Abstract
Hydrogen sulfide (H2S) is an important biomolecule, and controllable H2S donors are needed to investigate H2S biological functions. Here we utilize cysteine-mediated addition/cyclization chemistry to unmask an acrylate-functionalized caged thiocarbamate and release carbonyl sulfide (COS), which is quickly converted to H2S by carbonic anhydrase (CA).
Graphical Abstract
Cysteine-activated acrylate-functionalized caged thiocarbamate provides access to triggered COS/H2S donors
Hydrogen sulfide (H2S) has joined the gasotransmitter family since its first recognition as an endogenous neuromodulator in 1996.1 Four main enzymes, including cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), are responsible for endogenous H2S production, converting cysteine (Cys) and homocysteine (Hcy) to H2S.2 Significant efforts have been contributed to develop H2S releasing agents (H2S donors) because the regulation of H2S levels has been found to mediate a wide variety of physiological processes, including anti-inflammation, oxidative stress reduction, and vasorelaxation.3 Although sulfide salts, such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), have been widely used in the field, they are far from ideal donors because they release H2S spontaneously, resulting in a concentrated bolus of sulfide that oxidizes rapidly and does not mimic well-regulated endogenous H2S production. The use of these inorganic sulfide sources has even led to contradictory results,3c demonstrating the need for improved sources of H2S. These limitations suggest that controllable H2S donors, which are stable, only release H2S upon activation by certain stimuli, and have slower and controllable kinetics of sulfide release, are key research tools for H2S investigations.
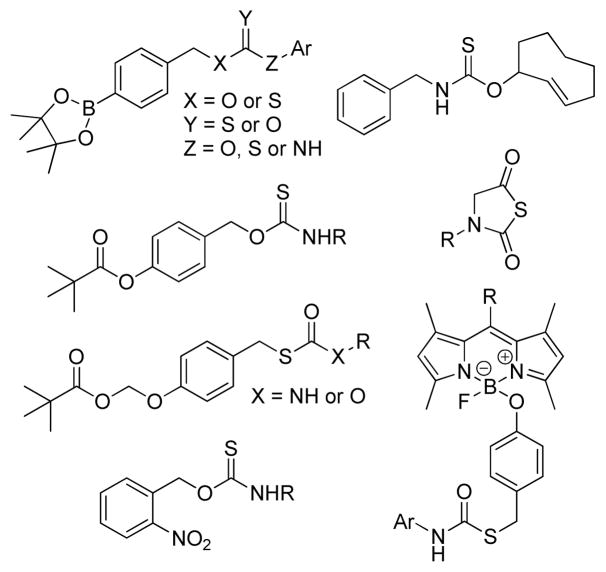
Aligned with this need, our group recently reported the use of caged-carbonyl sulfide (COS) molecules as new H2S donors.4 Unlike other known H2S donors, which directly release H2S as the activation product, COS-based donors are activated to release COS, which is quickly converted to H2S by the ubiquitous enzyme carbonic anhydrase (CA). We have demonstrated that caged-thiocarbamates and thiocarbonates can serve as promising COS donors and can be activated to release COS through a self-immolative cascade reaction.5 One important advantage of this strategy is that COS-releasing scaffolds can be designed to deliver H2S under well-defined conditions. For example, H2S delivery from these caged-COS donors can be modulated by judicious trigger selection, and the rate of release can be manipulated through modification of the donor structure.5 Following our initial report, we, as well as others, have expended this strategy to include donors activated by different triggers, such as reactive oxygen species (ROS),5–6 esterases,7 nucleophiles,8 click chemistry,9 and light10 (Figure 1).
Figure 1.
Examples of currently-available COS-based H2S donors that are activated by different triggering stimuli.
