Abstract
Underserved and minority populations suffer from a disproportionately high prevalence of obesity and related comorbidities. Effective obesity treatment programs delivered in primary care that produce significant weight loss are currently lacking. The purpose of this trial is to test the effectiveness of a pragmatic, high intensity lifestyle-based obesity treatment program delivered within primary care among an underserved population. We hypothesize that, relative to patients who receive usual care, patients who receive a high-intensity, health literacy- and culturally-appropriate lifestyle intervention will have greater percent reductions in body weight over 24 months. Eighteen clinics (N=803 patients) serving low income populations with a high proportion of African Americans in Louisiana were randomized to the intervention or usual car. Patients in the intervention participate in a high-intensity lifestyle program delivered by health coaches employed by an academic health center and embedded in the primary care clinics. The program consists of weekly (16 in-person/6 telephone) sessions in the first six months, followed by sessions held at least monthly for the remaining 18 months. Primary care practitioners in usual care receive information on weight management and the current Centers for Medicare and Medicaid Services reimbursement for obesity treatment. The primary outcome is percent weight loss at 24 months. Secondary outcomes include absolute 24-month changes in body weight, waist circumference, blood pressure, fasting glucose and lipids, health-related quality of life, and weight-related quality of life. The results will provide evidence on the effectiveness of implementing high-intensity lifestyle and obesity counselling in primary care settings among underserved populations.
Keywords: weight loss, intensive lifestyle intervention, underserved, safety net clinics, health coaches
1. Introduction
Obesity is a highly prevalent and serious medical condition in the United States (US), and Louisiana ranks highest among the states in the prevalence of obesity.1 Obesity increases the risk of type 2 diabetes, heart disease, stroke, gallbladder disease, respiratory problems, poor health-related quality of life, and several cancers.2 Indeed, Louisiana sits firmly in the “Chronic Disease Belt”, characterized by high prevalence of cancer,3 cardiovascular disease,4,5 diabetes,6 and obesity.7 Given that minorities and other underserved populations have a disproportionately high obesity prevalence,8,9 identifying strategies to reduce obesity in these populations is imperative for achieving national public health goals to reduce health inequities.10
While the high rates of obesity are of great concern, it is equally concerning that health care systems have not delivered medical interventions capable of producing even modest weight loss.11 With primary care practitioners (PCPs) being the cornerstone of medical care in the US, the US Preventive Services Task Force recommends that physicians offer intensive multi-component behavioral interventions to individuals with obesity.12 Further, the Centers for Medicare and Medicaid Services (CMS) covers intensive behavioral therapy for obesity by a PCP.13 However, the sole reliance on PCPs to deliver intensive behavioral therapy for obesity has limitations, in part due to time constraints during a typical primary care visit, and lack of training among PCPs in nutrition education, as well as in the delivery of behavioral therapy.11,14 A narrative review of obesity management in primary care indicated that obesity treatment options delivered in primary care have resulted in limited success, demonstrating only 1–3 kg weight loss over 6–24 months.11 As most studies typically only employed monthly or quarterly visits of 10–15 min duration, this low weight loss is likely due to the low intensity of the interventions.11 Indeed, there is evidence that higher-intensity interventions delivered by trained interventionists in primary care can produce greater weight loss.15
The 2013 American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) Guidelines for the Management of Overweight and Obesity in Adults16,17 assert that an intensive comprehensive lifestyle intervention is the centerpiece to effectively promote weight loss and improve health. These guidelines, based on an exhaustive systematic review,18 emphasize the gold standard of on-site, high-intensity (i.e., ≥14 sessions in 6 months) comprehensive intervention delivered in group or individual sessions by a trained interventionist. Treatment models based on the AHA/ACC/TOS Guidelines which are adaptable to real-life settings and which add effective and cost-conscious delivery methods for obesity treatment in the primary care setting are needed. The purpose of the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial is to test the effectiveness of a pragmatic, high intensity lifestyle-based obesity treatment program delivered within the primary care setting.
2. Methods
2.1. Overview of Study Design
This study is a cluster-randomized, two-arm controlled trial conducted in primary care clinics. A total of 18 primary care clinics inclusive of low income populations with a high percentage of African Americans from urban and rural areas across Louisiana were randomized to either a 1) high-intensity lifestyle intervention group or 2) usual care group. Outcomes are assessed at baseline and 6, 12, 18 and 24-month visits.
2.2. Aims
The primary aim of the PROPEL trial is to develop and test the effectiveness of a 24 month, patient-centered, pragmatic and scalable obesity treatment program delivered within primary care in an underserved population. We hypothesize that:
Relative to patients who receive usual care, patients who receive a high-intensity, health literacy-appropriate and culturally appropriate lifestyle intervention delivered by trained health coaches embedded in a primary care setting will have greater percent reductions in body weight; and
Relative to patients in usual care, patients who receive the high intensity lifestyle intervention will have significant improvements in health-related quality of life, functional capacity, satisfaction with medical care, and improved obesity co-morbidities (hypertension, dyslipidemia, insulin resistance, etc.).
The three secondary aims of the PROPEL trial are 1) to evaluate relationships between adherence to intervention components (physical activity, diet, sessions, etc.) and corresponding changes in body weight and secondary outcomes (post-hoc analyses); 2) to examine the effects of the intervention on system-level practices and patient satisfaction with care, and 3) to test the heterogeneity of effects across clinics and across subgroups of patients (men versus women, white versus African American, older versus younger adults).
2.3. Stakeholder and Patient Engagement
The design of the trial and all intervention materials was guided by extensive stakeholder and patient engagement. Stakeholders include Chief Executive Officers and medical directors of Federally Qualified Health Centers (FQHCs) in underserved areas of the state, in addition to PCPs and community leaders. The continuous engagement of stakeholders was initiated prior to the inception of the trial, and they continue to provide input to the investigative team through regular communication via email, phone, and in-person meetings. Patient engagement occurs largely through quarterly meetings with our three Patient Advisory Boards (PABs; located in North, South, and New Orleans Louisiana) who were instrumental in designing and adapting the intervention sessions and materials for the trial. The PABs are comprised of individuals representative of the PROPEL patient population or individuals who work with the patient population living in each of the three PROPEL study areas. The PABs provide significant input on patient recruitment and retention strategies, and also review our educational webinars for the PCPs. In addition to the PABs, a series of focus groups was conducted early in the trial design phase to gather feedback about perceptions of obesity treatment options among healthcare providers and low-income patients.19 A Community Monitoring Board (CMB), comprised of individuals representing community organizations, meets yearly to provide feedback on the overall direction of the trial and will also help disseminate the results to relevant partner organizations at the conclusion of the trial. Finally, two patient representatives attend monthly study project management committee meetings along with the study investigators and research staff.
