (2) MMP-2 interacts with TLR-2 to activate canonical NFκB signaling and upregulate TH2-promoting OX40L
Overall, our group has uncovered novel signaling mechanisms activated by MMP-2 that could actively affect inflammation, DC activation and tumor progression. Our discovery of enzymatically inactive MMP-2 as a novel endogenous alarmin for TLR-2 underscores the complexity of MMP-2 signaling and highlights its multifaceted roles in crafting the inflammatory landscape. A thorough examination of the TLR-2-MMP-2 axis and its contribution in melanoma enhancement will not only expand our global understanding of signaling events within the TME but also uncover new druggable targets for treating cancers.
NK cell function and tumor surveillance in melanoma
Natural killer (NK) cells are one of the cellular mediators of innate defense. They are lymphoid cells that, in the absence of pre-activation, can recognize and kill aberrant cells and rapidly produce soluble factors such as chemokines and cytokines that have antimicrobial or anti-tumor effects or prime other cells of the immune system [16]. NK cell precursors (NKP) which are primarily found in bone marrow, and also thymus, liver and/or spleen, have a role in the NK cell diversification that results in NK cell heterogeneity [16]. NKP mature to become NK cells under the influence of IL-2 or IL-15 [17] and acquire lytic and cytokine production capabilities [17]. NKP maturation is followed by the acquisition of maturation molecules, for example, KIR, CD57, CD85j [18].
Active NK cells have cytolytic potential and produce IFN-γ that allows them to function in first-line defense against tumors. Partial regression of primary growth has been correlated with higher NK cell infiltration and a more favorable prognosis in patients suffering from melanoma, renal cell carcinoma and esophageal squamous cell [19–21]. In fact, increased NK cell infiltration has been directly correlated with regression in melanoma patients [22]. Moreover, selective reduction of peripheral NK cells in transgenic mice was associated with an impaired acute in vivo reduction of tumor cells [23]. In addition, there is evidence of early benefit from NK cell adoptive cell therapies (ACT) in leukemia and melanoma patients [24, 25]
The role of peripheral blood NK cells has been described mainly during the course of acute and chronic infections where NK cells undergo transient or persistent modulation of activating receptor expression and function [26]. NK cells can become dysfunctional, resembling the phenotype previously described in T cells as a state of cellular exhaustion that arises as a consequence of continuous and chronic stimulation by viral or tumor antigens, as well as by immunosuppressive cytokines. NK cell dysfunction has been demonstrated during HIV, CMV, hepatitis C and hepatitis B infections [27–31]. We recently demonstrated, for the first time, that NK cells also become exhausted in the setting of advanced cancer. While NK cell dysfunction has been described in several human cancers [32, 33] and animal tumor models [34], we demonstrated that the aberrant function of blood NK cells in advanced melanoma resembles the panoply of dysfunction shown by exhausted T cells. This was shown by (1) reduction of proliferative capacity to IL-2 and cytotoxic activity against melanoma lines, as well as cytokine production (IFN-γ) in response to activation; (2) reduced expression of activation receptors (CD16, NKG2D, NKp46 and DNAM-1), IL-2R subunits and NK cell regulatory transcription factors (T-bet, Eomes), (3) upregulation of inhibitory receptors (KIR3DL1, KIR2DL3) and (4) expression of high levels of the checkpoint molecule Tim-3, but not CTLA-4 or PD-1, when compared to freshly isolated NK cells from healthy donors.
Interestingly, when the expression of the three IL-2 receptor (IL-2R) chains [α-(CD25), β-(CD122) and γ-(CD132)] in each NK cell subset (CD56bright and CD56dim) was analyzed, we noted low expression levels of CD25 in each subset of NK cells, slightly lower levels of CD122 and lower levels of CD132 in CD56dim and in total NK cells in melanoma patients compared to healthy donors. Altered IL-2R expression in melanoma donors (MD) generated different responses to IL-2 stimulation. After 6 days of in vitro IL-2 stimulation, freshly isolated NK cells from MD failed to upregulate CD25 expression and the overall expression of CD132 was substantially lower compared with the response of healthy donor (HD) NK cells.
