Abstract
Considering the current trends in life expectancy, women in the modern era are challenged with facing menopausal symptoms as well as heightened disease risk associated with increasing adiposity and metabolic dysfunction for up to three decades of life. Treatment strategies to combat metabolic dysfunction and associated pathologies have been hampered by our lack of understanding regarding the biological underpinnings of these clinical conditions and our incomplete understanding of the effects of estrogens and the tissue-specific functions and molecular actions of its receptors. In this review we provide evidence supporting a critical and protective role for the estrogen receptor α specific form in the maintenance of metabolic homeostasis and insulin sensitivity. Studies identifying the ER-regulated pathways required for disease prevention will lay the important foundation for the rational design of targeted therapeutics to improve women’s health while limiting complications that have plagued traditional hormone replacement interventions.
Keywords: Metabolic syndrome, Estrogen action, Insulin resistance, Obesity
1. Introduction
The National Vital Statistics report from the Centers for Disease Control indicates that life expectancy has increased by 68% to 80.6 years of age for Caucasian women over the past century, with a noted gender disparity – reduced life expectancy for males of almost 5 years. Considering that menopause occurs at ~51 years (National Institutes of Health, NIA www.nia.nih.gov), women in the modern era are challenged with facing menopausal symptoms including hot flashes, sleep and mood disorders, and sexual dysfunction as well as heightened disease risk associated with increasing adiposity and metabolic dysfunction for up to three decades of life. Arming women with knowledge about the health consequences of ovarian failure as well as furthering our understanding of the biological actions of estrogens in reproductive and non-reproductive tissues have become critical endeavors if we wish to improve the health of women around the world. Although many researchers and clinicians have focused on the impact of replacement estrogens to ameliorate clinical symptoms and provide protective health benefit, an incomplete understanding of the tissue-specific effects of estrogen action, and estrogen receptor (ER) distribution and function contributes to our confusion and failure to advance therapeutic strategies to combat female-associated pathologies.
Because of the dramatic increase in life expectancy since the turn of the century, the contribution of sex hormone deficiency to the pathogenesis of metabolic associated diseases has become a therapeutic challenge of the 21st century. Understanding how estrogens and estrogen receptor expression contribute to energy balance, glucose homeostasis, and adipose tissue development promises to yield novel therapeutic applications. Here we review evidence in humans and rodents supporting a role of estrogens and their receptors in regulating fuel homeostasis and insulin sensitivity for the preservation of disease-free health (Fig. 1). We will also present basic research suggesting that the estrogen receptor (ER), specifically the α form of the receptor, ERα, is an important target to combat metabolic dysfunction.
Fig. 1.
Schematic overview. The role of estrogen receptor (ER) α action in the maintenance of glucose homeostasis and insulin action for the protection against obesity and chronic diseases associated with metabolic dysfunction including atherosclerosis, type 2 diabetes and certain forms of cancer.
2. Molecular mechanisms of estrogen receptor (ER) action
Early studies in reproductive tissues investigating the actions of estradiol (E2) led to the paradigm of classical nuclear ERs as ligand-activated transcription factors (Fig. 2) (O’Malley, 1971). Although ERs exist in two main forms, α and β, which have multiple splice variants of unknown function, ERs exhibit tissue specificity in expression and function (Nilsson et al., 2001). The classical, or genomic mechanism of ER action, includes the ligand-activated dissociation of ER from its chaperone, receptor dimerization, and receptor binding either to estrogen response elements (ERE) in target gene promoters or to AP-1 or SP-1 response elements through association/tethering with other transcription factors bound to DNA (Fig. 2) (Safe and Kim, 2008). After binding, ER dimers interact with basal transcription factors leading to activation or repression of target gene expression. Overlap in binding sites for E2-liganded ERα and ERβ is observed when receptors are expressed individually; however, when both ERs are present, few sites are shared. Each ER restricts the binding site occupancy of the other, with ERα dominating (Charn et al., 2010). Moreover, ligand-activated ER promotes transcription in a cyclic fashion. The repeated cycling of the receptor complex on and off target promoters in the presence of continuous E2 stimulation may represent a mechanism of continuous sensing and adaptation to the external hormonal milieu to yield the appropriate transcriptional response (Shang et al., 2000).
Fig. 2.
Molecular actions of ERα to activate or repress target genes by classical, DNA binding, or non-genomic actions. ERE, estrogen response element in target gene promoters; P, phosphorylation; TF, transcription factor.
In addition to classical signaling, E2-ERα can act within minutes or seconds via extranuclear and membrane-associated forms of the receptor (Fig. 2) (Hammes and Levin, 2007). Membrane-associated receptors are localized to caveolae where they congregate with other signaling molecules, including G proteins, growth factor receptors, tyrosine kinases (Src), linker proteins (MNAR), and orphan G-protein coupled receptors (GPCRs). In a variety of cell types, membrane and extranuclear pools of ERs activate protein kinases that phosphorylate transcription factors to promote their nuclear translocation and transcriptional action (Filardo et al., 2000; Hammes and Levin, 2007; Tiano and Mauvais-Jarvis, 2012a). The G protein-coupled estrogen receptor (GPER), or GPR30, has been reported to respond to E2; however, its role as an ER is still controversial and thus will not be discussed in this review. Although it is thought that estrogen effects on reproductive function are almost exclusively mediated via classical nuclear ERs acting as ligand-activated transcription factors, a proportion of ER action related to energy metabolism may also involve extranuclear ERs (Fig. 2) (Liu and Mauvais-Jarvis, 2010). A central issue in the field pertaining to the tissue-specific sites of ERα action and the molecular mechanisms by which the receptor selectively activates or represses target genes remains to be resolved.
3. The impact of brain estrogen action in the regulation of energy intake and expenditure
Studies in humans and animal models have established important roles for estrogens in the regulation of metabolism. As estrogen levels decline during and following menopause, the prevalence of obesity escalates markedly (Carr, 2003; Flegal et al., 2002; Freedman et al., 2002). Ovariectomy (OVX), i.e. removal of the ovaries, leads to increased adiposity in rodents (Blaustein and Wade, 1976; Drewett, 1973; Wallen et al., 2001), that is prevented by estrogenization typically by subcutaneous implantation of a time-release estrogen pellet (Geary et al., 2001). Although OVX induces a transient increase in food intake, prevented by E2 normalization (Clegg et al., 2003a; Gao et al., 2007), hyperphagia cannot solely account for the changes in metabolism and the development of obesity (Wallen et al., 2001). Furthermore, mice of both sexes lacking the enzyme CYP19, aromatase (aromatase catalyzes the synthesis of estradiol), develop obesity in the absence of hyperphagia. Instead, aromatase-deficient mice exhibit reduced physical activity and diminished lean body mass (Jones et al., 2000). Similarly, mice of both genders with a homozygous null mutation for Esr1 (ERα) develop obesity in the absence of hyperphagia (Heine et al., 2000; Ribas et al., 2009). This work suggests that although endogenous E2 favors body weight homeostasis by increasing energy expenditure (Rogers et al., 2009a), exogenous estrogens may promote energy balance by influencing both energy intake and expenditure. Thus, loss of ERα action produces a predominant decrease in energy expenditure, while conversely, increasing ERα signaling by raising serum E2 concentrations both suppresses energy intake and increases energy expenditure as illustrated in Fig. 3 and will be discussed later.
Fig. 3.

The effects of ERα action in POMC and SF-1 neurons to control feeding and energy expenditure. Findings from Xu et al. (2011).
For over a century we have known that specific regions of the CNS control food intake, energy expenditure and weight gain, as lesioning of specific hypothalamic nuclei within the ventral medial hypothalamus (VMH) (Louis-Sylvestre, 1980; Smith, 2000) or lateral hypothalamus (LH) caused dramatic changes in these biological processes (Danguir and Nicolaidis, 1980; Milam et al., 1980). ERα is highly expressed in the rodent brain including the ventrolateral portion of the VMN, the ARC, the medial preoptic area (MPOA), and the paraventricular nuclei (PVN) (Merchenthaler et al., 2004; Osterlund et al., 1998; Shima et al., 2003; Simerly et al., 1990; Simonian and Herbison, 1997; Voisin et al., 1997; Wilkinson et al., 2002). ERβ is expressed in the same hypothalamic nuclei but at significantly lower levels by comparison. The effects of E2 on energy balance are thought to be primarily mediated by ERα, as women or female mice with mutations in the ERα gene become obese (Heine et al., 2000; Ohlsson et al., 2000). Moreover, ERα homozygosity in animals prevents the anti-obesity effects of estrogen replacement (Geary et al., 2001). These gene deletion studies are consistent with pharmacological interventions in ovariectomized (OVX) mice in which the selective ERα agonist PPT was shown to suppress food intake, in contrast to the selective ERβ agonist DPN which failed to influence feeding behavior (Liang et al., 2002; Roesch, 2006; Santollo et al., 2007).
The signaling mechanisms of ER action in hypothalamic neurons are not fully defined, but evidence suggests the involvement of both classical and extranuclear ER actions (Dhillon and Belsham, 2011; Gao et al., 2007). In the arcuate nucleus (ARC), ERα is highly expressed in pro-opiomelanocortin (POMC) neurons shown to modulate food intake, energy expenditure, and reproduction (Fig. 3) (Xu et al., 2011). POMC neurons secrete α melanocyte stimulating hormone (αMSH), which acts in the PVN and lateral hypothalamus on melanocortin 3 and melanocortin 4 (MC3/MC4) receptors to produce catabolism by reducing food intake and increasing energy expenditure (Elias et al., 1999, 2000; Elmquist et al., 1998a, 1999). ARC POMC mRNA levels fluctuate over the course of the estrous cycle, with a marked increase occurring coincident with proestrous when E2 concentration is the highest (Bohler et al., 1991; Gao et al., 2007; Korner et al., 1999; Slamberova et al., 2004; Wise et al., 1990). Conversely, POMC levels are reduced in ERα knockout mice (Hirosawa et al., 2008). These E2-induced synaptological rearrangements in POMC neurons are tightly paralleled with the effects of estrogens on food intake, energy expenditure and body weight (Gao et al., 2007), and appear mediated by MC4R (Polidori and Geary, 2002). Indeed, deletion of ERα in POMC neurons of mice leads to hyperphagia without affect to energy expenditure or adipose tissue distribution (Xu et al., 2011).