2.4. Cluster Randomization
All enrolled patients receive the intervention to which their clinic was assigned. Eighteen primary care clinics from across the state were randomized by the study statistician to either 1) a high-intensity lifestyle intervention group, or 2) a usual care group, after stratification by health system. The 18 clinics are from five health systems (one, three, four, and six clinics from each of four systems of FQHCs, respectively, and 4 clinics from one large, nonprofit academic multispecialty healthcare delivery system), and stratified randomization by health system was performed to ensure adequate local staffing of clinics assigned to the intervention arm and representation from all regions of the state and types of health systems in both arms of the trial. According to Federal Office of Rural Health Policy classification,20 14 clinics are in urban areas, while four are in rural areas. The size of the clinics (patients served) ranges from 1,100 to 35,000.
2.5. Patient Recruitment and Screening
Patients are recruited in the primary care clinics using a variety of approaches. Depending on the clinic, potentially eligible patients are initially identified through 1) interactions with their PCPs, 2) searches of electronic medical records, 3) responses to emails sent through their health care provider health portal, 4) responses to posters and brochures in clinic waiting areas and 5) interactions with PROPEL staff in the clinic.
A trained recruiter follows up with each potentially eligible patient to assess their interest in participating in the study. During this initial telephone screening call, their eligibility is assessed based on self-reported responses to several questions related to the inclusion/exclusion criteria. The inclusion and exclusion criteria for PROPEL are provided in Table 1. If the patient is eligible based on the initial screening criteria, a screening visit is scheduled. At this screening visit, they have their height and weight measured, and they answer several questionnaires to determine their eligibility. The body mass index (BMI) that is measured at the screening visit is used to determine eligibility, which may differ from the baseline BMI that is used for analysis. Thus, baseline BMI values may fall outside the range used to determine eligibility. Written informed consent is obtained prior to participation in the screening visit. Following the screening visit, if the patient is deemed eligible and permission to participate in the study is received from their PCP, he/she is formally enrolled in the study at the completion of a baseline visit.
Table 1.
Inclusion and exclusion criteria for the PROPEL trial.
| Inclusion Criteria |
| • Age 20.0 – 75.0 years |
| • BMI 30.0 – 50.0 kg/m2 |
| • Able to provide written informed consent |
| • Willing to change diet, physical activity and weight |
| • Patient of a participating clinic |
| • Able to participate in scheduled sessions |
| Exclusion Criteria |
| • Currently participating in a weight loss program |
| • Current use of weight loss medication or recent weight loss (>10 lbs in last six months) |
| • Plans to move from the area within 2 years |
| • Given birth within the past year, is currently pregnant or plans to become pregnant within 2 years |
| • Past bariatric surgery or plans for bariatric surgery within 2 years |
| • Current major depression |
| • History of suicidal behavior or diagnosed eating disorder (bulimia, anorexia) |
| • Hospitalization for mental disorder or substance abuse in the previous year |
| • Active cancer (except prostate, skin and thyroid if approved by physician) |
| • Serious arrhythmias or cardiomyopathy |
| • Severe congestive heart failure |
| • Stroke or heart attack in previous six months |
| • Chronic Inflammatory conditions, including but not limited to severe arthritis, lupus, or inflammatory bowel disease(i.e. Crohn’s disease or ulcerative colitis) |
| • Disease that is life threatening or that can interfere with or be aggravated by exercise or weight loss |
| • Discretion of primary care physician or principal investigator |
2.6. Primary and Secondary Outcome Measures
The primary outcome measure of the PROPEL trial is percent (%) change in body weight from baseline to month 24. Secondary outcome measures include absolute 24-month changes in body weight, waist circumference, blood pressure, fasting glucose and lipids, eating behaviors, physical activity, dietary intake, food security, health-related quality of life, and weight-related quality of life. All assessments are conducted by technicians who do not deliver the intervention. Table 2 provides an overview of the data measurement and collection schedule for PROPEL, as described below.
Table 2.
Data measurement and collection schedule for patient data.
| Measurement | Screening Visit | Baseline Visit | 6 Month | 12 Month | 18 Month | 24 Month |
|---|---|---|---|---|---|---|
| Anthropometry | ||||||
| Weight | X | X | X | X | X | X |
| Height | X | X | ||||
| Waist Circumference | X | X | X | X | X | |
| Risk Factors | ||||||
| Blood Pressure and Pulse | X | X | X | X | X | |
| Fasting Finger-stick Sample | X | X | X | |||
| Questionnaires | ||||||
| Demographics & Health History | X | |||||
| Change in Health History | X | X | X | X | ||
| REALM-SF | X | |||||
| Food Security | X | X | X | X | ||
| Eating Inventory | X | X | X | X | ||
| PROMIS-29 | X | X | X | X | ||
| IWQL – Lite | X | X | X | X | ||
| IPAQ-SF | X | X | X | X | ||
| Dietary Intake Questionnaire | X | X | X | X | ||
| Concomitant Medications | X | X | X | X | X | |
| Adverse Event Form | X | X | X | X | ||
| Patient-Provider Survey | X | X | ||||
| Intervention Satisfaction Survey* | X | X |
completed by patients in the active intervention arm only
2.6.1. Anthropometric Measurements
Standing height is measured in duplicate with a portable stadiometer (Seca Model 213) at the baseline visit. The patients are asked to remove their shoes and have their heels, buttocks and upper part of the back remain in contact with the stadiometer with their arms hanging naturally at their side. The patient is asked to inhale and hold their breath, while one technician lightly applies traction to the patient’s head in order to maintain alignment with the Frankfort Plane. The slide is lowered by a second technician until it reaches the vertex of the skull, and the reading from the indicator is recorded to the nearest 0.1 centimeter. Body weight is measured in duplicate with the patient in light clothing without shoes to the nearest 0.1 kg using a digital scale (Seca Model 876) at each assessment visit. The patient is instructed to stand still in the middle of the scale with head erect and eyes looking straight ahead. The accuracy of the scales and stadiometers is checked at scheduled monitoring visits using standard weights and a measuring stick. Waist circumference is measured in duplicate at all assessment visits using a non-elastic anthropometric tape (Graham Field Model 1340-2). The measurement is made by one technician while a second maintains a parallel position of the tape midway between the lower rib margin and the iliac crest, at the end of gentle expiration. If the two measurements differ by more than 0.5 cm, 0.5 kg, and 0.5 cm for height, weight and waist circumference, respectively, a third measurement is obtained and the two closest measurements are averaged for analysis.