Another characteristic that defined exhausted MD NK cells was the failure to reverse exhaustion even following long-term IL-2 stimulation. Exhausted NK cells from MD failed to produce IFN-γ in response to combinations of IL-12 and IL-18, or IL-15 cytokine alone, or after co-culture with K562 cells. Likewise, it was not possible to restore cytotoxic activity (as assessed by LAMP-1 expression) after stimulation by either IL-2 or IL-15, or proliferative capacity to IL-2, IL-12, IL-15 or IL-18. The immunoregulatory protein T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) has been described as a mediator of T cell exhaustion and to contribute to the suppression of immune responses in both viral infections and tumors. Tim-3 was significantly overexpressed at different stages of melanoma, I, II and III/IV, showing a pattern of gradually increasing Tim-3+ NK cells numbers (percentage) and MFI as the stage advanced. Tim-3 expression increased in those subjects who had poor prognostic factors, such as thickness >1 mm, mitotic rate ≥1/mm2 and ulceration. Moreover, the intensity of Tim-3 expression (MFI) was higher in patients with distal metastases. Therefore, the levels of Tim-3 appear to associate with functional NK cells defects in the setting of progressive disease. Significantly, exhausted NK cell phenotypes can be partially reversed in vitro after using Tim-3-blocking antibodies, restoring up to 30–65 % of NK cell function [35, 36]. These data suggest that other checkpoint molecules may be operative in conferring exhaustion to NK cells. Figure 2 represents a schematic view of NK cell exhaustion phenotype and its in vitro reversal after checkpoint inhibitor treatment.
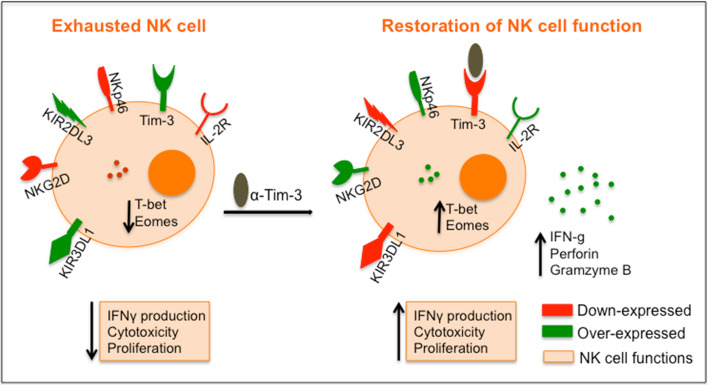
Fig. 2.
Schematic representation of NK cell exhaustion phenotype and its in vitro reversal after checkpoint inhibitor treatment. Exhausted NK cells in melanoma are characterized by (1) reduction of proliferative capacity to IL-2 and cytotoxic activity against melanoma lines, as well as cytokine production (IFN-γ) in response to activation; (2) reduced expression of activation receptors and IL-2R subunits and NK cell regulatory transcription factors (T-bet, Eomes), (3) upregulation of inhibitory receptors. In this figure, we made a schematic representation for an in vitro assay shown in Da Silva., et al. where after 1 h treatment with anti-Tim-3-blocking antibody freshly isolated NK cells from melanoma donors were able to reverse this phenotype and state of exhaustion in vitro, restoring 30–65 % of the NK cell dysfunction. The black oval ligand represents anti-Tim-3-blocking antibody. Blockade of Tim-3 in vitro was able to overexpress IL-2 receptor on NK cell surface, as well as expression of the transcription factors T-bet and Eomes. Moreover, blockade of Tim-3 is enough for exhausted NK cells (1) to recover their capacity for producing IFN-γ after IL-12 stimulation, (2) to recover their cytotoxic capacity for killing other targets cells and (3) to proliferate
The tumor microenvironment in melanoma is characterized with an increase in immunosuppression factors such as PD-L1, IDO, TGF beta, IL-10 and suppressor cells (regulatory T cells, myeloid-derived suppressor cells) that clearly contribute toward T cell exhaustion. Besides Tim-3, exhausted T cells are also characterized by overexpression of other checkpoint inhibitory molecules like PD-1, CTLA-4, LAG-3 and TIGIT [37, 38]. However, we found that, unlike T cells, exhausted NK cells in melanoma do not show significant differences in the expression levels of PD-1 and CTLA-4 between melanoma patients and healthy donors [35]. In fact, they have low levels of these checkpoint molecules. Despite this finding, we have preliminary data suggesting that anti-CTLA-4 antibody treatment can restore NK cell function in a subset of patients. Recent studies indicate that NK cells also have a distinct set of checkpoint molecules that in coordination with Tim-3 could participate in sustaining their global exhaustion such as Ceacam-1, CD96, TIGIT, Siglec-7 [39–41]. An in-depth understanding of the exact mechanisms that cause NK cell exhaustion to identify novel strategies for reversing this exhaustion will be required and is the subject of further study in our laboratory.