The ventral medial hypothalamus, VMH, is also thought to be an important neural circuit responsible for the homeostatic regulation of body weight and food intake, as estrogens have been shown to directly alter the electrophysiological properties of VMH neurons (Minami et al., 1990). Small hairpin (sh) RNA-mediated ERα gene silencing and selective ablation of ERα from SF-1 containing neurons of the VMH blunts E2-induced weight loss, promoting increased visceral fat deposition, and reductions in energy expenditure in the absence of hyperphagia (Fig. 3) (Musatov et al., 2007). These findings support the notion that ERα signaling in VMH neurons plays an important role in regulating physical activity, thermogenesis and fat distribution.
Since leptin was first described in 1994 (Zhang et al., 1994), it has proven to be a powerful catabolic signal in the brain, inhibiting food intake and increasing energy expenditure (Elmquist et al., 1999; Myers et al., 2010; Schwartz et al., 2000). The leptin receptor (Lepr) is localized in several brain areas including the VMH and the ARC, and colocalizes with several neuropeptides known to control food intake and reproduction (Elmquist et al., 1998b, 1998c; van Dijk et al., 1996). ERα crosstalks with Lepr in the ARC (Diano et al., 1998), which is consistent with estrogens inducing Lepr mRNA (Bennett et al., 1999), possibly by direct genomic action mediated by ERE binding in the promoter of the leptin receptor gene (Lindell et al., 2001). The extensive hypothalamic colocalization of these two receptors suggests a closely coupled interaction between peripheral derived signals in the regulation of energy homeostasis. Indeed, increased E2 is associated with enhanced central leptin sensitivity in rodents (Ainslie et al., 2001; Clegg et al., 2003b, 2006). Although circulating leptin levels do not change appreciably during the estrous cycle, ARC Lepr expression is highest during estrous and metestrous and is inversely correlated with neuropeptide Y (NPY) mRNA expression (Bennett et al., 1999). Moreover, OVX lowers the sensitivity to central leptin when compared to normally cycling females, and this leptin resistance is prevented by E2 replacement (Clegg et al., 2006). Analogously in male rodents, exogenous E2 administration also increases sensitivity to central leptin (Clegg et al., 2006). The differences in central leptin sensitivity caused by the presence or absence of estrogens may occur downstream of Lepr transcription and translation, possibly at the level of STAT3, as E2 decreases food intake and increases energy expenditure, resulting in a reduction in body weight in leptin-deficient (ob/ob) and leptin resistant (db/db) mice of both sexes (Gao et al., 2007).
NPY is an effective anabolic peptide considering that central administration of NPY potently increases food intake and decreases energy expenditure and fat oxidation (Chavez et al., 1995; Cone et al., 2001; Herzog, 2003; Levin, 1999). ARC neurons co-express NPY mRNA and Lepr protein. Leptin administration decreases, while leptin deficiency or leptin resistance increases NPY (and AgRP) mRNA, demonstrating that leptin is a critical determinant of ARC NPY function (Baskin et al., 1999). NPY neurons in the hypothalamus not only affect feeding, but also influence reproduction; therefore, E2 can modulate both of these neuroendocrine systems by regulating NPY gene expression, NPY Y1 receptor expression (Hill et al., 2004), and NPY release (Bonavera et al., 1994). Additionally, NPY/AgRP neurons are required to mediate the anorexigenic effects of E2 and hypothalamic expression of NPY and AgRP is tightly regulated across the estrus cycle, with the lowest levels during estrus concomitant with the E2 peak and feeding nadir in wild type mice (Olofsson et al., 2009). Importantly, the cyclic changes in food intake and E2-induced anorexia are blunted in mice with degenerated NPY/AgRP neurons (Olofsson et al., 2009), indicating that neurons co-expressing NPY and AgRP are functionally required for the cyclic changes in feeding across estrous cycle and that NPY/AgRP neurons are essential mediators of the anorexigenic function of E2. Interestingly, it was shown that ERα is completely excluded from NPY/AgRP neurons in the mouse hypothalamus (Olofsson et al., 2009), suggesting that E2 may regulate these neurons indirectly via unknown presynaptic neurons expressing ERα.
4. ERα expression in the control of adipose tissue distribution and obesity susceptibility
There is a well-described sexual dimorphism in body fat distribution as men have less total body fat but increased intra-abdominal adipose tissue compared with women who have more total fat but a higher proportion of gluteal/femoral subcutaneous (SC) adipose tissue which is shown to be less pathogenic (Bjorntorp, 1992a, 1992b, 1992c; Bouchard et al., 1993; Enzi et al., 1986). Following menopause and the decline in circulating E2, women gain intra-abdominal fat and develop male-pattern adiposity ameliorated by E2 replacement therapy (Gambacciani et al., 1997; Haarbo et al., 1991a, 1991b). Additionally, adipose tissue redistribution is reported in male–female transsexuals receiving E2 supplementation in which increased SC fat relative to intra-abdominal adipose tissue is observed (Elbers et al., 1999a). Thus, estrogens are thought to regulate fat distribution (Elbers et al., 1999a, 1999b), and this may contribute to improved metabolic profile in women receiving HRT.
Accumulation of central intra-abdominal or visceral fat (‘android’, or male-pattern obesity) is correlated with increased risk and mortality from diabetes and atherosclerosis (Wajchenberg, 2000). Intra-abdominal adipose tissue is thought to be metabolically and functionally different from SC adipose tissue. Indeed, compared with the SC fat, intra-abdominal adipose tissue has more efferent sympathetic axons and capillaries, per unit volume, and these capillaries drain into the hepatic portal system (Wajchenberg, 2000). Surgical removal of intra-abdominal adipose tissue in humans results in decreased insulin and glucose levels (Thorne et al., 2002) and prevents the onset of insulin resistance and glucose intolerance in male rodents (Gabriely et al., 2002). In contrast, surgical removal of SC fat tissue of equal weight has no appreciable impact on the same parameters (Gabriely et al., 2002). Teleologically, males may preferentially deposit adipose tissue in the intra-abdominal depot because of its ability to be rapidly mobilized providing a quick and abundant energy source during times of increased energy demand. In contrast, SC adipose tissue allows for efficient storage of maximal calories per unit volume of tissue. Lipid deposition into SC adipose tissue may provide an evolutionary advantage for females by extending protection during limited caloric supply in order to maintain reproductive function. Importantly, females also mobilize adipose tissue stored in SC depots to meet the caloric demands placed on the body during breast feeding. In contrast to android or male-pattern adiposity, ‘gynoid’ or female-pattern fat deposition is only weakly correlated with metabolic dysfunction and disease risk (Bjorntorp, 1996; Donahue and Abbott, 1987; Donahue et al., 1987; Lapidus et al., 1984; Ohlson et al., 1985). In fact, transplantation of SC fat from donor mice into visceral regions of recipient mice decreases total fat and improves glucose homeostasis suggesting that adipose-secreted factors may act systemically to improve metabolism (Tran et al., 2008).
In addition to ovarian production, estrogens are also synthesized by adipocytes (via aromatization of androgenic precursors by CYP19), and circulating levels are elevated in proportion to total body adiposity (Schneider et al., 1979; Tchernof et al., 1995). ERα is highly expressed in adipose tissue (Mizutani et al., 1994; Price and O’Brien, 1993), and targeted global deletion of the ERα gene (ERαKO) in mice of both genders promotes increased adiposity, with a near doubling of the visceral depots compared with age-matched wildtype (WT) mice (Fig. 4) (Heine et al., 2000). In contrast, mice with a homozygous deletion of ERβ (βERKO) show a body composition identical to WT mice, suggesting that ERβ may play a limited role in adipose tissue development and metabolism (Ogawa et al., 1999). Reduced ERα expression or impaired ERα function due to genetic alteration has been linked with increased prevalence of specific features of the metabolic syndrome including obesity in both male and female humans and rodents (Casazza et al., 2010; Deng et al., 2000; Nilsson et al., 2007; Okura et al., 2003a, 2003b, 2003c; Smith et al., 1994; Yamada et al., 2002). The role of ERα in adipocytes and the phenotypic outcomes of adipose-specific ERα deletion in mice are currently under investigation by several laboratories, however initial phenotypic studies indicate that adipose tissue specific deletion of ERα increases adiposity (Drewet al., 2015; Kim et al., 2014). Whether the obesity phenotype observed in whole body ERαKO mice or in women harboring polymorphisms in ESR1 manifests as a consequence of impairment in ERα action in adipose tissue specifically and or as a secondary phenotype of ERα inactivation in other metabolic tissues remains unclear.
Fig. 4.
ERαKO metabolic phenotype. Animals with a homozygous deletion of ERα develop insulin resistance, impaired glucose tolerance and obesity as early as 3–6 months of age and are more susceptible to the deleterious effects of HFD on metabolism and insulin action than age-matched WT mice (Heine et al., 2000; Ribas et al., 2010a).
5. Estrogen action and insulin sensitivity
Insulin resistance is a central disorder in the pathogenesis of type 2 diabetes and a defining feature of the metabolic syndrome, a clustering of metabolic abnormalities including obesity, hypertension, glucose intolerance and dyslipidemia (DeFronzo et al., 1992; Miranda et al., 2005). Normally cycling women show enhanced insulin sensitivity compared to men when normalized to lean mass, and this is a likely contributor to the reduced incidence of type 2 diabetes in pre-menopausal women (Park et al., 2003; Yki-Jarvinen, 1984). Moreover, although a 40–50% reduction in insulin-mediated glucose disposal is consistently observed in male mice following high fat feeding (Choi et al., 2007; Hevener et al., 2007), E2-replete females, humans and rodents, are often protected against a high fat diet and acute fatty acid-induced insulin resistance (Djouadi et al., 1998; Frias et al., 2001; Hevener et al., 2002; Hong et al., 2009). Following menopause or OVX, a precipitous decline in insulin sensitivity coincides with increased fat mass, and elevated circulating inflammatory markers, LDL, triglycerides, and fatty acids (Carr, 2003; Pfeilschifter et al., 2002; Sites et al., 2002). OVX mice and rats are insulin resistant, show impaired exercise-stimulated glucose disposal into muscle (Campbell and Febbraio, 2002), and are more susceptible to the deleterious effects of high fat diet or lipid oversupply, and these physiological consequences of OVX are prevented by restoration of circulating E2 within a physiological concentration (Stubbins et al., 2012).