2.6.2. Blood Pressure and Pulse Measurements
Resting systolic and diastolic blood pressures and resting pulse are measured using a validated automated Omron device (HEM-907XL Digital Blood Pressure Monitor) at baseline and all follow-up visits. After the patient has been sitting quietly for at least 5 minutes prior to the first measurement, two sitting measurements are made one minute apart. A third measurement is obtained if the first two systolic measurements are greater than 20 mmHg apart, or the first two diastolic measurements are greater than 10 mmHg apart, and the two closest measurements are averaged for analysis.
2.6.3. Fasting Glucose and Lipids
Finger-stick blood samples are obtained to measure fasting levels of lipids (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) and glucose using a validated Alere Cholestech LDX® Analyzer at baseline, month 12 and month 24 visits. The patients are asked to arrive at the clinic after fasting for at least 10 hours. The Cholestech Analyzer is checked for accuracy using standard controls each day before it is used to analyze patient blood samples.
2.6.4. Concomitant Medications
Patients are asked to bring their medications or a list from their pharmacist/PCP to the screening visit and all subsequent assessment visits. A concomitant medications form is completed at each visit to track changes in the patient’s medications and dosages throughout the trial.
2.6.5. Adverse Events
An adverse event surveillance form is used to screen for adverse events and serious adverse events (SAEs) at each assessment visit. All SAEs are recorded, and those that are unexpected and related or are possibly related to the study are reported to the IRB in accordance with their requirements.
2.6.6. Questionnaires
2.6.6.1. Health Literacy
The REALM short form (SF) is a seven-item health literacy assessment that is administered at the screening visit.21 The patient is asked to read health-related words aloud. Two words (“flu” and “pill”) are not scored but are used in this study to identify patients who are ineligible due to their inability to read the informed consent documents. The technician records the number of words pronounced correctly, with a possible score range of 0–7. Raw scores can be converted to reading grade levels. Those scoring below 7 are considered to have low health literacy (reading below a 9th grade level). Those scoring below 4 are considered to have very low health literacy (reading below a 3rd grade level).19 Patients scoring 0–3 on the REALM SF are provided assistance in completing the other questionnaires using a semi-structured interview. Patients scoring 4–7 on the REALM SF complete the other questionnaires on their own, but assistance is provided as required or requested.
2.6.6.2. Demographics and Health History
A self-report demographic and health history questionnaire is used to collect information about age, sex, race/ethnicity, use of tobacco and alcohol, health insurance status, postmenopausal status, income and employment, education level, and history of chronic diseases. Further, the questionnaire collects information on the patients’ physical home address and nearest cross-street for the purpose of GIS analyses. This questionnaire is administered at the screening visit. Changes in health outcomes, physical address, and post-enrollment participation in weight loss programs other than PROPEL are captured by self-report at the 6, 12, 18 and 24-month assessment visits.
2.6.6.3. Food Security
Household food security is measured at baseline and at the 6, 12 and 24-month assessment visits using a 6-item subscale of the 12-month Food Security Scale Questionnaire.22 Scores range from 0–6, and a score of 2 or higher indicates food insecurity.
2.6.6.4. Health-Related Quality of Life
Health-related quality of life is measured using the PROMIS-2923 questionnaire, which is a 29-item questionnaire that includes questions covering health-related domains of physical function, anxiety, depression, fatigue, sleep disturbance, ability to participate in social roles and activities, pain interference and pain intensity. The PROMIS items were developed using a rigorous methodology funded by the National Institutes of Health (NIH) with a goal to develop valid, reliable, brief standardized questionnaires to measure patient–reported outcomes.24 PROMIS surveys were designed and tested to be easy to understand and navigate. The PROMIS-29 is administered at the baseline visit, and at the 6, 12, and 24-month visits.
2.6.6.5. Weight-Related Quality of Life
The Impact of Weight on Quality of Life – Lite (IWQOL-Lite) is a 31-item questionnaire designed to measure obesity-specific aspects of quality of life, which produces a total score and separate scores for physical function, self-esteem, sexual life, public distress, and work or daily activities.25,26 The IWQOL-Lite is administered at baseline and at the 6, 12 and 24-month assessment visits.
2.6.6.6. Physical Activity
Physical activity levels are self-reported at baseline and at the 6, 12 and 24-month assessment visits using the International Physical Activity Questionnaire – Short Form (IPAQ-SF)27 which asks questions related to physical activity performed over the previous 7 days. The IPAQ-SF has acceptable reliability27 and has been shown to be sensitive to change within the context of a weight loss intervention.28 The results are reported as a continuous measure in median metabolic equivalent of task (MET)-minutes per week of physical activity.
2.6.6.7. Dietary Intake
A questionnaire that assesses dietary fat, fruit, vegetable and alcohol intake is administered at baseline and at the 6, 12 and 24-month assessment visits. The questionnaire contains scales derived from several sources. The National Cancer Institute (NCI) fat screener estimates the percentage of energy from fat by asking patients to report the frequency of consuming specific foods over the past 12 months.29 A standard 7-item fruit and vegetable screener developed by the NCI and National 5 a Day Program grantees asks how often fruit and vegetables were consumed in the past month.30,31 Three questions related to the frequency of alcohol intake (beer, wine, hard liquor) were adapted from the Brief Questionnaire to Assess Habitual Beverage Intake (BEVQ-15).32
2.6.6.8. Eating Attitudes and Behaviors
The Three-Factor Eating Inventory is designed to measure different dimensions of eating attitudes and behaviors. Three factor-analyzed subscales (Cognitive Restraint, Disinhibition, and Hunger) are derived from the questionnaire.33 For the purpose of the PROPEL trial, only the Cognitive Restraint and Disinhibition sub-scales is administered to patients. This questionnaire is administered at baseline and at the 6, 12 and 24-month assessment visits.
2.6.6.9. Patient-Provider Relationships
A patient-provider relationship survey is completed by each patient at the baseline visit and at the 24-month assessment visit. This is an 11-item instrument that was adapted from the Hopkins Practice-based Opportunities for Weight Reduction (POWER) trial.34 The POWER investigators compiled the patient-provider survey from several sources, including six questions adapted from the validated Consumer Assessment of Healthcare Providers and Systems (CAHPS) 2.0 Adult Core Survey, which asks patients how often their providers explained things clearly, listened carefully, showed respect, provided easy to understand instructions, knew their medical history and spent enough time with them.35 A CAHPS global rating of the provider (rating from 0–10) is also included. Finally, a question addressing patient-centeredness or the perception of “being known as a person” has been identified as an important domain in the patient–provider relationship.36 The framing of all questions in the survey was adapted by asking patients about their care over the past 2 years given that both the POWER and PROPEL trials were 2 years in duration. A summary patient–provider relationship score (14–32) is computed by adding up the total responses from the 8 questions (and reversing the 2 opposite scales).