Vaccine strategies to prime effective dendritic cell adjuvant function
DCs are natural adjuvants and their activity in priming T cells has been well documented in our laboratory and several others. In the first controlled clinical trial, we showed that DCs were essential for stimulating immunity against peptide and protein antigens [42] and that maturation was required to prevent the induction of tolerance [43]. Subsequently, we have made efforts to iteratively test adjuvants that can activate DC in vivo, focusing in particular upon the ligation of specific Toll-like receptors. In a series of studies, we explored the immunogenicity of the cancer testis antigen NY-ESO-1 given in combination with TLR agonists in the adjuvant setting of melanoma. Subjects, who were disease free, were immunized with topical imiquimod and NY-ESO-1 protein [44]. In a study of nine patients, who tolerated the intervention well, we observed both humoral and cellular responses in a significant fraction of patients. Topical imiquimod-induced dermal mononuclear cell infiltrates in all patients composed primarily of T cells, monocytes, macrophages, myeloid DCs, NK cells and, to a lesser extent, plasmacytoid DCs with evidence of DC activation. However, no CD8+ T cell responses were detected. Subsequently, we compared the adjuvant CpG ODN 7909, a TLR-9 agonist, this time delivered with the water in oil emulsion Montanide ISA-51 to ensure that adjuvant and protein could be introduced as a form of depot [45]. This combination delivered subcutaneously led to the development of antigen-specific humoral and CD4+ TH1immunity in almost all patients (an integrated immune response) and CD8+ T cell responses in about 50 % of patients. Vaccine-induced antibodies promoted in vitro cross-presentation of NY-ESO-1 protein by dendritic cells to vaccine-induced CD8+ T cells, suggesting that a humoral response may benefit the induction of a cellular response through immune complex formation, although this has yet to be proved in vivo.
These studies did not evaluate the relative contribution of a TLR agonist vs. Montanide, however. Therefore, we next evaluated the immunogenicity of NY-ESO-1 given in combination with Montanide subcutaneously with or without the topically administered TLR-7 agonist resiquimod in patients with high-risk melanoma [46]. The majority of patients in both arms tolerated the treatment well and developed NY-ESO-1-specific CD4+ T cell responses and antibodies. CD8+ T cell responses, however, were only seen in 3 of 12 patients in the arm receiving antigen together with Montanide and resiquimod. Notably those patients who expressed the TLR-7 SNP rs179008 had a greater likelihood of developing NY-ESO-1-specific CD8+ responses. This study determined that NY-ESO-1 protein in combination with Montanide with or without topical resiquimod is safe and induces both antibody and CD4+ T cell responses in the majority of patients. Resiquimod appeared to have a small benefit in terms of inducing CD8+ T cell responses, but larger studies will be needed to assess the contribution of the TLR7 SNP rs179008 toward the activity of resiquimod. Most recently, in an ongoing study we are comparing the safety and immunogenicity of NY-ESO-1 protein given in combination with Poly-ICLC, a TLR-3 and MDA5 agonist, with or without Montanide, injected subcutaneously. The purpose of the study was to determine whether a TLR agonist can suffice to induce potent immunity in the absence of Montanide. Patients tolerated the vaccines well. Most developed an integrated antigen-specific humoral and CD4+ T cell response. The determination of CD8+ T cell immunity is ongoing although these studies will shed light on which adjuvants induce potent immunity and determine the contribution of Montanide.