Although chronic administration of E2 is shown to improve insulin sensitivity, at least in rodents, the acute action of E2 to promote insulin-stimulated glucose uptake into muscle remains disputed; this despite consistent observations of E2-induced activation of Akt and AMP-activated Protein Kinase (AMPK) (Gorres et al., 2011; Rogers et al., 2009b). Furthermore, although administration of intravenous conjugated estrogens and E2 to postmenopausal women or OVX rats, respectively, is shown to stimulate a significant increase in glucose disposal during hyperinsulinemic–euglycemic clamp studies (Alonso et al., 2010; Van Pelt et al., 2003), ex vivo treatment of skeletal muscle with E2 failed to recapitulate the same increase in insulin-stimulated glucose disposal (Rogers et al., 2009b). This is also in contrast to short-term E2 effects on insulin action in myotubes from postmenopausal women and age-matched men (Salehzadeh et al., 2011). Thus, two questions remain: does E2 enhance insulin sensitivity and what are the critical tissues of E2 action that confer protection against insulin resistance induced by nutrient oversupply?
Similar to findings for ovarian failure in women and rodents, a reduction in circulating estrogens resulting from rare inactivating mutations or experimental deletion of Cyp19 (aromatase) in mice confers an obesity-insulin resistance phenotype (Guercio et al., 2009; Jones et al., 2000, 2007; Maffei et al., 2004, 2007; Morishima et al., 1995; Rochira et al., 2007; Smith et al., 1994; Takeda et al., 2003). The physiological and genetic evidence argues that E2 and ERα favor insulin sensitivity in rodents and humans of both sexes when E2 is maintained within a tight physiological concentration. Indeed, replacement or augmentation of E2 to supraphysiological levels or over-stimulation of ERs is thought to induce insulin resistance secondary to hyperinsulinemia and or a reduction in total GLUT4 expression in muscle (Barros et al., 2008; Nadal et al., 2009). In fact, two studies have reported that in postmenopausal women, higher plasma levels of E2 were prospectively associated with increased risk of developing T2D (Ding et al., 2007; Kalyani et al., 2009). Clearly, additional studies in rodents and humans using a dose–response strategy are necessary to better understand the interplay of steroid hormones including E2, testosterone, and progesterone, on the regulation of metabolism and insulin action in glucoregulatory tissues.
Although, several laboratories have characterized the whole body ERαKO mouse (Fig. 4, phenotype overview), many questions still remain including the tissues responsible for conferring the severe insulin resistance-obesity phenotype. Does obesity arise from loss of ERα within adipocytes specifically or can it be driven as a secondary phenotype resulting from a loss of ERα in brain, skeletal muscle, liver, or even selective immune cells? Furthermore, does a loss of ERα specifically from myocytes drive skeletal muscle insulin resistance, or does this phenotype arise as a secondary consequence of increased adiposity and altered adipokine/cytokine release?
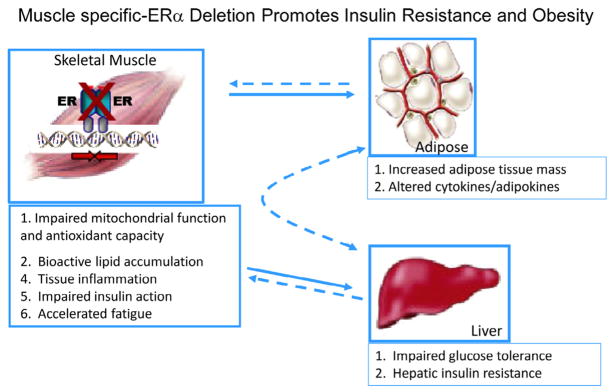
Although two forms of ER are expressed in many of the glucoregulatory tissues, ERα is found in much higher abundance than ERβ, as ERβ transcript is nearly undetectable in human and rodent muscle (Baltgalvis et al., 2010; Salehzadeh et al., 2011; Wiik et al., 2005). Consistent with these observations, homozygous deletion of ERβ failed to produce insulin resistance (Ohlsson et al., 2000) in contrast to the marked skeletal muscle insulin resistance observed in ERαKO animals (Fig. 4) (Riant et al., 2009; Ribas et al., 2010a). The underlying mechanism contributing to impaired insulin action in ERαKO animals remains disputed as findings reported by Bryzgalova et al. (2006), suggesting reduced total GLUT4 levels in muscle as an underlying cause for the ERαKO insulin resistance phenotype was not supported by Ribas et al. (2010a). Furthermore, despite maintenance of GLUT4 mRNA and protein, Ribas et al. reported more dramatic skeletal muscle insulin resistance in ERαKO mice than Bryzgalova et al. Work by Hevener and colleagues suggests that the skeletal muscle insulin resistance observed in ERαKO mice is predominantly due to direct effects of ERα deletion on mitochondrial function, pro-inflammatory signaling, and proximal insulin signal transduction.
Indeed, emerging findings in muscle from muscle-specific ERα knockout mice and myotubes with ERα knockdown show no alteration in GLUT4 mRNA or protein despite a marked impairment in insulin-stimulated glucose disposal. These observations in the muscle-specific ERα mouse are entirely consistent with those of whole body ERα KO mice (Figs. 4 and 5) (Ribas et al., 2010a, 2010b). Furthermore, additional studies by Barros et al. (2006, 2008) investigating alteration in GLUT4 expression in response to ovariectomy and E2 supplementation are in conflict with other published studies of similar design (Alonso et al., 2009; Baltgalvis et al., 2010; Campbell et al., 2003; Hansen et al., 1996; Salehzadeh et al., 2011). Given the lack of consensus ERE in the GLUT4 promoter (Barros and Gustafsson, 2011) and absence of confirmatory findings in cellular reporter and chromatin immunoprecipitation assays, the regulation of GLUT4 expression by ERα requires further investigation. GLUT4 expression is regulated by several redundant transcriptional pathways (Murgia et al., 2009; Zorzano et al., 2005). Redundancy in regulation is likely responsible for the maintenance of total GLUT4 transcript and protein levels in the absence of ERα. Maintenance of GLUT4 total protein is often observed even in the context of obesity and type 2 diabetes (Fu et al., 2009; Garvey et al., 1992, 1998; Hoeg et al., 2009). Considering the concomitant increase in ERα and GLUT4 expression observed in muscle of exercise-trained humans and mice, it is conceivable that while ERα deletion fails to impact GLUT4 expression, increased ERα action may mediate in part the training-induced increase in total GLUT4 content (Banks et al., 1992; Brozinick et al., 1993, 1994; Dela et al., 1994; Fu et al., 2009; Lemoine et al., 2002a; Rodnick et al., 1990).
Fig. 5.
The impact of skeletal muscle-specific ERα deletion on insulin sensitivity and adiposity in female mice (Ribas et al., 2010b).
Myocyte enhancer factor 2 (MEF2) expression and a functional MEF2 element in the GLUT4 promoter are critical for GLUT4 gene expression (Mora and Pessin, 2000). Furthermore, reciprocal positive regulation between ERα and MEF2 can be observed in cardiomyocytes via ERα interaction with class II HDAC (van Rooij et al., 2010). Despite complex transcriptional signal integration in the regulation of GLUT4 expression (Gan et al., 2011; Moreno et al., 2003; Murgia et al., 2009; Oshel et al., 2000; Smith et al., 2008; Zorzano et al., 2005), it is reasonable to speculate that elevated ERα action could promote increased GLUT4 transcription via heightened protein tethering with MEF2 on the GLUT4 promoter or by indirect action via AMPK (Gong et al., 2011; Rogers et al., 2009b). It is important to note that transcriptional activity of the GLUT4 promoter is quite low under basal conditions and other ovarian hormones, e.g. progesterone, are shown to play an antagonistic role in the regulation of GLUT4 expression (Campbell and Febbraio, 2002). These issues as well as the dose of E2 administration during interventional studies (presumably off-target effects of supraphysiological doses of E2 are deleterious to metabolism), the age and hormone status of the human subjects and rodents used are important considerations when interpreting the literature. Given the varying roles that muscle and adipose tissue play in controlling whole body metabolic homeostasis, it is likely that the interplay of transcriptional regulators of GLUT4 vary markedly between tissues. Taken together, these data suggest a potential role for ERα in enhancing GLUT4 transcription in muscle under certain conditions such as exercise, but not necessarily obligatory in the direct regulation of GLUT4 expression under basal conditions.
Collectively, work by Ribas et al. suggests that the skeletal muscle insulin resistance observed in whole body ERαKO mice and animals with a muscle-specific deletion of ERα is predominantly the result of impaired insulin signal transduction (Ribas et al., 2010b). A role for ERα in the regulation of proximal insulin signal transduction has been suggested previously as E2 administration to insulin resistant rodents increases insulin receptor substrate (IRS)-1 abundance and insulin-stimulated tyrosine phosphorylation and as well as the phosphotylation of Akt at Ser473 (Ordonez et al., 2008; Riant et al., 2009). Akt serves many functions in myocytes including ERα-induced regulation of myogenic differentiation (Galluzzo et al., 2009), suppression of muscle-atrophy associated ubiquitin ligases via FOXO1 inhibition (Stitt et al., 2004), and induction of genes associated with myocellular proliferation (Enns and Tiidus, 2008; Enns et al., 2008; Galluzzo et al., 2009; Kamanga-Sollo et al., 2010; Thomas et al., 2010). In breast cancer cell lines, endothelial cells and cortical neurons, ERα-specific binding and activation of PI3kinase as well as suppression of the tumor suppressor and PI3kinase inhibitory protein, PTEN, are well-established (Lee et al., 2005; Mannella and Brinton, 2006; Noh et al., 2011; Simoncini et al., 2000, 2002); however, studies on these direct interactions in skeletal muscle are limited. Additionally, E2 acting via ERα is also shown to promote phosphorylation of p38 MAPK (Ronda et al., 2010a, 2010b), a signaling cascade shown to enhance GLUT4 intrinsic activity and glucose uptake (Furtado et al., 2002; Niu et al., 2003; Sweeney et al., 1999). Furthermore, it is possible that ERα activation of Akt and MAPK pathways may underlie in large part the E2-mediated protection of muscle against age-induced sarcopenia (Chen et al., 2005; Leger et al., 2008; Messier et al., 2011; Sipila et al., 2001; Sorensen et al., 2001; Teixeira et al., 2003; Tiidus, 2000), exercise-induced muscle damage (Dieli-Conwright et al., 2009; Sotiriadou et al., 2006; Thomas et al., 2010; Tiidus, 2000), and myocyte apoptosis in the face of a variety of cellular perturbations (Boland et al., 2008; McLoughlin et al., 2009; Vasconsuelo et al., 2008; Wang et al., 2010). Thus, ERα stimulation of muscle growth and insulin sensitivity via direct and indirect interaction with these pathways requires further exploration.