2.6.6.10. Intervention Satisfaction
A self-report questionnaire that asks about the patient’s experience with the intervention and satisfaction with care is administered to patients enrolled in the active intervention arm of the trial at the 6-month and 24-month assessment visits. This questionnaire was developed by the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) study37 and includes questions obtained from the Veterans Affairs Diabetes Prevention Program (VA DPP), Rural Lifestyle Eating and Activity Program (Rural LEAP) and POWER trial.
2.6.6.11. Provider Demographics and Knowledge
A survey is completed by each patient’s primary care provider (not the patient) prior to the trial beginning and at the trial’s end. The provider survey was developed for the POWER trial34 and has been adapted for PROPEL. The questionnaire assesses the primary care provider’s demographic information (age, race, training, specialty, years in practice, weight, and height), and knowledge about weight management practices (calculation of BMI, weight loss counseling and referral, etc.).
2.7. Interventions
2.7.1. High-Intensity Lifestyle Intervention Group
Trained health coaches, recruited and hired by an academic health center from local communities and embedded in the primary care clinics, deliver a comprehensive, high-intensity intervention program as recommended by the 2013 AHA/ACC/TOS Obesity Guidelines.17 This intervention program was designed based on the Diabetes Prevention Program (DPP),38 Look AHEAD,39 and CALERIE40 studies. However, given that Louisiana is ranked 49th nationally among the states with respect to health literacy,41 all intervention materials and intervention approaches were adapted to be health-literacy appropriate through extensive consultation with three regional PABs reflecting the three study areas (North, South, New Orleans Louisiana) and the team’s health literacy experts (TCD and CA). Further, health coaches were trained in principles of health literacy and motivational interviewing techniques to enhance patient communication and education and encourage patient activation.42,43 The health coaches had post-secondary degrees in areas related to nutrition, physical activity, or behavioral medicine.
Patients in the intervention arm attend weekly (16 in-person) sessions in the first six months, in addition to receiving 6 phone sessions. Sessions then occur at least monthly and alternate between in-person and phone sessions for the remaining 18 months, though increased contact with the health coach via in-person or phone sessions is an option that is utilized if patients are struggling to meet their goals. More frequent in-person or telephone sessions are encouraged during the maintenance phase for participants who report that they would like such continued support or who have struggled to achieve or maintain their weight goals. The type of contact is determined on a case-by-case basis between the coach and the patient and usually involves a mix of in-person and telephone sessions. A patient can request to meet with a coach as often as the patient wishes assuming that the coach’s schedule could accommodate it and there are no contraindications identified by the intervention team.
Session topics include the use of portion controlled foods and meal replacements, increasing physical activity, calorie balance, self-monitoring, structured diets, healthy snacking, and dealing with stress, among other topics (See Supplementary Table 1 for a full list of session topics). The intervention is delivered in individual and small group sessions in the clinic (2–4 patients, based on scheduling availability) during the initial 6-month phase. In the final 18 months, sessions alternate between individual phone sessions and individual or small group in-person sessions.
Given that greater initial weight loss is associated with long-term weight loss and weight loss maintenance,44,45 a strong focus of the intervention is on maximizing the initial (three month) weight loss in this group. The meal plan for weeks 1–4 includes portion-controlled foods, which includes items that are easily available and affordable, such as fruits (bananas, apples) and soups. Additionally, pre-packaged portion-controlled foods and meal-replacement shakes are provided during weeks 1–4 and are also available as part of our toolbox approach for patients who are interested in using them (see below). After week 5, portion-controlled foods are utilized as needed, though the meal plan becomes less structured over time as the patient learns to purchase, prepare, and package foods to manage portion size and energy intake. A limited amount of prepackaged food may be distributed to a patient as a toolbox option and this food is provided if it is clear that the patient might benefit and if other attempts to secure prepackaged or similar foods have failed. These foods are provided to the patient on a time-limited scale, usually one to two weeks, while additional solutions to the patient’s challenge are identified. The meal plans are consistent with dietary guidelines, i.e., ~55% carbohydrate, ~15% protein, and ~30% fat,46 include nutrient-dense foods (e.g., whole grains, fruits, lean protein), and are formatted for easy creation and dissemination through clinics. Patients are provided with measuring cups and portion-appropriate plates embossed with the study logo to help them attain their dietary goals.
An important component of the behavioral intervention is shared short-term goal setting between the health coach and the patient in order to reach the broader study goals. Two important goals in this trial are related to weight loss and physical activity. A personal goal of 10% weight loss is set for patients, and they are coached to set their own goals and develop eating and physical activity action plans to meet that goal. Given the importance of physical activity for successful weight loss and weight loss maintenance, the second goal for this trial is for patients to achieve at least 175 minutes per week of moderate-to-vigorous physical activity. A major focus of the physical activity intervention is on increasing lifestyle activities, such as taking the stairs instead of the elevator, parking further away, actively commuting (walking or riding bicycle), and replacing sedentary activities with more active options. A pedometer (New Lifestyles Model SW200) is provided to all intervention participants along with directions on how to increase their daily step counts.
A novel aspect of the PROPEL intervention is the addition of a computer tracking system (CTS), which facilitates tailoring the intervention to the individual patient and promoting intervention fidelity among clinics, health coaches and patients.40 A weight loss calculator is used to 1) calculate personalized daily energy targets for each patient that, if met, will result in 10% weight loss over six months, and 2) create a weight graph that displays each patient’s predicted weight loss over time. This weight graph includes a zone that reflects being adherent to the diet. By plotting the patient’s weight over time in relation to the zone, it determines if they are meeting their energy intake target and losing weight at the rate expected (See Figure 1).47–50 The zone includes a 10% weight loss target represented by a line in the middle of the zone, with lower and upper bounds that represent ~7.5% and ~12.5% weight loss. The upper and lower bounds of the graph accommodate biological variation and error in the prediction model. Patients are provided with an electronic scale (BodyTrace©) and are encouraged to weigh themselves daily. The scale directly uploads data via a cellular signal in real-time to the internet and the CTS. In turn, the data are plotted onto their personal weight graph, available to the patient and his/her health coach and the research team. The CTS is used by the health coaches to identify patients who are struggling to meet their weight loss goals, which triggers the health coach to deploy strategies from the “toolbox” (described below). Additionally, reports generated from the CTS provide objective data on intervention adherence and engagement, including session attendance and dietary adherence, the latter of which is quantified by participants’ weight in relation to their zone of adherence. Specifically, the patient’s weights are plotted on their weight graph and they are considered adherent to the diet if their weight is in the zone, and they are considered non-adherent to the diet if their weight is out of the zone.