Cellular nucleophiles play crucial roles in biological systems. Among these, thiol species, such as Cys and reduced glutathione (GSH), attract the most attention due to their cellular abundance and potent reactivity. Cys and GSH have been widely used to trigger biologically active molecules and prodrugs to release caged compounds, including sulfur dioxide (SO2),11 nitroxyl (HNO),12 and anti-cancer drugs.13 Importantly, thiol activation strategies have been adopted in H2S donor development and several thiol labile H2S donors exhibit promising protections in animal models with some of them currently in clinical trials.3b, 3c, 3e Motivated by these findings, we report here the first example of a Cys-activated COS/H2S donor through functionalization of a thiocarbamate with a Cys-reactive acrylate moiety. We envision that such thiocarbamate compounds will expand the current COS-based H2S donor family and serve as promising research tools for H2S studies.
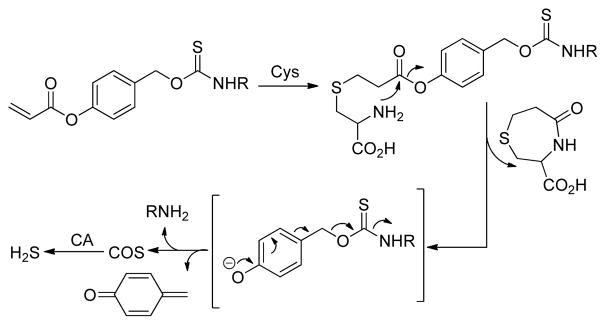
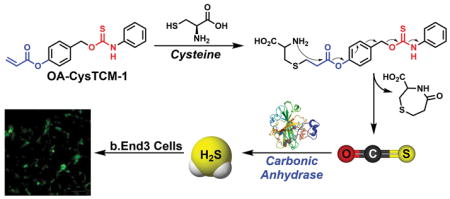
The reactions of Cys with acrylates in the preparation of substituted 1,4-thiazepines have been known for decades.14 The initial attack by Cys on the acrylate generates a thioether, which then undergoes an intramolecular cyclization to yield 1,4-thiazepines. This cyclization strategy has been leveraged by Strongin,15 as well as others,16 to design a series of acrylate-based fluorescent probes for Cys detection. Similarly, the Berreau group has recently used a similar approach to develop a class of Cys-responsive CO donors.17 Building from these approaches, we adopt the Cys-acrylate reaction as a triggering mechanism to access new COS/H2S donors in which an aryl acrylate-functionalized thiocarbamate is activated through a Cys-mediated addition/cyclization sequence. The resultant phenolic intermediate then undergoes a 1,6-elimination to release COS, which is quickly converted to H2S by CA (Scheme 1).
Scheme 1.
General design of Cys-triggered COS/H2S release from caged-thiocarbamate donors.
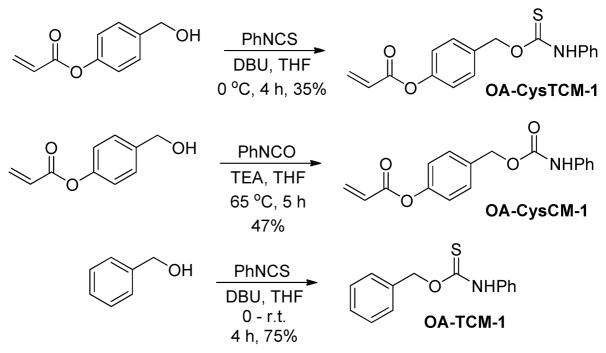
To test our hypothesis that acrylate-functionalized thiocarbamates could serve as Cys-triggered COS/H2S donors, we prepared O-alkyl cysteine-sensitive thiocarbamate (OA-CysTCM-1) with an aryl acrylate trigger and an aniline payload by reacting 4-(hydroxymethyl)phenyl acrylate and phenyl isothiocyanate. Upon activation, OA-CysTCM-1 releases COS, which is quickly hydrolyzed to H2S by CA. In addition to OA-CysTCM-1, we also prepared the corresponding carbamate (OA-CysCM-1) and triggerless thiocarbamate (OA-TCM-1)18 control compounds. OA-CysCM-1, obtained from the reaction between 4-(hydroxymethyl)phenyl acrylate and phenyl isocyanate, is expected to undergo the same Cys activation but would release CO2 instead of COS. OA-TCM-1, on the other hand, maintains the thiocarbamate scaffold but lacks the acrylate trigger, and thus is not expected to react with Cys or decompose to otherwise release COS (Scheme 2).