In contrast to the protective effects of ERα, it is suggested that ERβ may promote insulin resistance in skeletal muscle when the ERβ:ERα ratio is elevated. In OVX mice, while ERα-specific activation by PPT improved muscle insulin action (Gorres et al., 2011), conversely, ligand-specific activation of ERβ by DPN failed to ameliorate insulin resistance (Gorres et al., 2011). Moreover, ovariectomy of hyperestrogenic female ERα-deficient mice was shown to improve glucose tolerance and insulin sensitivity presumably through elimination of ERβ activation by endogenous E2 (Naaz et al., 2002). Similarly, administration of an ERβ-selective agonist to male E2-deficient androgen receptor knockout (ArKO) mice decreased glucose uptake (Barros et al., 2006). Finally, evidence indicates that ERβ-deficiency protects against diet-induced insulin resistance in male mice by increasing PPARγ signaling in adipocytes, which indirectly improves skeletal muscle insulin action by promoting lipid accumulation in adipose tissue and diminishing ectopic lipid deposition in muscle (Barros et al., 2009; Foryst-Ludwig et al., 2008). A role for ERβ in the pathogenesis of human insulin resistance remains unknown and there is still much work to do in determining the tissue-specific interactions of these transcription factors under more physiological conditions.
6. ERα and skeletal muscle fatty acid metabolism and inflammation
Normally cycling women are protected against acute lipid-induced insulin resistance compared with estrogen-deficient women and men (Frias et al., 2001; Hoeg et al., 2011). Furthermore, muscle from premenopausal women shows enhanced insulin sensitivity despite 47% higher triglyceride content compared with age-matched men (Hoeg et al., 2009). These observations are consistent with a reduced respiratory quotient and greater reliance on the oxidation of fatty acids as a fuel source in women (Cortright and Koves, 2000). Interesting similarities between E2 replete women and exercise trained subjects including elevated muscle ERα expression (Lemoine et al., 2002a, 2002b; Wiik et al., 2005), heightened insulin sensitivity (Torgan et al., 1993), elevated muscle lipid tolerance (Amati et al., 2011), and enhanced oxidative capacity (Maher et al., 2010a; Turcotte et al., 1992) are consistently observed. Estrogen supplementation is shown to enhance lipid oxidation in men during acute endurance exercise (Hamadeh et al., 2005) as well as palmitate oxidation inmyotubes from male subjects ex vivo (Salehzadeh et al., 2011). The effect of E2 to increase the expression of fatty acid transport protein FAT/CD36 and FABP as well as transcription factors and key enzymes that regulate oxidative metabolism (Campbell et al., 2003; Fu et al., 2009; Maher et al., 2010b) likely underlie these observations in male subjects. In addition, exercise and E2 are shown to rapidly stimulate AMPK phosphorylation in both muscle and myotubes (D’Eon et al., 2008; Rogers et al., 2009b). AMPK is considered a central regulator of many cellular processes including growth, mitochondrial biogenesis, and oxidative metabolism (Hardie, 2011; Mihaylova and Shaw, 2011). Similar to the effects of E2, the ERα-selective agonist PPT stimulates AMPK phosphorylation in muscle of female rats (Gorres et al., 2011) while OVX or whole body ERα deletion is associated with reduced skeletal muscle levels of phosphorylated AMPK (Kim et al., 2010; Ribas et al., 2010a). Muscle PPARα, PPARδ, and UCP2 expression are also reduced in whole body ERαKO mice suggesting that ERα is essential in the regulation of a coordinated program regulating oxidative metabolism. Importantly, although the impairment in muscle fatty oxidation was recapitulated in the muscle-specific ERαKO mice (MERKO), no alteration in basal p-AMPK, PPARα, PPARδ, or UCP2 was observed (Ribas et al., 2010b), thus suggesting that these specific alterations in muscle gene expression in ERαKO mice are secondary to the loss of ERα in other metabolic tissues, likely the CNS, adipose or liver. Despite these model differences, the skeletal muscle insulin resistance and bioactive lipid accumulation (triacylglycerol, diacylglycerol and ceramides) was surprisingly similar between ERαKO and MERKO. Consistent with these observations, oxygen consumption rates in C2C12 myotubes with ERα knockdown were reduced significantly. In addition, mitochondria from muscle cells depleted of ERα produced high levels of reactive oxygen species thus precipitating increased cellular oxidative stress. Collectively, these data support the notion that ERα is critical for maintaining fatty acid oxidation in skeletal muscle by mechanisms including the regulation of: (1) fatty acid transport into the cell, (2) activation of intermediary signaling critical for shifting substrate metabolism, (3) transcriptional regulators of fatty acid metabolism and mitochondrial function. Thus, ERα expression in skeletal muscle may be a central regulator of adiposity by indirect action as MERKO mice reproduced the obesity phenotype observed in the whole body ERαKO (Fig. 5).
Moreover, E2 treatment reduces HFD-induced insulin resistance in skeletal muscle by 50% (assessed by hyperinsulinemic–euglycemic clamp) in an ERα-dependent manner (Riant et al., 2009). The mechanistic link between the accumulation of lipid intermediates, activation of inflammatory signaling cascades, and impaired insulin action is shown inmyocytes and rodent muscle, and indeed these factors are observed concurrently in obese, type 2 diabetic subjects (Adams et al., 2004; Itani et al., 2002; Wellen and Hotamisligil, 2005; Yang et al., 2009a), as well as muscle from whole body and muscle-specific ERαKO mice (Ribas et al., 2010a). Bioactive lipid intermediates including diacylglycerol and ceramides are believed to activate stress kinases including IKKβ, c-Jun-N-terminal kinase (JNK), and certain nPKCs (Holland et al., 2007a, 2007b; Itani et al., 2002; Summers, 2006). Indeed, muscle from normal chow fed whole body ERαKO mice showed heightened inflammatory signaling as reflected by markedly increased JNK phosphorylation and TNFα transcript (Ribas et al., 2010a). In addition to the increase in bioactive lipid intermediates found in ERαKO muscle, the production of reactive oxygen species as well as the possible ERα de-repression of selective inflammatory targets within the nucleus are likely mediators of heightened muscle inflammation.
Markers of inflammation and oxidative stress are elevated in rodent models of obesity and in patients with type 2 diabetes (Donath and Shoelson, 2011; Hotamisligil, 2008). Myotubes and skeletal muscle with ERα deletion showed a marked reduction in Gpx3 expression, an antioxidant enzyme reported to scavenge hydrogen peroxide and diminish oxidative stress (Baltgalvis et al., 2010; Ribas et al., 2010a). E2 replacement in OVX animals also increased Gpx3 expression in skeletal muscle (Baltgalvis et al., 2010). Given that Gpx3 expression levels in skeletal muscle are elevated in females compared to male (Borras et al., 2003), reduced in T2DM patients (Chung et al., 2009), are associated with insulin action and metabolic function (Chung et al., 2009), and the gene is now identified as a causal candidate for obesity (Yang et al., 2009b), additional work studying the direct role of estrogen action in the regulation of anti-oxidant enzymes appears warranted.
Although reductions in mitochondrial number and function have been implicated in the pathobiology of insulin resistance (Befroy et al., 2007; Morino et al., 2005; Patti et al., 2003; Petersen et al., 2004), and indeed gender dimorphisms in mitochondrial biology have been described (Gomez-Perez et al., 2008), whether E2/ERα preserves insulin action by maintenance of mitochondrial integrity remains unknown. Emerging unpublished findings from the Hevener laboratory indicate that skeletal muscle ERα is critical for the maintenance of mitochondrial function and the turnover of damaged organelles. However, the mechanisms underlying these observations remain incompletely understood at the present time.
7. ERs and hepatic insulin sensitivity
Hepatic insulin resistance contributes to impaired glucose tolerance and fasting hyperglycemia of type 2 diabetes by unrestrained hepatic glucose production. Although a direct role of the liver in the insulin resistance phenotype induced by E2 deficiency or tissue-selective ablation of ERα remains unclear. Bryzgalova et al. showed that the global insulin resistance of female mice with a homozygous ERα null mutation was due almost exclusively to impaired suppression of hepatic glucose production (HGP) during euglycemic–hyperinsulinemic clamp studies in anesthetized mice (Bryzgalova et al., 2006). In contrast, Ribas et al. reported in the same ERα-deficient female mice only modest reduction in liver insulin sensitivity during euglycemic–hyperinsulinemic clamp studies in conscious animals (Ribas et al., 2009). Thus, the possibility exists that anesthesia artificially contributed to the severe hepatic insulin resistance phenotype observed by Bryzgalova et al. in ERα-deficient mice (Bryzgalova et al., 2006). Studies in liver-specific ERα-deficient mice should provide the definitive findings required to determine the role of liver ERα in controlling hepatic insulin action and glucose tolerance. Still, E2 and PPT treatments ameliorate insulin resistance in genetically obese leptin resistant and high fat diet-fed mice (Bryzgalova et al., 2008; Riant et al., 2009). Numerous studies are in agreement that E2 suppresses lipogenic gene expression, triglyceride accumulation, and steatosis in liver of HFD-fed (Bryzgalova et al., 2008; Hewitt et al., 2004) and leptin-resistant female mice (Fig. 6) (Gao et al., 2006). Interestingly, this effect is not always reproduced by the ERα, selective agonists PPT (Lundholm et al., 2008). A significant limitation of studies inducing chronic E2 elevation is the inability to ascribe specific actions of estradiol or ERα activation to a select tissue or cell type as circulating E2 impacts all metabolic tissues producing numerous secondary phenotypes due to extensive tissue crosstalk. Moreover, E2, and possibly ERα, cyclicity appears critical in the regulation of gene expression and cellular function, and this cyclicity is eliminated during chronic E2 or ERα agonist administration (Villa et al., 2012). Together, these data suggest that ERα activation protects from hepatic insulin resistance by preventing ectopic lipid accumulation in liver (lipotoxicity), but the direct involvement of ERα and the molecular mechanisms of action in hepatocytes require further investigation.
Fig. 6.

Proposed actions of E2 and ERα in the protection against hepatic triglyceride accumulation and insulin resistance (Della Torre et al., 2011; Villa et al., 2012; Zhu et al., 2014).