Figure 1.
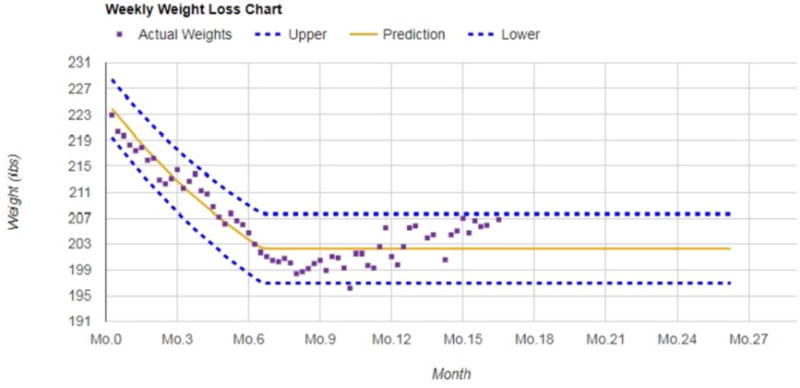
Example of a personal weight loss graph for a single patient followed for over 15 months.
Another novel aspect of the intervention is the expansion of the “toolbox” weight loss approach to the primary care setting. The toolbox has been successfully deployed in previous clinical trials and shown to lead to improvements in intervention efficacy.38–40 The weight graphs are available in real time to both the coach and the patient via any Internet-connected device and thus provide a platform to intervene quickly (e.g., between sessions) should the patient need additional support. This approach also fosters treatment fidelity among health coaches and across clinics. Additionally, the toolbox enables health coaches to tailor treatment to address patient preferences and lifestyles, as well as regional and cultural differences among patients. The intervention is tailored to the needs of the patient, with specific nutritional, physical activity and behavioral strategies selected from the toolbox. Examples include increased contact, increasing dietary fiber, modifying recipes to decrease energy density, adding novel foods to relieve boredom, strategies to avoid impulse eating, limiting calories in restaurants, parties or at work, adding variety to physical activity routines, and so on. Selections from the toolbox depend on the patient’s success in achieving adherence, and on overcoming problems that arise. This approach is highly individualized, and the health coach works with the patient to develop acceptable strategies, including how long they will be deployed, and how to evaluate their effectiveness.
As previously noted, intervention fidelity is facilitated by hiring coaches who reside in the communities where the clinics are located, providing extensive initial training and yearly updated training sessions to the coaches by the intervention team at the coordinating center, and utilizing the CTS and toolbox to help standardize treatment delivery. The initial coach training occurred over 1.5 days and included intervention-specific topics, including the theoretical framework of the intervention and health literacy. The coaches learned about the rationale for using a weight graph to track progress in the intervention, the history and utility of a toolbox approach for addressing patients’ challenges, information on the meal plans, and the importance of changing energy intake/diet and exercise to lose weight and keep it off. This training also included the coaches observing and participating in mock counselling sessions. The annual training sessions include a brief review of the theoretical rationale of the intervention and the importance of continuing to rely on the intervention’s infrastructure (e.g., Computer Tracking System, toolbox) during the two years that patients are treated. This training also stresses the importance of retention, and the coaches shared ways in which they were able to retain and re-engage patients who were not regularly attending sessions. Finally, the training included mock sessions of challenging participants that the coaches have encountered to initiate discussion of how to address common challenges. Importantly, the annual trainings involve much more coach involvement and feedback and one aim is to encourage the coaches to share their experiences with each other to better treat their patients.
In addition to formal training sessions, the health coaches regularly audio record sessions that are reviewed by the intervention team to ensure that they are delivering the intervention similarly across clinics and utilizing the CTS and toolbox as intended. Finally, a weekly case conferencing meeting is held via webinar for coaches to staff cases via the CTS with the research team and to share intervention strategies with each other. These weekly webinars are designed to maintain fidelity of the intervention and high quality of the health coaching. All coaches attend the weekly webinars. The webinars are also attended by a Master’s level clinician, one or more health literacy collaborators, a doctoral level intervention leader, one administrative staff member from the study, and a student worker or other staff member. The first part of the webinar involves administrative items, such as answering record keeping questions and resolving scheduling problems. The remainder of the webinar is dedicated to case conferencing, addressing the coaches’ questions or concerns, and encouraging discussion among the coaches so they can learn what is and is not working with patients across the study. Each coach typically staffs ~3 cases, including cases selected by the coach, the intervention leader, and frequently a case that is randomly selected. Finally, the webinar includes demonstrating different counseling techniques (e.g., motivational interviewing).
In addition to the patient-centered, intensive lifestyle intervention program, PCPs of patients in the intervention receive an obesity science education program. This education includes information on the management of obesity, the co-management of morbidities such as type 2 diabetes and hypertension, minimizing bias and stigma related to obesity, and principles of health literacy. The PCP education program is delivered in a series of webinars and face-to-face brown-bag seminars conducted in the clinics by experienced obesity, patient engagement and health literacy experts. All intervention PCPs receive access to the webinar series via a password protected website.
2.7.2. Usual Care Group
Patients in the usual care group receive their normal, usual care from their primary care team throughout the 24-month period. To maintain contact and aid in the retention of patients in the usual care arm, patients receive three newsletters per year on selected topics and a listing of health promotion events offered in their community. Potential topics that are covered in the newsletters include the importance of reducing sedentary behavior, sleep hygiene, household money management, family coping skills, smoking cessation, etc.
A baseline presentation is conducted for the PCPs in clinics randomized to usual care which describes weight management in primary care settings and the current CMS approach to reimbursing for obesity treatment.13 Additionally, an informational brochure on the current CMS approach to reimbursing for obesity treatment is sent to the PCPs at least once per year throughout the trial. All PCPs also have access to the baseline webinar presentation and the informational brochure throughout the study via a password protected website. If updates to CMS policies occur during the course of the study, webinars are scheduled to inform the PCPs of the changes.