Finally, we are testing the efficacy of TLR agonists when delivered intra-tumorally. In a case report, we showed how the injection of intra-tumoral Poly-ICLC can dramatically impact tumor burden in a subject with facial embryonal rhabdomyosarcoma with extension to the brain [47]. A follow-up pilot study in patients with head and neck squamous cell cancers, still being evaluated, demonstrated that intra-tumoral Poly-ICLC modulates the tumor microenvironment, changing it from a T cell poor to a T cell rich environment. We speculate that Poly-ICLC is effective in this regard due to its multifactorial effects: It has direct antineoplastic effects by inducing apoptosis and also an immune stimulatory effect through activation of NK cells, macrophages and DCs. This results in the release of cytokines and chemokines and also T cell priming. In situ cell activation and priming may therefore enhance anti-tumor immunity. In our study, there was evidence of DC infiltration and activation, upregulation of PDL-1 and systemic immune activation. Larger studies are in progress to confirm these findings in several histologies.
Conclusion
In summary, we are developing immunotherapeutic strategies and vaccine platforms that are targeted to prime DC function, TH1 differentiation and restore NK cell activity, thereby promoting immune activation and preventing immune suppression. In Fig. 3, we show a schematic representation of different vaccine strategies. Our team is actively exploring ways to improve DC adjuvant function, including mobilizing DCs in vivo with Flt3L, deriving DC subsets from CD34+ progenitor cells to yield distinct populations (plasmacytoid DC, CD1c+, CD141+ DCs) and testing them in combination with other modalities, e.g., cytokines, preconditioning methods or novel sources of antigens, e.g., neoantigens. The overall goal is to tip the balance in the TME from being immunosuppressive to pro-inflammatory by using these interventions, eventually in combination with checkpoint blockade, which promises to be the platform for many cancer histologies.
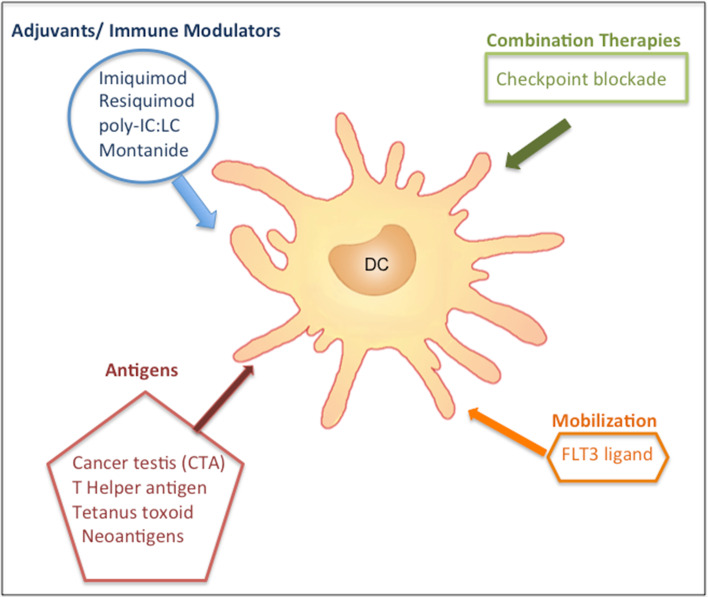
Fig. 3.