8. Estrogen action, immunity and the metabolic syndrome
Estrogens affect many immune and inflammatory conditions including auto-immune diseases (Atwater et al., 2002; Dulos et al., 2010; Gold et al., 2009; Spence and Voskuhl, 2012; Tiwari-Woodruff and Voskuhl, 2009; Yang et al., 2010) as well as immuno-modulatory responses to parasitic and bacterial infection (Calippe et al., 2010; Douin-Echinard et al., 2011; Hepworth et al., 2010; Klein et al., 2008; Lezama-Davila et al., 2008; Vegeto et al., 2010). Following OVX, immune cell infiltration and increased tissue inflammation (TNFa, iNOS and CD11c) coincided with an ~ 4-fold increase in perigonadal and inguinal fat. The T-cell marker CD3 and the Th1 cytokine interferon γ were also elevated in perigonadal fat from ovariectomized female mice (Rogers et al., 2009a) suggesting that the absence of E2 promotes immune cell inflammation. Indeed, similar to findings in rodents, circulating levels of pro-inflammatory cytokines, are elevated in women following natural or surgical menopause (Pfeilschifter et al., 2002). In line with these studies, work by the laboratory of Pierre Gourdy showed that E2 heightens the inflammatory response to intraperitoneal injection of thioglycollate or lipopolysaccharide, and that ERα is critical in mediating these actions as well as reducing bacterial burden through phagocytosis (Calippe et al., 2010). Taken together, these data suggest that ERα expression in immune cells is critical for mediating a variety of cellular responses necessary for normal innate and adaptive immunity.
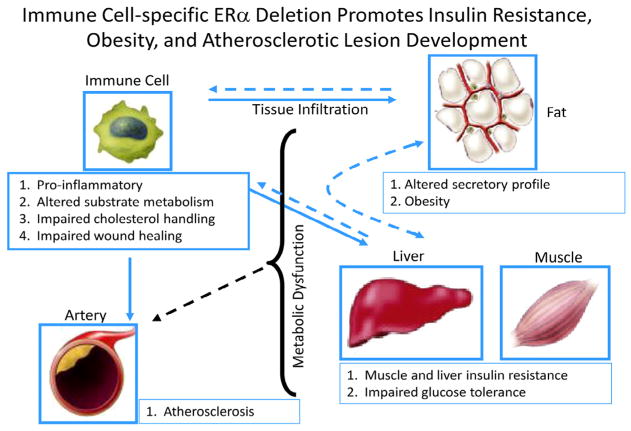
ERα is expressed in macrophages and other immune cells known to exert dramatic effects on glucose homeostasis. Macrophages are central effector cells of innate and adaptive immunity, and over the past decade their role in modulating whole body metabolism and insulin sensitivity has taken on increasing interest (Chawla et al., 2011; Olefsky and Glass, 2010). Recently Ribas et al. investigated the impact of ERα expression on macrophage function to determine whether hematopoietic or myeloid-specific ERα deletion manifests an obesity-induced insulin resistance phenotype in mice (Ribas et al., 2011). This group sought to determine the contribution of myeloid cells to the whole body ERαKO phenotype. Indeed, altered plasma adipokine and cytokine levels, glucose intolerance, insulin resistance, and increased adipose tissue mass were observed in animals harboring a hematopoietic or myeloid-specific deletion of ERα (Fig. 7) (Ribas et al., 2011). A similar obese phenotype with increased atherosclerotic lesions was produced in LDLR-KO mice transplanted with ERαKO bone marrow. In isolated macrophages, Ribas et al. found that ERα is necessary for repression of inflammation, maintenance of oxidative metabolism, IL4-mediated induction of alternative activation, full phagocytic capacity in response to LPS, and oxidized LDL-induced expression of ApoE and Abca1 (Fig. 7) (Ribas et al., 2011). Moreover, bone marrow-derived macrophages lacking ERα secrete factors that induce skeletal muscle and adipocyte insulin resistance in culture (Ribas et al., 2011). A major limitation in the field is the failed identification of these pro-inflammatory insulin-resistance producing substance secreted from immune cells. Thus, metabolomic and proteomic analyses will be necessary to move the field forward in this regard. It is likely that these macrophage-secreted factors include a combination of cytokines, fatty acids, and reactive oxygen/nitrogen species that act to alter metabolic function of adjacent cells in contact with or in close proximity to tissue resident macrophages.
Fig. 7.
Myeloid-specific ERα deletion promotes obesity, insulin resistance, and atherosclerosis susceptibility in female mice.
Taken together, these data suggest that ERα expression in immune cells is critical for mediating a variety of cellular responses necessary for normal innate and adaptive immunity. When E2 levels are low or ERα action is impaired, disease susceptibility increases as the functionality and responsiveness of critical immune cell types become compromised. Although a few direct ERα targets in myeloid cells have been identified, considering the intricate and diverse signaling by ERα as well as the complex nature and crosstalk between cell types, the impact of sex steroids on immunometabolism requires further and more sophisticated dissection.
9. Estrogen action and pancreatic β-cell function
The role of estrogens and ERs in β-cell function and the protection of β-cell mass has been previously reviewed (Tiano and Mauvais-Jarvis, 2012a), so we will focus only on the more recent developments. In rodent models, treatment with E2 protects pancreatic β-cells against various injuries encountered in both type 1 diabetes mellitus (T1DM) and T2DM, including oxidative stress, amyloid polypeptide toxicity, lipotoxicity and apoptosis (Tiano and Mauvais-Jarvis, 2012a). Three receptors: ERα, ERβ and GPER – have been identified in rodent and human β cells. Unlike the classical nuclear ERs acting as ligand-activated transcription factors in breast or uterine cells, β-cell ERs reside mainly in extranuclear locations. They promote their effect via cytosolic interactions with kinases such as Src, ERK, and AMPK or transcription factors of the STAT family (Tiano and Mauvais-Jarvis, 2012, 2012a, 2012b; Tiano et al., 2011; Wong et al., 2010). Activation of ERα enhances glucose-stimulated insulin biosynthesis (Alonso-Magdalena et al., 2008; Wong et al., 2010) through a pathway involving Src, ERK and the stimulation of the nuclear translocation and binding to the insulin promoter of the insulintropic transcription factor NeuroD1 (Fig. 8) (Wong et al., 2010). Activation of ERα reduces de novo synthesis of fatty acids and restrains lipogenesis and accumulation of toxic lipid intermediates in islets (Fig. 8) (Tiano and Mauvais-Jarvis, 2012, 2012b; Tiano et al., 2011). This anti-lipogenic action involves extranuclear ERα activation and promotes the nuclear translocation of STAT3 leading to inhibition in the expression of the master regulator of lipogenesis, the liver X receptor (LXR)β, and hence the expression of its transcriptional targets, sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate response element binding protein (ChREBP). The suppression of LXRβ and SREBP1c mRNA may be mediated via a membrane associated ERα working through Src and STAT3 (Tiano and Mauvais-Jarvis, 2012b). In βcells, chronic LXRβ activation promotes lipogenesis associated with lipotoxicity and apoptosis (Choe et al., 2007). Thus, ERα suppression of LXRβ expression in β-cells may account for the inhibition of lipogenesis and prevention of islet lipotoxicity (Fig. 8) (Tiano and Mauvais-Jarvis, 2012b). Moreover, ERα can also activate AMP-kinase to suppress SREBP-1c gene and protein expression (Tiano and Mauvais-Jarvis, 2012b). Thus, taken together, ERα acts via STAT3 and AMPK pathways to decrease expression and activity of the master effector of lipogenesis under conditions of glucose surplus (Tiano et al., 2011).
Fig. 8.

The effects of islet-specific ERα deletion on pancreatic function and glucose homeostasis (Tiano and Mauvais-Jarvis, 2012a).
Additionally, ERα promotes β-cell survival from most proapoptotic stimuli encountered in the diabetic condition (Le May et al., 2006; Liu and Mauvais-Jarvis, 2009; Liu et al., 2009). Anti-apoptotic mechanisms involve a combination of rapid ERα actions mediated by protein phosphorylation (Liu and Mauvais-Jarvis, 2009; Liu et al., 2009), and a more classical genomic mechanism inducing an anti-inflammatory cascade via liver receptor homolog-1 (LRH-1) (Baquie et al., 2011). Activation of ERβ seems to predominantly enhance glucose-stimulated insulin secretion (Soriano et al., 2009, 2012) via a membrane pathway and activation of the ANF receptor promoting closure of KATP channels (Soriano et al., 2009). GPER activation, however, protects β-cells from lipid accumulation (Tiano and Mauvais-Jarvis, 2012) and promotes cell survival (Balhuizen et al., 2010; Kumar et al., 2011; Liu et al., 2009). GPER activation also enhances glucose-stimulated insulin secretion (Balhuizen et al., 2010; Sharma and Prossnitz, 2011) via activation of the epidermal growth factor receptor (EGFR) and ERK (Sharma and Prossnitz, 2011), but has no effect on insulin biosynthesis (Wong et al., 2010). However, it has been proposed that GPER induces the expression of ERα36, a splice variant of the classical long isoform of ERα66 (Kang et al., 2010), as both ERα66 and ERα36 are expressed in β-cells (Tiano et al., 2011). Thus, it is unclear whether GPER effects on β-cells are due to intrinsic GPER action or indirect effects of GPER collaborating with ERα36 at the membrane. Importantly, the beneficial effects of ER ligands on β-cell survival, function, and nutrient homeostasis described earlier in rodents, are all observed in human β-cells (Contreras et al., 2002; Kumar et al., 2011; Liu et al., 2009; Tiano and Mauvais-Jarvis, 2012a; Tiano et al., 2011).
Perhaps the translational potential of E2 therapy in β-cell protection would be best achieved in the context of pancreatic islet transplantation (PIT). Fertile women with T1DM show E2 deficiency compared to non-diabetic women (Salonia et al., 2006), suggesting that islet survival in T1DM women undergoing islet transplantation could be enhanced by short-term augmentation of circulating E2. To explore this hypothesis, Mauvais-Jarvis and colleagues used a T1DM model with xenotransplantation of human islets in nude mice rendered insulin-deficient by streptozotocin. In this model a 4 week E2 treatment protected β-cell mass and enhanced islet revascularization and engraftment (Liu et al., 2010). Thus, transient E2 treatment provided an immediate therapeutic impact to improve PIT and achieve insulin independence with fewer islets. Studies in human subjects are warranted and considering the short-term duration of E2 administration, the risk of secondary complications arising in reproductive tissues is likely minimal.