2.8. Sample Size and Statistical Power
According to the 2013 AHA/ACC/TOS Obesity Guidelines, as little as 3–5% weight loss is likely to result in clinically meaningful health outcomes.17 The sample size calculations for this primary outcome assume the study should be powered to detect a differences in mean percent of baseline weight loss in the intervention arm of 3.5% at 24 months, relative to usual care. Power calculations used a nominal 0.05 significance level with a two-tailed test with minimal power of 80% for the planned subgroup analyses (men and women; white and African American; older versus younger adults). Based on the LOSS study,51 the standard deviation for weight change for patients nested within clinics was 8.4%. For the subgroup analyses, a net sample size of 184 patients (92/arm) is sufficient for detecting a 3.5% weight loss with 80% power when randomization is performed at the patient level. After accounting for the design effect (estimated to be 1.8 from the LOSS study51), at least 134 patients per arm are required to realize 80% power in a trial randomized at the clinic level. Allowing for 30% attrition, at least 192 patients per arm are needed with a total (2 arms) enrollment of at least 384 patients per subgroup analysis, which translates into a total sample size of at least 768 patients, which will provide at least 97% power to detect a mean weight loss in the intervention arm of 3.5% at 24 months, relative to usual care in the total sample.
2.9. Planned Statistical Analyses
To address the primary aim, weight loss will be analyzed as mean percent of baseline weight loss at months 6, 12, 18 and 24 in the context of linear mixed (fixed and random) effects multi-level models. In addition to intervention group, clinic, assessment time, and their interaction terms, the model may include patient-level (weight, age, sex, race, etc.) and clinic-level (clinic size, % African American, % Medicaid/Medicare, etc.) variables as explanatory covariates. Preliminary analytical findings will be used to choose the most plausible and efficient repeated measures covariance structure (e.g., compound symmetric, autoregressive, unstructured) to use. Although substantial effort is employed to minimize missing data, it is inevitable that some missing data will occur. An intention-to-treat analysis that includes all randomized patients, regardless of the number of assessments obtained, will be conducted. The primary analysis will use the mixed effects model mentioned above and employ restricted maximum likelihood using all available data. The analytical plans are flexible and will be adapted as scientific perspectives are advanced.
3. Results
The flow of participants from initial screening to enrollment (at baseline) is described in Figure 2. A total of 1958 patients were screened for initial eligibility at the 18 randomized clinics, and of that total, 362 declined participation, 669 did not meet the inclusion/exclusion criteria, and 124 were not enrolled as they were in the screening pipeline at the time recruitment ended. Further, an additional 102 patients were screened but were deemed ineligible due to not having a PCP at a participating clinic. These patients were not allocated to a study arm. The most common reason for ineligibility was having a BMI outside the range of 30 to 50 kg/m2. The final sample size at baseline was 803 (452 intervention; 351 usual care). With the exception of two clinics (n=2 and n=4), the clinic-level sample size ranged from 15 to 89 patients, with an overall median of 43.5 patients per clinic.
Figure 2.
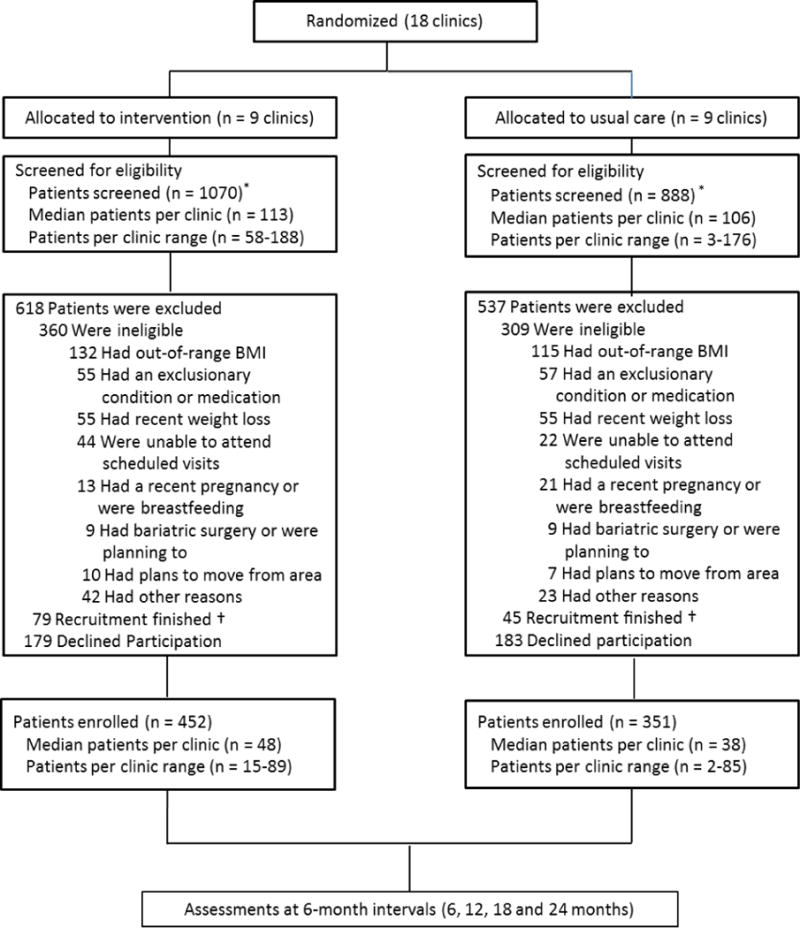
Patient flow from screening to study enrollment. *an additional 102 patients (not shown) were ineligible due to not having a PCP at a participating clinic and were not allocated to a study arm. †124 patients were not enrolled as they were in the screening pipeline at the time recruitment was complete.
Table 3 provides a summary of the descriptive characteristics of the sample at baseline. Approximately 67% of the sample is African American; 40.4% reported an annual household income below $20,000, while 64.1% reported an annual income under $40,000. The mean age of the sample is 49.4 years, with a range of 21 to 74 years. The mean BMI is 37.2 kg/m2, with a range of 29.0 to 49.8 kg/m2. A total of 30.8% of the sample scored 6 or lower on the REALM-SF health literacy assessment which corresponds to less than a 9th grade education, and is considered low literacy, and 29.9% had food insecurity.
Table 3.