Vaccine strategies to prime effective dendritic cell adjuvant function. Strategies for priming classical DCs in general by using (1) adjuvants or immune modulators, (2) combined therapies, (3) DC antigens and (4) mobilization modulators. Our vaccine trials in particular have been designed to target classical DCs (Poly-ICLC) and plasmacytoid DCs (imiquimod and resiquimod)
Acknowledgments
This work was supported by research funding for Dr. Bhardwaj: CLIP award from the Cancer Research Institute R01 AI081848 NIH; R37 AI044628 NIH; R01CA180913 NIH. Dr. Saxena has been supported by: NIH/NIAD 2T32A1007647-15-Virus–Host Interactions Training Grant, Icahn School of Medicine at Mount Sinai, 2015–2016; NIH/NIAD T32A1007605-Immunology Training Grant, Icahn School of Medicine at Mount Sinai, 2014–2015.
Abbreviations
- ACT
Adoptive cell therapies
- CMV
Cytomegalovirus
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DC
Dendritic cell
- DNAM-1
DNAX accessory molecule-1
- ECM
Extracellular matrix
- Eomes
Eomesodermin transcription factor
- Flt3L
Feline sarcoma virus-related tyrosine kinase3 ligand
- GATA-3
GATA binding protein 3
- HD
Healthy donor
- HIV
Human immunodeficiency virus
- IDO
Indoleamine 2, 3-dioxygenase
- IFN-γ
Interferon gamma
- IFNAR1
Type I interferon receptor1
- IL
Interleukin
- KIR
Killer cell immunoglobulin-like receptors
- KIR2DL3
Killer cell immunoglobulin-like receptor 2DL3
- KIR3DL1
Killer cell immunoglobulin-like receptor 3DL1
- LAG-3
Lymphocyte-activation gene 3
- LAMP-1
Lysosomal-associated membrane protein 1
- MD
Melanoma donor
- MDA5
Melanoma differentiation-associated protein 5
- MFI
Mean fluorescence intensity
- MMP-2
Matrix metalloproteinase-2
- MYD88
Myeloid differentiation primary response 88
- NFκB
Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1
- NK Cells
Natural killer cells
- NKG2D
Natural killer group 2, member D
- NKP
Natural killer cells precursors
- NKp46
Natural killer cell p46-related protein
- ODN
Oligonucleotide
- OVA
Ovalbumin
- OX40L
OX40 ligand
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein 1 ligand
- Poly-ICLC
Polyinosinic-polycytidylic acid, and poly-l-lysine
- SNP
Single-nucleotide polymorphisms
- Stat1
Signal transducer and activator of transcription 1
- T-bet
T-box transcription factor T-box 21
- TGF beta
Transforming growth factor beta
- TH1
T helper type 1
- TH2
T helper type 2
- TIGIT
T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibition motif domains
- TILs
Tumor-infiltrating lymphocytes
- Tim3
T cell immunoglobulin- and mucin-domain-containing molecule-3
- TLR
Toll-like receptor
- TME
Tumor microenvironment
- TNF-α
Tumor necrosis factor-alpha
- WT
Wild type
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Elena Gonzalez-Gugel and Mansi Saxena are contributed equally to the paper.