10. Conclusions and perspectives
Over the past decade, a vast literature of molecular targets has emerged promising the prospect of pharmacological intervention for the restoration of metabolic homeostasis and insulin action in humans with type 2 diabetes. The inherent simplicity and elegance of using E2 or ER agonists as therapeutic agents, at least in women, is underscored by decades of research and in depth knowledge related to biological/clinical efficacy and toxicity profiles obtained by in vivo studies of preclinical animal models and humans. Estradiol and ERα-specific agonists promote energy homeostasis, improve body fat distribution, and ameliorate insulin resistance, βcell dysfunction and inflammation. The challenge with estrogen supplementation, however, is the relatively narrow therapeutic index that is required when administered chronically. Thus, although successful in rodents, the translation of basic science advances showing amelioration of complications associated with metabolic dysfunction by E2 described in this review becomes problematic when extending to clinical practice. However, 10 years after the WHI concluded that the risks of hormone therapy outweighed benefit, reevaluation of the WHI findings determined that the risks of breast cancer, coronary heart disease, stroke, and pulmonary embolism with estrogen-progestin treatment were overstated, and a revised position statement was issued by the North American Menopause Society (2012). The revised position statement indicating that HRT has a role in short-term treatment of menopausal symptoms led to the renewed interest in investigating the therapeutic benefits of HRT. Thus, moving forward, it will be important to determine whether short-term HRT during early menopause offers protection against metabolic dysfunction.
Additionally, it is imperative that we determine mechanistically how best to modulate specific ERα-regulated pathways involved in energy balance and glucose homeostasis and develop targeted estrogen mimetics yielding metabolic benefit without unwanted side effects. This could be achieved possibly by fusion peptides (Finan et al., 2011, 2012) or through novel SERMs that retain the beneficial metabolic effects of E2 in desired tissues, while exerting minimal or antagonistic effects on ERs in breast and uterus. With regard to whole body metabolism and obesity, future studies should focus on identifying the critical brain sites where ERs regulate body weight homeostasis and delineate the intracellular signaling pathways that are required for estrogen action. Additionally, determining the functional role and molecular mechanism(s) of ER action in islets, immune cells, skeletal muscle, liver and adipose tissue as well as the metabolic crosstalk between these tissues may reveal additional pharmacological targets for therapeutic intervention. A major limitation in our understanding and interpretation of E2 action is the lack of information regarding the contribution of extranuclear vs. nuclear ER actions, as well as ligand vs. non-ligand-mediated functions of estrogen receptors in metabolic tissues. Delineation of these pathways and the tissue specificity with which these signaling pathways are engaged will be critical in moving the field forward and advancing therapeutic strategies to improve women’s health.
Acknowledgments
We attempted to reference the many laboratories who have contributed in advancing the field of research and apologize to those investigators we were unable to cite in this Review.
ALH is supported by grants from National Institutes of Health (NIH) DK89109 and DK063491, UCLA Department of Medicine, UCLA Iris-Cantor Women’s Health Foundation, and the UCLA Jonsson Comprehensive Cancer Center. DJC is supported by NIH/NIDDK grant P01 088761-01 and the Klarman Foundation for Eating Disorders. FMJ is supported by NIH (R01 DK074970), the American Diabetes Association (7-13-BS-101) and the Price-Goldsmith Endowed Chair in Nutrition.
References
- Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53(1):25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25(11):1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- Alonso A, Ordonez P, Fernandez R, Moreno M, Llaneza P, Patterson AM, et al. 17beta-estradiol treatment is unable to reproduce p85 alpha redistribution associated with gestational insulin resistance in rats. J Steroid Biochem Mol Biol. 2009;116(3–5):160–170. doi: 10.1016/j.jsbmb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Alonso A, Gonzalez-Pardo H, Garrido P, Conejo NM, Llaneza P, Diaz F, et al. Acute effects of 17 beta-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age (Dordr) 2010;32(4):421–434. doi: 10.1007/s11357-010-9148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;3(4):e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60(10):2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I, Gondos B, DiBartolomeo R, Bazaes R, Jovanovic L. Pregnancy hormones prevent diabetes and reduce lymphocytic infiltration of islets in the NOD mouse. Ann Clin Lab Sci. 2002;32(1):87–92. [PubMed] [Google Scholar]
- Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320(1–2):16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS ONE. 2010;5(4):e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks EA, Brozinick JT, Jr, Yaspelkis BB, 3rd, Kang HY, Ivy JL. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol. 1992;263(5 Pt 1):E1010–E1015. doi: 10.1152/ajpendo.1992.263.5.E1015. [DOI] [PubMed] [Google Scholar]
- Baquie M, St-Onge L, Kerr-Conte J, Cobo-Vuilleumier N, Lorenzo PI, Jimenez Moreno CM, et al. The liver receptor homolog-1 (LRH-1) is expressed in human islets and protects {beta}-cells against stress-induced apoptosis. Hum Mol Genet. 2011;20(14):2823–2833. doi: 10.1093/hmg/ddr193. [DOI] [PubMed] [Google Scholar]
- Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Barros RP, Morani A, Moriscot A, Machado UF. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol Cell Endocrinol. 2008;295(1–2):24–31. doi: 10.1016/j.mce.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297(1):E124–E133. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- Barros RPA, Machado UF, Warner M, Gustafsson J-Å. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA. 2006;103(5):1605–1608. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte DJ, Breininger JF, et al. Leptin receptor long form splice variant protein expression in neuron cell bodies of the brain and colocalization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69(6):417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992;24(1):15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol. 1992;20(Suppl 8):S26–S28. [PubMed] [Google Scholar]
- Bjorntorp P. Hormonal effects on fat distribution and its relationship to health risk factors. Acta Paediatr Suppl. 1992;383:59–60. discussion 1. [PubMed] [Google Scholar]
- Bjorntorp P. The android woman – a risky condition. J Intern Med. 1996;239(2):105–110. doi: 10.1046/j.1365-2796.1996.364690000.x. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Bohler HC, Jr, Tracer H, Merriam GR, Petersen SL. Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology. 1991;128(3):1265–1269. doi: 10.1210/endo-128-3-1265. [DOI] [PubMed] [Google Scholar]
- Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17beta-Estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids. 2008;73(9–10):859–863. doi: 10.1016/j.steroids.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134(6):2367–2370. doi: 10.1210/endo.134.6.8194462. [DOI] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev. 1993;14(1):72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, 3rd, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol. 1993;265(3 Pt 1):E419–E427. doi: 10.1152/ajpendo.1993.265.3.E419. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, 3rd, Ivy JL. Glucose uptake and GLUT-4 protein distribution in skeletal muscle of the obese Zucker rat. Am J Physiol. 1994;267(1 Pt 2):R236–R243. doi: 10.1152/ajpregu.1994.267.1.R236. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185(2):1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282(5):E1139–E1146. doi: 10.1152/ajpendo.00184.2001. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17beta-Estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol. 2003;31(1):37–45. doi: 10.1677/jme.0.0310037. [DOI] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Casazza K, Page GP, Fernandez JR. The association between the rs2234693 and rs9340799 estrogen receptor alpha gene polymorphisms and risk factors for cardiovascular disease: a review. Biol Res Nurs. 2010;12(1):84–97. doi: 10.1177/1099800410371118. [DOI] [PubMed] [Google Scholar]
- Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24(1):47–59. doi: 10.1210/me.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M, van Dijk G, Arkies BJ, Woods SC. Third ventricular insulin infusion attenuates NPY-induced feeding at the level of the paraventricular nucleus. Obes Res. 1995;3:335s. [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Bassford T, Green SB, Cauley JA, Jackson RD, LaCroix AZ, et al. Postmenopausal hormone therapy and body composition – a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- Choe SS, Choi AH, Lee JW, Kim KH, Chung JJ, Park J, et al. Chronic activation of liver X receptor induces beta-cell apoptosis through hyper-activation of lipogenesis: liver X receptor-mediated lipotoxicity in pancreatic beta-cells. Diabetes. 2007;56(6):1534–1543. doi: 10.2337/db06-1059. [DOI] [PubMed] [Google Scholar]
- Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, et al. Over-expression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117(7):1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Kim M, Youn BS, Lee NS, Park JW, Lee IK, et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol Cell Biol. 2009;29(1):20–30. doi: 10.1128/MCB.00544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Benoit SC, Barrera JG, Woods SC. Estrogen mediates body fat distribution and brain sensitivity to adiposity signals. Diabetes. 2003;52(Suppl 1) [Google Scholar]
- Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52(3):682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74(9):1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- Cortright RN, Koves TR. Sex differences in substrate metabolism and energy homeostasis. Can J Appl Physiol. 2000;25(4):288–311. doi: 10.1139/h00-023. [DOI] [PubMed] [Google Scholar]
- Danguir J, Nicolaidis S. Cortical activity and sleep in the rat lateral hypothalamic syndrome. Brain Res. 1980;185(2):305–321. doi: 10.1016/0006-8993(80)91070-7. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43(7):862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- Della Torre S, Rando G, Meda C, Stell A, Chambon P, Krust A, et al. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13(2):205–214. doi: 10.1016/j.cmet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Deng HW, Li J, Li JL, Dowd R, Davies KM, Johnson M, et al. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85(8):2748–2751. doi: 10.1210/jcem.85.8.6728. [DOI] [PubMed] [Google Scholar]
- D’Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring) 2008;16(6):1284–1288. doi: 10.1038/oby.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812(1–2):256–259. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009;107(3):853–858. doi: 10.1152/japplphysiol.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest. 1998;102(6):1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RP, Abbott RD. Central obesity and coronary heart disease in men. Lancet. 1987;2(8569):1215. doi: 10.1016/s0140-6736(87)91357-2. [DOI] [PubMed] [Google Scholar]
- Donahue RP, Orchard TJ, Becker DJ, Kuller LH, Drash AL. Sex differences in the coronary heart disease risk profile: a possible role for insulin. The Beaver County Study. Am J Epidemiol. 1987;125(4):650–657. doi: 10.1093/oxfordjournals.aje.a114578. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Douin-Echinard V, Calippe B, Billon-Gales A, Fontaine C, Lenfant F, Tremollieres F, et al. Estradiol administration controls eosinophilia through estrogen receptor-alpha activation during acute peritoneal inflammation. J Leukoc Biol. 2011;90(1):145–154. doi: 10.1189/jlb.0210073. [DOI] [PubMed] [Google Scholar]
- Drew BG, Hamidi H, Zhou Z, Villanueva CJ, Krum SA, Calkin AC, et al. Estrogen receptor (ER)α-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J Biol Chem. 2015;290(9):5566–5581. doi: 10.1074/jbc.M114.606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242(5398):476–477. doi: 10.1038/242476a0. [DOI] [PubMed] [Google Scholar]