Baseline characteristics of patients enrolled in the PROPEL trial.
| Usual Care Group
|
Intervention Group
|
|||
|---|---|---|---|---|
| N | Mean (SD) or % | N | Mean (SD) or % | |
| Female (%) | 280 | 79.8 | 398 | 88.1 |
| African American (%) | 208 | 59.0 | 332 | 73.2 |
| Annual Family Income (%) | ||||
| < $10,000 | 70 | 19.9 | 86 | 19.0 |
| $10,000 – $19,999 | 73 | 20.8 | 95 | 21.0 |
| $20,000 – $39,999 | 79 | 22.5 | 112 | 24.8 |
| $40,000 – $59,999 | 48 | 13.7 | 69 | 15.3 |
| ≥ $60,000 | 71 | 20.2 | 83 | 18.4 |
| Missing | 10 | 2.9 | 7 | 1.6 |
| REALM-SF Score [0–7] | 351 | 6.4 (1.1) | 452 | 6.4 (1.2) |
| ≤ 6 (≤ 8th grade) (%) | 106 | 30.2 | 141 | 31.2 |
| 7 (≥ 9th grade) (%) | 245 | 69.8 | 311 | 68.8 |
| Household Food Insecurity (0–5) | 351 | 1.2 (1.7) | 452 | 1.1 (1.6) |
| Food Secure (%) | 238 | 67.8 | 325 | 71.9 |
| Food Insecure (%) | 113 | 32.2 | 127 | 28.1 |
| Diabetes (%) | 104 | 29.6 | 103 | 22.8 |
| Age (y) | 351 | 49.8 (13.6) | 452 | 48.8 (12.7) |
| BMI (kg/m2) | 351 | 37.2 (4.8) | 452 | 37.3 (4.6) |
| Waist Circumference (cm) | 349 | 113.9 (12.6) | 451 | 113.1 (12.4) |
| Systolic Blood Pressure (mmHg) | 350 | 122.6 (16.5) | 452 | 123.1 (16.3) |
| Diastolic Blood Pressure (mmHg) | 350 | 78.4 (10.6) | 452 | 79.7 (10.6) |
| Glucose (mg/dL) | 344 | 112.3 (39.9) | 438 | 106.5 (31.9) |
| Total Cholesterol (mg/dL) | 340 | 180.0 (36.7) | 433 | 179.6 (37.5) |
| HDL-cholesterol (mg/dL) | 343 | 47.7 (14.4) | 437 | 50.4 (14.4) |
| Triglycerides (mg/dL) | 333 | 131.6 (69.4) | 410 | 125.3 (72.8) |
| LDL-cholesterol (mg/dL) | 328 | 106.7 (31.5) | 401 | 105.7 (32.9) |
4. Discussion
The PROPEL trial aims to test the effectiveness of implementing a pragmatic, high-intensity lifestyle intervention in a primary care setting. A unique aspect of the trial is the adaptation of an evidence-based approach to low-income, underserved populations in Louisiana. Results of the trial should have broad generalizability to other underserved populations across the US, and will add to a growing body of literature on the effectiveness of different obesity treatment and prevention programs in primary care in low income, minority and/or low health literacy populations.52–54 There was substantial research infrastructure developed and maintained to support the implementation and evaluation of the PROPEL trial. This investment was required in order to test the effectiveness of the weight loss approach, maintain the fidelity of the intervention, and to collect process measures that will help determine the most effective components of the intervention. However, we believe that the resulting intervention approach is scalable and achievable in most primary care settings.
The PROPEL trial is being conducted in parallel with the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) trial in the Midwestern US.37 Both trials are large pragmatic investigations of obesity treatment options in primary care settings, and have many outcome measures in common. While RE-POWER focuses on the rural, Midwestern population, PROPEL addresses the issue of obesity treatment in the underserved, low-income population of the Deep South with a high prevalence of obesity (the average BMI in PROPEL is approximately 37 kg/m2). While the two trials are both considered pragmatic, they differ in the degree of pragmatic versus explanatory “attitudes” in study design.55 Both trials have minimal inclusion/exclusion criteria to allow for broad generalizability to their patient populations. However, PROPEL relies on highly trained research staff embedded within the primary care environment to deliver the intervention and assess the outcomes, whereas RE-POWER relies on clinic staff to conduct these tasks. Taken together, the results of these two trials will advance our understanding of the effectiveness of different treatment options in primary care.
PROPEL is uniquely positioned to explore the role of primary care in facilitating weight loss in underserved populations. The sample is 67% African American, and approximately 31% of the patients have low health literacy. However, a potential limitation is the unbalanced race distribution across the groups at baseline: approximately 59% and 73% of the usual care and intervention groups, respectively, are African American (Table 2). Further, the sample as a whole is predominantly female, which may limit our ability to perform sub-group analyses that are stratified by sex. The median sample size per clinic is 43.5 patients. However, two clinics had low sample sizes (n=2 and n=4). These two clinics were located in rural areas of the state, and we were hampered by low population density in addition to low PCP engagement at these two sites. We continue to follow the patients at these two clinics, and we plan to analyze the data with and without them to determine the potential effects on the overall results and interpretation.
Although the prevalence of adult obesity has increased significantly in recent decades,8 analysis of data from National Ambulatory Medical Care Survey revealed a 41% lower odds of weight-related counselling among patients with obesity in 2007–2008 compared to 1995–1996.56 While we know that modest weight loss is achievable in some settings such as in academic health science centers,38,57 to date there has been no successful translation of this achievement to primary care practices. The results of the PROPEL trial will have a direct impact on improving healthcare delivery and outcomes. Effective obesity treatment programs currently exist; however, a “translation-gap” also exists where the current science has not been implemented in primary care in any scalable format. The adaptation of an intensive lifestyle intervention, with significant patient and stakeholder input for deployment in primary care settings, has the potential to significantly address this gap.
Acknowledgments
The authors would like to acknowledge the patients and stakeholders who are members of the PROPEL Patient Advisory Boards, Community Monitoring Board, and the Project Management Committee, who significantly impacted the trial’s design and conduct. The authors are also indebted to the PROPEL assessment technicians and health coaches, without whom this study would have been possible. The authors also gratefully acknowledge the contributions of Willie C. White III and the David Raines Community Health Centers, Dr. Gary Wiltz and the Teche Action Clinic sites, Michael G. Griffith and Dr. Robert Post and Daughters of Charity Services of New Orleans, and the management and staff of the Access Health Ruth U. Fertel/Tulane Community Health Center. This research was supported by the Patient-Centered Outcomes Research Institute (PCORI) Contract #OB–1402–10977 (PI: P. Katzmarzyk). Additional support was provided by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health that funds the Louisiana Clinical and Translational Science Center, and NORC Center Grant # P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: ClinicalTrials.gov Identifier NCT02561221
References
- 1.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System 2015. 2017 Available on-line at: https://www.cdc.gov/brfss/brfssprevalence/index.html.