Scheme 2.
Synthesis of OA-CysTCM-1, OA-CysCM-1, and OA-TCM-1.
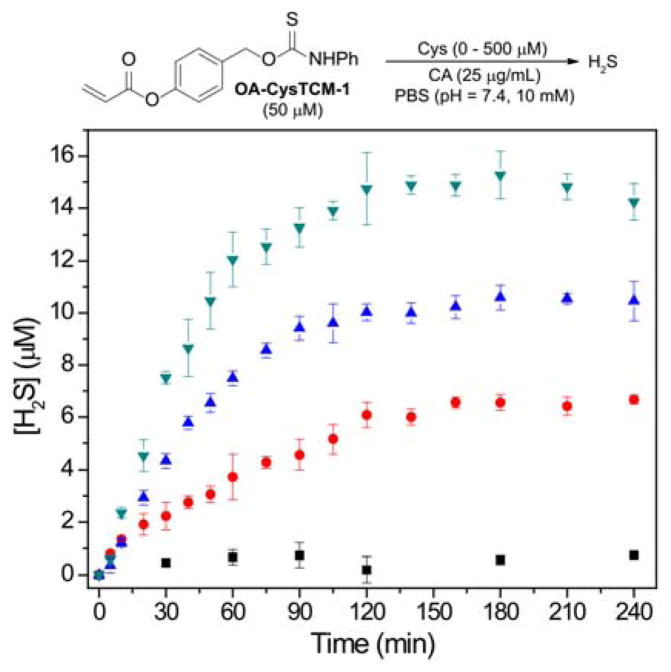
To evaluate Cys-activated H2S release from the donor motif, we used the methylene blue (MB) assay to monitor H2S release from OA-CysTCM-1 (50 μM) in the presence of Cys (0 – 500 μM) in PBS buffer (pH 7.4, 10 mM) containing cellularly-relevant concentrations of CA (25 μg/mL). The MB assay was chosen to measure H2S production since it has been widely used to detect H2S from previously developed Cys-activated H2S donors. In the absence of Cys, OA-CysTCM-1 was stable in aqueous buffer and did not release COS/H2S spontaneously. By contrast, the addition of Cys led to a dose-dependent COS/H2S release from OA-CysTCM-1 (Figure 2). These results demonstrate that OA-CysTCM-1 can be activated by Cys and the resultant COS is quickly converted to H2S in the presence of CA.
Figure 2.
COS/H2S Release from OA-CysTCM-1 (50 μM) in the presence of 0 μM (black), 50 μM (red), 250 μM (blue), and 500 μM (green) Cys. The experiments were performed in triplicate and results are expressed as mean ± SD (n = 3).
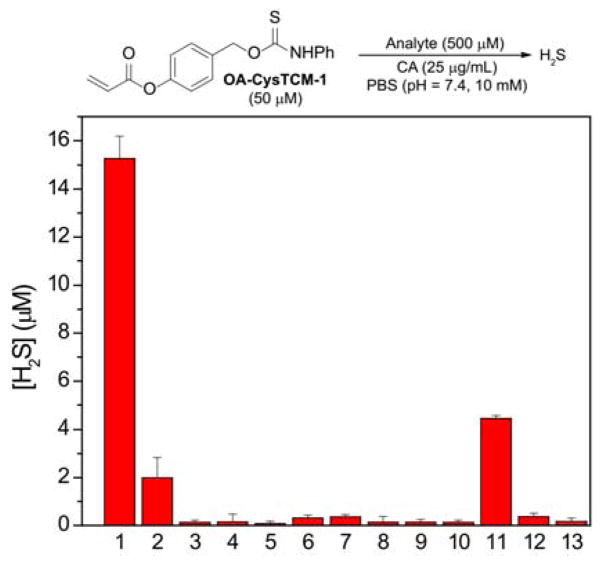
To further demonstrate that the observed H2S release is due to Cys activation via the proposed mechanism, we pretreated Cys with N-ethylmaleimide (NEM), a Cys scavenger, for 20 min, followed by the addition of OA-CysTCM-1. When compared to the regular activation conditions (Figure 3, bar 1), NEM pretreatment significantly diminished H2S release from OA-CysTCM-1, confirming that Cys was required for donor activation (Figure 3, bar 2). No H2S release was observed in the absence of CA, and, similarly, pretreatment of CA with acetazolamide (AAA), a CA inhibitor, also failed to provide H2S, confirming that H2S release from OA-CysTCM-1 proceeds through a COS-dependent pathway (Figure 3, bars 3 and 4).