- Dulos J, Vijn P, van Doorn C, Hofstra CL, Veening-Griffioen D, de Graaf J, et al. Suppression of the inflammatory response in experimental arthritis is mediated via estrogen receptor alpha but not estrogen receptor beta. Arthritis Res Ther. 2010;12(3):R101. doi: 10.1186/ar3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2 Pt 1):E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- Elbers JM, de Roo GW, Popp-Snijders C, Nicolaas-Merkus A, Westerveen E, Joenje BW, et al. Effects of administration of 17beta-oestradiol on serum leptin levels in healthy postmenopausal women. Clin Endocrinol (Oxf) 1999;51(4):449–454. doi: 10.1046/j.1365-2265.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423(2):261–281. [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104(2):347–353. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 2008;194(1):81–93. doi: 10.1111/j.1748-1716.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44(6):739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Finan B, Yang B, Gelfanov V, Ottaway N, Pfluger P, Perez-Tilve D, et al. Estrogen conjugates of GLP-1 demonstrate enhanced efficacy in diet-induced obese mice. Keystone Symposia Type 2 Diabetes, Insulin Resistance and Metabolic Dysfunction; January 12–17.2011. [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18(12):1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4(6):e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288(14):1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50(6):1344–1350. doi: 10.2337/diabetes.50.6.1344. [DOI] [PubMed] [Google Scholar]
- Fu MH, Maher AC, Hamadeh MJ, Ye C, Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17beta-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics. 2009;40(1):34–47. doi: 10.1152/physiolgenomics.00115.2009. [DOI] [PubMed] [Google Scholar]
- Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80(5):569–578. doi: 10.1139/o02-156. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51(10):2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17beta-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha-mediated signals. Am J Physiol Cell Physiol. 2009;297(5):C1249–C1262. doi: 10.1152/ajpcell.00188.2009. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82(2):414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- Gan Z, Burkart-Hartman EM, Han DH, Finck B, Leone TC, Smith EY, et al. The nuclear receptor PPARbeta/delta programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011;25(24):2619–2630. doi: 10.1101/gad.178434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20(6):1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13(1):89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992;41(4):465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101(11):2377–2386. doi: 10.1172/JCI1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142(11):4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Gold SM, Sasidhar MV, Morales LB, Du S, Sicotte NL, Tiwari-Woodruff SK, et al. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha) Lab Invest. 2009;89(10):1076–1083. doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Perez Y, Amengual-Cladera E, Catala-Niell A, Thomas-Moya E, Gianotti M, Proenza AM, et al. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem. 2008;22(5–6):539–548. doi: 10.1159/000185538. [DOI] [PubMed] [Google Scholar]
- Gong H, Xie J, Zhang N, Yao L, Zhang Y. MEF2A binding to the Glut4 promoter occurs via an AMPKalpha2-dependent mechanism. Med Sci Sports Exerc. 2011;43(8):1441–1450. doi: 10.1249/MSS.0b013e31820f6093. [DOI] [PubMed] [Google Scholar]
- Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor alpha increases insulin-stimulated skeletal muscle glucose uptake. J Physiol. 2011;589(Pt 8):2041–2054. doi: 10.1113/jphysiol.2010.199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio G, Di Palma MI, Pepe C, Saraco NI, Prieto M, Saure C, et al. Metformin, estrogen replacement therapy and gonadotropin inhibition fail to improve insulin sensitivity in a girl with aromatase deficiency. Horm Res. 2009;72(6):370–376. doi: 10.1159/000249165. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Hansen BF, Christiansen C. Hormone replacement therapy prevents coronary artery disease in ovariectomized cholesterol-fed rabbits. APMIS. 1991;99(8):721–727. doi: 10.1111/j.1699-0463.1991.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40(12):1323–1326. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- Hamadeh MJ, Devries MC, Tarnopolsky MA. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab. 2005;90(6):3592–3599. doi: 10.1210/jc.2004-1743. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hansen PA, McCarthy TJ, Pasia EN, Spina RJ, Gulve EA. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol. 1996;80(5):1605–1611. doi: 10.1152/jappl.1996.80.5.1605. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Hardman MJ, Grencis RK. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur J Immunol. 2010;40(2):406–416. doi: 10.1002/eji.200939589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480(1–3):21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51(6):1907–1912. doi: 10.2337/diabetes.51.6.1907. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KN, Pratis K, Jones MEE, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145(4):1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- Hill JW, Urban JH, Xu M, Levine JE. Estrogen induces neuropeptide Y (NPY) Y1 receptor gene expression and responsiveness to NPY in gonadotrope-enriched pituitary cell cultures. Endocrinology. 2004;145(5):2283–2290. doi: 10.1210/en.2003-1368. [DOI] [PubMed] [Google Scholar]
- Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochem Biophys Res Commun. 2008;371(2):320–323. doi: 10.1016/j.bbrc.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Hoeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol. 2009;107(3):824–831. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- Hoeg LD, Sjoberg KA, Jeppesen J, Jensen TE, Frosig C, Birk JB, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60(1):64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2):S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl 7):S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97(23):12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, McInnes KJ, Boon WC, Simpson ER. Estrogen and adiposity – utilizing models of aromatase deficiency to explore the relationship. J Steroid Biochem Mol Biol. 2007;106(1–5):3–7. doi: 10.1016/j.jsbmb.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanga-Sollo E, White ME, Hathaway MR, Weber WJ, Dayton WR. Effect of estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 2010;39(1):54–62. doi: 10.1016/j.domaniend.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Jo KJ, Kim OS, Kim BJ, Kang DW, Lee KH, et al. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010;87(11–12):358–366. doi: 10.1016/j.lfs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Kim M, Neinast MD, Frank AP, Sun K, Park J, Zehr JA, et al. ERα upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Mol Metab. 2014;3(6):642–651. doi: 10.1016/j.molmet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PW, Easterbrook JD, Lalime EN, Klein SL. Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med. 2008;5(4):423–433. doi: 10.1016/j.genm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology. 1999;70(6):377–383. doi: 10.1159/000054499. [DOI] [PubMed] [Google Scholar]
- Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17{beta}-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology. 2011;152(7):2568–2579. doi: 10.1210/en.2010-1361. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103(24):9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336(4):1221–1226. doi: 10.1016/j.bbrc.2005.08.256. [DOI] [PubMed] [Google Scholar]
- Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Granier P, Tiffoche C, Berthon PM, Thieulant ML, Carre F, et al. Effect of endurance training on oestrogen receptor alpha expression in different rat skeletal muscle type. Acta Physiol Scand. 2002;175(3):211–217. doi: 10.1046/j.1365-201X.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Granier P, Tiffoche C, Berthon PM, Rannou-Bekono F, Thieulant ML, et al. Effect of endurance training on oestrogen receptor alpha transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174(3):283–289. doi: 10.1046/j.1365-201x.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Levin BE. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol. 1999;276(2 Pt 2):R382–R387. doi: 10.1152/ajpregu.1999.276.2.R382. [DOI] [PubMed] [Google Scholar]
- Lezama-Davila CM, Isaac-Marquez AP, Barbi J, Cummings HE, Lu B, Satoskar AR. Role of phosphatidylinositol-3-kinase-gamma (PI3K-gamma)-mediated pathway in 17beta-estradiol-induced killing of L. mexicana in macrophages from C57BL/6 mice. Immunol Cell Biol. 2008;86(6):539–543. doi: 10.1038/icb.2008.39. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26(8):1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- Lindell K, Bennett PA, Itoh Y, Robinson IC, Carlsson LM, Carlsson B. Leptin receptor 5′ untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol Cell Endocrinol. 2001;172(1–2):37–45. doi: 10.1016/s0303-7207(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1(3):273–275. doi: 10.4161/isl.1.3.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–864. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58(10):2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kilic G, Mauvais-Jarvis F. Estrogens improve angiogenesis during pancreatic islet transplantation (Abstract). Keystone Symposia on islet Biology Whistler; BC, Canada. 2010. [Google Scholar]
- Louis-Sylvestre J. Neuroendocrinology of hyperphagias and obesities. Reprod Nutr Dev. 1980;20(5B):1545–1562. doi: 10.1051/rnd:19800901. [DOI] [PubMed] [Google Scholar]
- Lundholm L, Bryzgalova G, Gao H, Portwood N, Falt S, Berndt KD, et al. The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199(2):275–286. doi: 10.1530/JOE-08-0192e. [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67(2):218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- Maher AC, Akhtar M, Vockley J, Tarnopolsky MA. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. PLoS ONE. 2010;5(8):e12025. doi: 10.1371/journal.pone.0012025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics. 2010;42(3):342–347. doi: 10.1152/physiolgenomics.00016.2010. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26(37):9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol. 2009;297(3):C548–C555. doi: 10.1152/ajpcell.00502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68(4):331–336. doi: 10.1016/j.maturitas.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam KM, Stern JS, Storlien LH, Keesey RE. Effect of lateral hypothalamic lesions on regulation of body weight and adiposity in rats. Am J Physiol. 1980;239(3):R337–R343. doi: 10.1152/ajpregu.1980.239.3.R337. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17 beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519(1–2):301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Nishikawa Y, Adachi H, Enomoto T, Ikegami H, Kurachi H, et al. Identification of estrogen receptor in human adipose tissue and adipocytes. J Clin Endocrinol Metab. 1994;78(4):950–954. doi: 10.1210/jcem.78.4.8157726. [DOI] [PubMed] [Google Scholar]
- Mora S, Pessin JE. The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. J Biol Chem. 2000;275(21):16323–16328. doi: 10.1074/jbc.M910259199. [DOI] [PubMed] [Google Scholar]
- Moreno H, Serrano AL, Santalucia T, Guma A, Canto C, Brand NJ, et al. Differential regulation of the muscle-specific GLUT4 enhancer in regenerating and adult skeletal muscle. J Biol Chem. 2003;278(42):40557–40564. doi: 10.1074/jbc.M306609200. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol. 2009;587(17):4319–4327. doi: 10.1113/jphysiol.2009.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(7):2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34(11–12):758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1–2):63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Dahlman I, Ryden M, Nordstrom EA, Gustafsson JA, Arner P, et al. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond) 2007;31(6):900–907. doi: 10.1038/sj.ijo.0803528. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Niu W, Huang C, Nawaz Z, Levy M, Somwar R, Li D, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003;278(20):17953–17962. doi: 10.1074/jbc.M211136200. [DOI] [PubMed] [Google Scholar]