- 2.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35(8):1999–2010. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Cancer Statistics Working Group. Incidence and Mortality Web-based Report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. United States Cancer Statistics: 1999-2010. Available at: http://www.cdc.gov/uscs. [Google Scholar]
- 4.Brown JR, O’Connor GT. Coronary heart disease and prevention in the United States. N Engl J Med. 2010;362(23):2150–3. doi: 10.1056/NEJMp1003880. [DOI] [PubMed] [Google Scholar]
- 5.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 7.Sherry B, Blanck HM, Galuska DA, Pan L, Dietz WH, Balluz L. Vital Signs: State-specific obesity prevalence among adults-United States, 2009. Morb Mortal Wkly Rep. 2010 Aug 3;59:1–5. [PubMed] [Google Scholar]
- 8.Flegal K, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slack T, Myers CA, Martin CK, Heymsfield SB. The geographic concentration of US adult obesity prevalence and associated social, economic, and environmental factors. Obesity. 2014;22(3):868–74. doi: 10.1002/oby.20502. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. Healthy People 2020 Framework: The Vision, Mission, and Goals of Healthy People 2020. 2011 Available at www.healthypeople.gov. www.healthypeople.gov (accessed September 22 2012)
- 11.Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: A narrative review. Ann NY Acad Sci. 2013;1281:191–206. doi: 10.1111/nyas.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–8. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 13.Decision Memo for Intensive Behavioral Therapy for Obesity. 2011 Accessed at: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253.
- 14.Pagoto SL, Pbert L, Emmons K, Farber D, Society of Behavioral Medicine Public Policy Leadership Group Policy Brief: The Society for Behavioral Medicine position statement on the CMS decision memo on intensive behavioral therapy for obesity. Transl Behav Med. 2012;2:381–3. doi: 10.1007/s13142-012-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312(17):1779–91. doi: 10.1001/jama.2014.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen MD, Ryan DH. New obesity guidelines: Promise and potential. JAMA. 2014;311(1):23–4. doi: 10.1001/jama.2013.282546. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Heart Lung and Blood Institute. Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel. US Department of Health and Human Services; 2013. 2013. Available at www.nhlbi.nih.gov/guidelines: [Google Scholar]
- 19.Kennedy BM, Kennedy KB, Sarpong DF, Katzmarzyk PT. Perceptions of obesity treatment options among healthcare providers and low-income primary care patients. Ochsner J. 2016;16:158–165. [PMC free article] [PubMed] [Google Scholar]
- 20.Federal Office of Rural Health Policy, Health Resources and Services Administration. List of Rural Counties And Designated Eligible Census Tracts in Metropolitan Counties: Updated Census 2010. 2018 Available at: https://www.hrsa.gov/sites/default/files/ruralhealth/resources/forhpeligibleareas.pdf. Accessed January 19, 2018.
- 21.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026–33. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 22.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service; 2000. Revised 2000. [Google Scholar]
- 23.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–71. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 26.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–11. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Fuller NR, Williams K, Shrestha R, et al. Changes in physical activity during a weight loss intervention and follow-up: A reandomized controlled trial. Clinical Obesity. 2014;4:127–35. doi: 10.1111/cob.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson FE, Midthune D, Subar AF, Kipnis V, Kahle LL, Schatzkin A. Development and evaluation of a short instrument to estimate usual dietary intake of percentage energy from fat. J Am Diet Assoc. 2007;107(5):760–7. doi: 10.1016/j.jada.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Havas S, Heimendinger J, Damron D, et al. 5 A Day for better health–nine community research projects to increase fruit and vegetable consumption. Public Health Rep. 1995;110(1):68–79. [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson FE, Kipnis V, Subar AF, et al. Evaluation of 2 brief instruments and a food-frequency questionnaire to estimate daily number of servings of fruit and vegetables. Am J Clin Nutr. 2000;71(6):1503–10. doi: 10.1093/ajcn/71.6.1503. [DOI] [PubMed] [Google Scholar]
- 32.Hedrick VE, Savla J, Comber DL, et al. Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): sugar-sweetened beverages and total beverage energy intake. J Acad Nutr Diet. 2012;112(6):840–9. doi: 10.1016/j.jand.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stunkard AJ, Messick S. Eating Inventory Manual. San Antonio, TX: The Psychological Corporation; 1988. [Google Scholar]
- 34.Bennett WL, Wang NY, Gudzune KA, et al. Satisfaction with primary care provider involvement is associated with greater weight loss: Results from the practice-based POWER trial. Patient Educ Couns. 2015 doi: 10.1016/j.pec.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hargraves JL, Hays RD, Cleary PD. Psychometric properties of the Consumer Assessment of Health Plans Study (CAHPS) 2.0 adult core survey. Health Serv Res. 2003;38(6 Pt 1):1509–27. doi: 10.1111/j.1475-6773.2003.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21(6):661–5. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Befort CA, VanWormer JJ, DeSouza C, et al. Protocol for the Rural Engagement in Primary Care for Optimizing Weight Reduction (RE-POWER) trial: comparing three obesity treatment models in rural primary care. Contemp Clin Trials. 2016;47:304–14. doi: 10.1016/j.cct.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 38.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diab Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Look AHEAD Research Group. The Look AHEAD Study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rickman AD, Williamson DA, Martin CK, et al. The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials. 2011;32:874–81. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute for Literacy. State of Literacy in America: Estimates at the Local, State and National levels. Washington, DC: U.S. Government Printing Office; 1998. [Google Scholar]
- 42.Weiss B, Schwartzberg J, Davis T, Parker R, Sokol P, Williams M. Health literacy and patient safety: Help patients understand: Manual for clinicians. AMA Foundation; 2007. [Google Scholar]
- 43.US Department of Health and Human Services. Office of Disease Prevention and Health Prmotion National Action Plan to Improve Health Literacy. Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 44.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD Study: Factors associated with long-term success. Obesity. 2011;19:1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neiberg RH, Wing RR, Bray GA, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD study. Obesity. 2012;20:2048–56. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2010. 7th. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- 47.Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Math Biosci Eng. 2009;6(4):873–87. doi: 10.3934/mbe.2009.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas DM, Das SK, Levine JA, et al. New fat free mass - fat mass model for use in physiological energy balance equations. Nutr Metab. 2010;7:39. doi: 10.1186/1743-7075-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas DM, Martin CK, Heymsfield S, Redman LM, Schoeller DA, Levine JA. A simple model predicting individual weight change in humans. J Biol Dyn. 2011;5(6):579–99. doi: 10.1080/17513758.2010.508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92(6):1326–31. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: Results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146–54. doi: 10.1001/archinternmed.2009.508. [DOI] [PubMed] [Google Scholar]
- 52.Bennett GG, Foley P, Levine E, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA Intern Med. 2013;173(19):1770–7. doi: 10.1001/jamainternmed.2013.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172(7):565–74. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faruqi N, Stocks N, Spooner C, El Haddad N, Harris MF. Research protocol: Management of obesity in patients with low health literacy in primary health care. BMC Obes. 2015;2:5. doi: 10.1186/s40608-015-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care. 2013;51(2):186–92. doi: 10.1097/MLR.0b013e3182726c33. [DOI] [PubMed] [Google Scholar]
- 57.The Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with Type 2 diabetes mellitus. Arch Intern Med. 2011;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]