Figure 3.
COS/H2S Release from OA-CysTCM-1 (50 μM) in the presence of cellular nucleophiles (500 μM): (1) Cys, (2) Cys + NEM (10 mM), (3) Cys - CA, (4) Cys + AAA (10 μM), (5) Hcy, (6) NAC, (7) GSH (5.0 mM), (8) Ser, (9) Lys, (10) GSSG, and (11) PLE (1 U/mL). Cys (500 μM) effects on OA-CysCM-1 (12), and OA-TCM-1 (13) toward COS/H2S release. H2S concentration was measured after 3-h incubation. The experiments were performed in triplicate and the results were expressed as mean ± SD (n = 3).
In addition to Cys, other biologically relevant nucleophiles, such as GSH, oxidized glutathione (GSSG), Hcy, N-acetylcysteine (NAC), serine (Ser), and lysine (Lys), were evaluated towards donor activation. As expected, none of these species triggered OA-CysTCM-1, and no COS/H2S release was observed due to the lack of the addition/cyclization activation sequence (Figure 3, bars 5–10). Considering the high abundance of GSH in cellular environment, we also evaluated Cys-triggered COS/H2S release from OA-CysTCM-1 in the presence of GSH. In these experiments, OA-CysTCM-1 (50 μM) was co-incubated with Cys (500 μM) and GSH (0 – 1000 μM) and COS/H2S release was monitored by MB assay (Figure S2). A decrease of H2S release was observed as the GSH concentration increased, indicating a potential GSH-induced donor consumption. Although the effects of GSH were not significant in aqueous buffer, it should be taken into consideration when applying donors in biological systems. Since the acrylate trigger may be prone to esterase-catalyzed hydrolysis, we also incubated OA-CysTCM-1 with porcine liver esterase (PLE) to determine whether common esterases could generate COS/H2S release. Although we did observe H2S release, it was significantly less efficient than Cys activation (Figure 3, bar 11). As expected, treatment of control compounds OA-CysCM-1 and OA-TCM-1 with Cys in the presence of CA failed to generate H2S, demonstrating that both the aryl-acrylate trigger and the caged COS-containing thiocarbamate scaffold are crucial for COS release from this scaffold (Figure 3, bars 12 and 13). Taken together, these selectivity studies demonstrate that OA-CysTCM-1 is highly sensitive towards Cys activation to release COS/H2S and inert to activation by other biomolecules, such as GSH, GSSG, Hcy, NAC, Ser, and Lys.
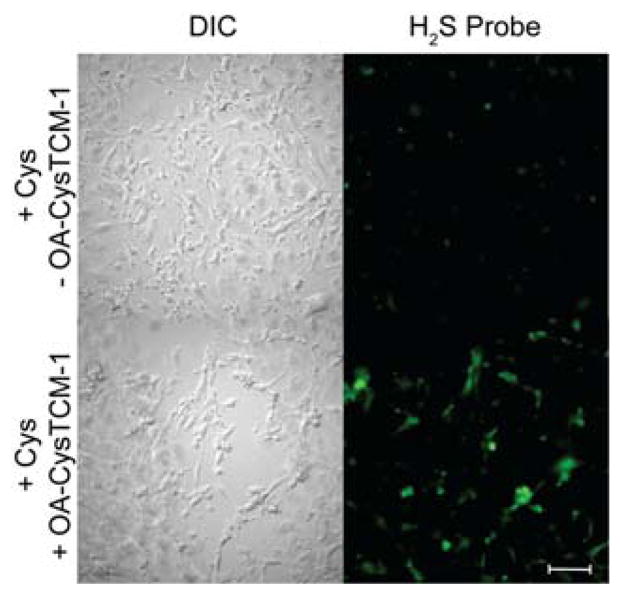
We next sought to confirm that OA-CysTCM-1 would release COS/H2S upon reaction with Cys in a cellular environment. We incubated bEnd.3 cells with OA-CysTCM-1 in the presence of Cys and visualized H2S-release using SF7-AM, a cell-trappable H2S-responsive fluorescent probe.19 In the absence of OA-CysTCM-1, negligible SF7-AM fluorescence was observed, suggesting a minimum amount of endogenous H2S present in bEnd.3 cells. By contrast, addition of OA-CysTCM-1 resulted in a significant increase in SF7-AM fluorescence, confirming that OA-CysTCM-1 can be activated by Cys to release H2S in a cellular environment (Figure 4). These results demonstrate that OA-CysTCM-1 is a potent COS/H2S donor and Cys-triggered H2S delivery can be visualized in complex biological systems, indicating applications of OA-CysTCM-1 as a potential H2S-related therapeutic or research tool.