- Noh EM, Lee YR, Chay KO, Chung EY, Jung SH, Kim JS, et al. Estrogen receptor alpha induces down-regulation of PTEN through PI3-kinase activation in breast cancer cells. Mol Med Rep. 2011;4(2):215–219. doi: 10.3892/mmr.2011.412. [DOI] [PubMed] [Google Scholar]
- North American Menopause Society. The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96(22):12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27(9):1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Shimokata H. Relationships of resting energy expenditure with body fat distribution and abdominal fatness in Japanese population. J Physiol Anthropol Appl Human Sci. 2003;22(1):47–52. doi: 10.2114/jpa.22.47. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Tanaka M, Shimokata H. Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum Genet. 2003;113(5):432–436. doi: 10.1007/s00439-003-0983-8. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci USA. 2009;106(37):15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW. Mechanisms of action of steroid hormones. N Engl J Med. 1971;284(7):370–377. doi: 10.1056/NEJM197102182840710. [DOI] [PubMed] [Google Scholar]
- Ordonez P, Moreno M, Alonso A, Llaneza P, Diaz F, Gonzalez C. 17beta-Estradiol and/or progesterone protect from insulin resistance in STZ-induced diabetic rats. J Steroid Biochem Mol Biol. 2008;111(3–5):287–294. doi: 10.1016/j.jsbmb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Oshel KM, Knight JB, Cao KT, Thai MV, Olson AL. Identification of a 30-base pair regulatory element and novel DNA binding protein that regulates the human GLUT4 promoter in transgenic mice. J Biol Chem. 2000;275(31):23666–23673. doi: 10.1074/jbc.M001452200. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54(1):175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23(9):1697–1700. doi: 10.1016/s0196-9781(02)00112-2. [DOI] [PubMed] [Google Scholar]
- Price TM, O’Brien SN. Determination of estrogen receptor messenger ribonucleic acid (mRNA) and cytochrome P450 aromatase mRNA levels in adipocytes and adipose stromal cells by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab. 1993;77(4):1041–1045. doi: 10.1210/jcem.77.4.8408452. [DOI] [PubMed] [Google Scholar]
- Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2009;298:E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Soleymani T, Daraei P, Hevener A. Skeletal muscle specific ER alpha deletion is causal for the metabolic syndrome. Endocr Rev. 2010;31(3):S5. [Google Scholar]
- Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA. 2011;108(39):16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet Med. 2007;24(12):1491–1495. doi: 10.1111/j.1464-5491.2007.02304.x. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes. 1990;39(11):1425–1429. doi: 10.2337/diab.39.11.1425. [DOI] [PubMed] [Google Scholar]
- Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87(1):39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382(4):646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda AC, Buitrago C, Boland R. Role of estrogen receptors, PKC and Src in ERK2 and p38 MAPK signaling triggered by 17beta-estradiol in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;122(4):287–294. doi: 10.1016/j.jsbmb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Ronda AC, Vasconsuelo A, Boland R. Extracellular-regulated kinase and p38 mitogen-activated protein kinases are involved in the antiapoptotic action of 17beta-estradiol in skeletal muscle cells. J Endocrinol. 2010;206(2):235–246. doi: 10.1677/JOE-09-0429. [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41(5):263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehzadeh F, Rune A, Osler M, Al-Khalili L. Testosterone or 17{beta}-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol. 2011;210(2):219–229. doi: 10.1530/JOE-10-0497. [DOI] [PubMed] [Google Scholar]
- Salonia A, Lanzi R, Scavini M, Pontillo M, Gatti E, Petrella G, et al. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care. 2006;29(2):312–316. doi: 10.2337/diacare.29.02.06.dc05-1067. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152(8):3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor beta mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003;78(2):85–97. doi: 10.1046/j.0022-7722.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Fornari L, Mannella P, Varone G, Caruso A, Liao JK, et al. Novel non-transcriptional mechanisms for estrogen receptor signaling in the cardiovascular system. Interaction of estrogen receptor alpha with phosphatidylinositol 3-OH kinase. Steroids. 2002;67(12):935–939. doi: 10.1016/s0039-128x(02)00040-5. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J Neuroendocrinol. 1997;9(11):803–806. doi: 10.1046/j.1365-2826.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101(2):147–157. [PubMed] [Google Scholar]
- Sites CK, Toth MJ, Cushman M, L’Hommedieu GD, Tchernof A, Tracy RP, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77(1):128–135. doi: 10.1016/s0015-0282(01)02934-x. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Hnatczuk OC, Vathy I. Expression of proopiomelanocortin and proenkephalin mRNA in sexually dimorphic brain regions are altered in adult male and female rats treated prenatally with morphine. J Pept Res. 2004;63(5):399–408. doi: 10.1111/j.1399-3011.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab. 2008;295(3):E698–E704. doi: 10.1152/ajpendo.00747.2007. [DOI] [PubMed] [Google Scholar]
- Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9(10):622–626. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, et al. Rapid regulation of K(ATP) channel activity by 17{beta}-estradiol in pancreatic {beta}-cells involves the estrogen receptor {beta} and the atrial natriuretic peptide receptor. Mol Endocrinol. 2009;23(12):1973–1982. doi: 10.1210/me.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S, Alonso-Magdalena P, Garcia-Arevalo M, Novials A, Muhammed SJ, Salehi A, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor beta. PLoS ONE. 2012;7(2):e31109. doi: 10.1371/journal.pone.0031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriadou S, Kyparos A, Albani M, Arsos G, Clarke MS, Sidiras G, et al. Soleus muscle force following downhill running in ovariectomized rats treated with estrogen. Appl Physiol Nutr Metab. 2006;31(4):449–459. doi: 10.1139/h06-008. [DOI] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33(1):105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274(15):10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]
- Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176(2):237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Despres JP, Dupont A, Belanger A, Nadeau A, Prud’homme D, et al. Relation of steroid hormones to glucose tolerance and plasma insulin levels in men. Importance of visceral adipose tissue. Diabetes Care. 1995;18(3):292–299. doi: 10.2337/diacare.18.3.292. [DOI] [PubMed] [Google Scholar]
- Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, Flint-Wagner HG, et al. Resistance training in postmenopausal women with and without hormone therapy. Med Sci Sports Exerc. 2003;35(4):555–562. doi: 10.1249/01.MSS.0000058437.17262.11. [DOI] [PubMed] [Google Scholar]
- Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf) 2010;198(1):81–89. doi: 10.1111/j.1748-1716.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26(2):193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- Tiano J, Mauvais-Jarvis F. Selective estrogen receptor modulation in pancreatic beta-cells and the prevention of type 2 diabetes. Islets. 2012;4(2):173–176. doi: 10.4161/isl.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- Tiano JP, Mauvais-Jarvis F. Molecular mechanisms of estrogen receptors’ suppression of lipogenesis in pancreatic beta-cells. Endocrinology. 2012;153(7):2997–3005. doi: 10.1210/en.2011-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121(8):3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25(4):274–287. doi: 10.1139/h00-022. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286(1–2):81–85. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgan CE, Brozinick JT, Jr, Banks EA, Cortez MY, Wilcox RE, Ivy JL. Exercise training and clenbuterol reduce insulin resistance of obese Zucker rats. Am J Physiol. 1993;264(3 Pt 1):E373–E379. doi: 10.1152/ajpendo.1993.264.3.E373. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262(6 Pt 1):E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- van Dijk G, Thiele TE, Donahey JCK, Campfield LA, Smith FJ, Burn P, et al. Central infusion of leptin and GLP-1 (7–36) amide differentially stimulate c-Fos-like immunoreactivity in the rat brain. Am J Physiol. 1996;271:R1096–R1100. doi: 10.1152/ajpregu.1996.271.4.R1096. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Fielitz J, Sutherland LB, Thijssen VL, Crijns HJ, Dimaio MJ, et al. Myocyte enhancer factor 2 and class II histone deacetylases control a gender-specific pathway of cardioprotection mediated by the estrogen receptor. Circ Res. 2010;106(1):155–165. doi: 10.1161/CIRCRESAHA.109.207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–E317. doi: 10.1152/ajpendo.00490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconsuelo A, Milanesi L, Boland R. 17Beta-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2008;196(2):385–397. doi: 10.1677/JOE-07-0250. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Cuzzocrea S, Crisafulli C, Mazzon E, Sala A, Krust A, et al. Estrogen receptor-alpha as a drug target candidate for preventing lung inflammation. Endocrinology. 2010;151(1):174–184. doi: 10.1210/en.2009-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Della Torre S, Stell A, Cook J, Brown M, Maggi A. Tetradian oscillation of estrogen receptor α is necessary to prevent liver lipid deposition. Proc Natl Acad Sci USA. 2012;109(29):11806–11811. doi: 10.1073/pnas.1205797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78(1):215–228. doi: 10.1016/s0306-4522(96)00551-9. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131(9):2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55(5):1172–1178. doi: 10.1161/HYPERTENSIONAHA.110.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik A, Gustafsson T, Esbjornsson M, Johansson O, Ekman M, Sundberg CJ, et al. Expression of oestrogen receptor alpha and beta is higher in skeletal muscle of highly endurance-trained than of moderately active men. Acta Physiol Scand. 2005;184(2):105–112. doi: 10.1111/j.1365-201X.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson HA, Dahllund J, Liu H, Yudkovitz J, Cai SJ, Nilsson S, et al. Identification and characterization of a functionally distinct form of human estrogen receptor beta. Endocrinology. 2002;143(4):1558–1561. doi: 10.1210/endo.143.4.8829. [DOI] [PubMed] [Google Scholar]
- Wise PM, Scarbrough K, Weiland NG, Larson GH. Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: a possible role in cyclic luteinizing hormone secretion. Mol Endocrinol. 1990;4(6):886–892. doi: 10.1210/mend-4-6-886. [DOI] [PubMed] [Google Scholar]
- Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, et al. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA. 2010;107(29):13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density of the femoral neck in elderly Japanese women. J Mol Med. 2002;80(7):452–460. doi: 10.1007/s00109-002-0348-0. [DOI] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297(1):E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Deignan JL, Qi H, Zhu J, Qian S, Zhong J, et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. 2009;41(4):415–423. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Ngo D, Jones M, Simpson E, Fritzemeier KH, Morand EF. Endogenous estrogen regulation of inflammatory arthritis and cytokine expression in male mice, predominantly via estrogen receptor alpha. Arthritis Rheum. 2010;62(4):1017–1025. doi: 10.1002/art.27330. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism. 1984;33(11):1011–1015. doi: 10.1016/0026-0495(84)90229-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab. 2014;306:E1188–E1197. doi: 10.1152/ajpendo.00579.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Palacin M, Guma A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183(1):43–58. doi: 10.1111/j.1365-201X.2004.01380.x. [DOI] [PubMed] [Google Scholar]