This paper is a Focussed Research Review based on a presentation given at the Thirteenth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 11th–13th May, 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Lauerova L, Dusek L, Simickova M, Kocak I, Vagundova M, Zaloudik J, Kovarik J. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49(3):159–166. [PubMed] [Google Scholar]
- 2.Dye DE, Medic S, Ziman M, Coombe DR. Melanoma biomolecules: independently identified but functionally intertwined. Front Oncol. 2013;3:252. doi: 10.3389/fonc.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Distinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell lines. Oncol Rep. 2009;21(3):821–826. [PubMed] [Google Scholar]
- 4.Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology. 2008;124(1):42–50. doi: 10.1111/j.1365-2567.2007.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polette M, Gilbert N, Stas I, Nawrocki B, Noel A, Remacle A, et al. Gelatinase A expression and localization in human breast cancers. An in situ hybridization study and immunohistochemical detection using confocal microscopy. Virchows Arch. 1994;424(6):641–645. doi: 10.1007/BF00195779. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58(5):1048–1051. [PubMed] [Google Scholar]
- 7.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 9.Renaud V, Godefroy E, Larrieu P, Fleury F, Jotereau F, Guilloux Y. Folding of matrix metalloproteinase-2 prevents endogenous generation of MHC class-I restricted epitope. PLoS ONE. 2010;5(7):e11894. doi: 10.1371/journal.pone.0011894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godefroy E, Moreau-Aubry A, Diez E, Dreno B, Jotereau F, Guilloux Y. alpha v beta3-dependent cross-presentation of matrix metalloproteinase-2 by melanoma cells gives rise to a new tumor antigen. J Exp Med. 2005;202(1):61–72. doi: 10.1084/jem.20042138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godefroy E, Manches O, Dreno B, Hochman T, Rolnitzky L, Labarriere N, et al. Matrix metalloproteinase-2 conditions human dendritic cells to prime inflammatory T(H)2 cells via an IL-12- and OX40L-dependent pathway. Cancer Cell. 2011;19(3):333–346. doi: 10.1016/j.ccr.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15(6):620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Godefroy E, Gallois A, Idoyaga J, Merad M, Tung N, Monu N, et al. Activation of toll-like receptor-2 by endogenous matrix metalloproteinase-2 modulates dendritic-cell-mediated inflammatory responses. Cell Rep. 2014;9(5):1856–1870. doi: 10.1016/j.celrep.2014.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3(5):413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 17.Jaleco AC, Blom B, Res P, Weijer K, Lanier LL, Phillips JH, et al. Fetal liver contains committed NK progenitors, but is not a site for development of CD34 + cells into T cells. J Immunol. 1997;159(2):694–702. [PubMed] [Google Scholar]
- 18.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16 + NK-cell subset. Blood. 2010;116(19):3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl) 2012;90(1):55–66. doi: 10.1007/s00109-011-0806-7. [DOI] [PubMed] [Google Scholar]
- 20.Hsia JY, Chen JT, Chen CY, Hsu CP, Miaw J, Huang YS, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J. 2005;28(5):335–340. [PubMed] [Google Scholar]
- 21.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577–583. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.McKay K, Moore PC, Smoller BR, Hiatt KM. Association between natural killer cells and regression in melanocytic lesions. Hum Pathol. 2011;42(12):1960–1964. doi: 10.1016/j.humpath.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97(6):2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 25.Beelen DW, Ottinger HD, Ferencik S, Elmaagacli AH, Peceny R, Trenschel R, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105(6):2594–2600. doi: 10.1182/blood-2004-04-1441. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol. 2012;25(4):329–332. doi: 10.1089/vim.2011.0096. [DOI] [PubMed] [Google Scholar]
- 28.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39(6):733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer JL, Li H, Evans TI, Estes JD, Reeves RK. Accumulation of cytotoxic CD16 + NK Cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol. 2015;89(13):6887–6894. doi: 10.1128/JVI.00660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alter G, Jost S, Rihn S, Reyor LL, Nolan BE, Ghebremichael M, et al. Reduced frequencies of NKp30 + NKp46 + , CD161 + , and NKG2D + NK cells in acute HCV infection may predict viral clearance. J Hepatol. 2011;55(2):278–288. doi: 10.1016/j.jhep.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonorino P, Ramzan M, Camous X, Dufeu-Duchesne T, Thelu MA, Sturm N, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51(3):458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Investig. 2011;121(9):3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer. 2011;2:443–457. doi: 10.7150/jca.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 2012;119(24):5758–5768. doi: 10.1182/blood-2012-03-415364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2(5):410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallois A, Silva I, Osman I, Bhardwaj N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. 2014;3(12):e946365. doi: 10.4161/21624011.2014.946365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 38.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15(5):431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 41.Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Demoulins T, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Investig. 2014;124(4):1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Investig. 1999;104(2):173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Investig. 2000;105(6):R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181(1):776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104(21):8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F, et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res. 2015;3(3):278–287. doi: 10.1158/2326-6066.CIR-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2(8):720–724. doi: 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]