Figure 4.
H2S Release from OA-CysTCM-1 in bEnd.3 cells. Top: DIC (left) and GFP (right) channels with cysteine (250 μM) and SF7-AM (5 μM). Bottom: DIC (left) and GFP (right) channels with cysteine (250 μM), OA-CysTCM-1 (100 μM), and SF7-AM (5 μM). Scale bar represents 100 μM.
In summary, we prepared and evaluated OA-CysTCM-1 as a Cys-triggered COS/H2S donor. Our studies demonstrate that OA-CysTCM-1 is stable in aqueous media and does not release COS/H2S until being activated by Cys. Importantly, H2S delivery from OA-CysTCM-1 is observed in a cellular environment, indicating OA-CysTCM-1 can be used as a new efficacious Cys labile COS/H2S donor in complex biological systems. Taken together, our investigations demonstrate that H2S delivery from OA-CysTCM-1 can be controlled and regulated through a COS-dependent pathway, making OA-CysTCM-1 a new member of COS-based H2S donor family with potential applications in the study of both H2S and COS chemical biology, especially when used in combination with available Cys-activated H2S donors. Further applications of this as well as other COS-releasing constructs are currently ongoing in our laboratory.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (MDP; R01GM113030), Dreyfus Foundation, and NSF/GRFP (AKS; DGE-1309047). NMR spectroscopy and fluorescence microscopy instrumentation in the CAMCOR facility is supported by the NSF (CHE-1427987, CHE-1531189).
Footnotes
Electronic Supplementary Information (ESI) available: experimental details, H2S release measurements, NMR spectra. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare
Notes and references
- 1.Abe K, Kimura H. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Szabo C. Antioxid Redox Signal. 2012;17:68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang R. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]; (c) Filipovic MR, Zivanovic J, Alvarez B, Banerjee R. Chem Rev. 2018;118:1253–1337. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Hartle MD, Pluth MD. Chem Soc Rev. 2016;45:6108–6117. doi: 10.1039/c6cs00212a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Szabo C, Papapetropoulos A. Pharmacol Rev. 2017;69:497. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao Y, Biggs TD, Xian M. Chem Commun. 2014;50:11788–11805. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao Y, Pacheco A, Xian M. Handb Exp Pharmacol. 2015;230:365–388. doi: 10.1007/978-3-319-18144-8_18. [DOI] [PubMed] [Google Scholar]; (e) Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Med Res Rev. 2018;38:57–100. doi: 10.1002/med.21433. [DOI] [PubMed] [Google Scholar]
- 4.(a) Steiger AK, Pardue S, Kevil CG, Pluth MD. J Am Chem Soc. 2016;138:7256–7259. doi: 10.1021/jacs.6b03780. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Steiger AK, Zhao Y, Pluth MD. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Henthorn HA, Pluth MD. J Am Chem Soc. 2017;139:16365–16376. doi: 10.1021/jacs.7b09527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Pluth MD. Angew Chem Int Ed. 2016;55:14638–14642. doi: 10.1002/anie.201608052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Steiger AK, Marcatti M, Szabo C, Szczesny B, Pluth MD. ACS Chem Biol. 2017;12:2117–2123. doi: 10.1021/acschembio.7b00279. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chauhan P, Bora P, Ravikumar G, Jos S, Chakrapani H. Org Lett. 2017;19:62–65. doi: 10.1021/acs.orglett.6b03336. [DOI] [PubMed] [Google Scholar]
- 8.Powell CR, Foster JC, Okyere B, Theus MH, Matson JB. J Am Chem Soc. 2016:13477–13480. doi: 10.1021/jacs.6b07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiger AK, Yang Y, Royzen M, Pluth MD. Chem Commun. 2017;53:1378–1380. doi: 10.1039/c6cc09547j. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zhao Y, Bolton SG, Pluth MD. Org Lett. 2017;19:2278–2281. doi: 10.1021/acs.orglett.7b00808. [DOI] [PubMed] [Google Scholar]; (b) Sharma AK, Nair M, Chauhan P, Gupta K, Saini DK, Chakrapani H. Org Lett. 2017;19:4822–4825. doi: 10.1021/acs.orglett.7b02259. [DOI] [PubMed] [Google Scholar]
- 11.(a) Malwal SR, Sriram D, Yogeeswari P, Konkimalla VB, Chakrapani H. J Med Chem. 2012;55:553–557. doi: 10.1021/jm201023g. [DOI] [PubMed] [Google Scholar]; (b) Pardeshi KA, Malwal SR, Banerjee A, Lahiri S, Rangarajan R, Chakrapani H. Bioorg Med Chem Lett. 2015;25:2694–2697. doi: 10.1016/j.bmcl.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Silva Sousa EH, Ridnour LA, Gouveia FS, Jr, Silva da Silva CD, Wink DA, de Franca Lopes LG, Sadler PJ. ACS Chem Biol. 2016;11:2057–2065. doi: 10.1021/acschembio.6b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalzoppo D, Di Paolo V, Calderan L, Pasut G, Rosato A, Caccuri MA, Quintieri L. Anticancer Agents Med Chem. 2017;17:4–20. [PubMed] [Google Scholar]
- 14.(a) Blondeau P, Gauthier R, Berse C, Gravel D. Can J Chemistry. 1971;49:3866–3876. [Google Scholar]; (b) Leonard NJ, Ning RY. J Org Chem. 1966;31:3928–3935. doi: 10.1021/jo01350a011. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Guo Y, Strongin RM. Angew Chem Int Ed. 2011;50:10690–10693. doi: 10.1002/anie.201103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Nawimanage RR, Prasai B, Hettiarachchi SU, McCarley RL. Anal Chem. 2017;89:6886–6892. doi: 10.1021/acs.analchem.7b01384. [DOI] [PubMed] [Google Scholar]; (b) Qi Y, Huang Y, Li B, Zeng F, Wu S. Anal Chem. 2018;90:1014–1020. doi: 10.1021/acs.analchem.7b04407. [DOI] [PubMed] [Google Scholar]; (c) Wang JF, Li B, Zhao WY, Zhang XF, Luo X, Corkins ME, Cole SL, Wang C, Xiao Y, Bi XM, Pang Y, McElroy CA, Bird AJ, Dong YZ. Acs Sensors. 2016;1:882–887. [Google Scholar]; (d) Wu Q, Wang K, Wang Z, Sun Y, Cao D, Liu Z, Guan R, Zhao S, Yu X. Talanta. 2018;181:118–124. doi: 10.1016/j.talanta.2017.12.062. [DOI] [PubMed] [Google Scholar]
- 17.Soboleva T, Esquer HJ, Benninghoff AD, Berreau LM. J Am Chem Soc. 2017;139:9435–9438. doi: 10.1021/jacs.7b04077. [DOI] [PubMed] [Google Scholar]
- 18.Steiger AK, Zhao Y, Choi WJ, Crammond A, Tillotson MR, Pluth MD. Biochem Pharmacol. 2018;149:124–130. doi: 10.1016/j.bcp.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin VS, Lippert AR, Chang CJ. Proc Natl Acad Sci U S A. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.