Abstract
Despite previous inquiry into the fermentative bacterial community of kimchi, there has been little insight into the impacts of starting ingredients on the establishment and dynamics of the microbial community. Recently some industrial producers have begun to utilize vegan production methods that omit fermented seafood ingredients. The community-level impacts of this change are unknown. In this study, we investigated the differences in the taxonomic composition of the microbial communities of non-vegan kimchi and vegan kimchi prepared through quick fermentation at room temperature. In addition to tracking the community dynamics over the fermentation process, we looked at the impact of the constituent ingredients and the production facility environment on the microbial community of fermenting kimchi. Our results indicate that the bacterial community of the prepared vegan product closely mirrors the progression and final structure of the non-vegan final product. We also found that room temperature-fermented kimchi differs minimally from more traditional cold-fermented kimchi. Finally, we found that the bacterial community of the starting ingredients show a low relative abundance of the lactic acid bacteria in fermented kimchi, whereas the production facility is dominated by these bacteria.
Keywords: Kimchi, Vegan, Microbiome, High-Throughput Sequencing, Fermentation, Artisan, Probiotics
1. Introduction
Kimchi, a fermented food commonly made from cabbage, radish, and various seasonings, is a staple of traditional Korean cuisine (Jung et al., 2011). It is also growing in popularity in the United States and around the world, where both commercial and artisanal preparations are sold in Asian supermarkets and high-end retail stores. The process of making kimchi is thought to be largely dependent on microbial composition, the ingredients used, and fermentation conditions (Cheigh and Park, 1994; Ick, 2003). Because kimchi is made without the use of a starter culture, the raw ingredients play a key role in establishing the bacterial community that is responsible for fermenting kimchi (Jung et al., 2011; S. H. Lee et al., 2015). Temperature is also a known determinant of microbial composition in kimchi (J.-Y. Jang et al., 2015). Warmer temperatures pose problems for kimchi fermentation and preservation, so kimchi has historically been produced during the fall and winter (Cho et al., 2006). This cold-fermented process usually takes about thirty days (Jung et al., 2011; J.-S. Lee et al., 2005; M. Lee et al., 2017). This practice has been extended throughout the year with the advent of modern refrigeration (Cho et al., 2006; D. J. Jang et al., 2015). Today, industrial refrigeration is used to produce kimchi at near-freezing temperatures, mimicking traditional fermentation conditions (Cho et al., 2006). Fermentation at room temperature can expedite kimchi production; however, it is unknown whether the development of microbial communities in these fast-fermented preparations differs from that of more traditional kimchi (M. Lee et al., 2017).
Within Korea, the ingredients used vary by the region in which the kimchi is produced (M. Lee et al., 2017). Cabbage and radish are the main ingredients used to prepare kimchi, but seasonings frequently include ginger, scallion, onion, red pepper, radish, salt, and fermented seafood (jeotgal) (Jung et al., 2011). Previous studies have shown that the microbial composition of kimchi is impacted by the types and amounts of ingredients used (Ahmadsah et al., 2015; Cheigh and Park, 1994; Ick, 2003; M. Lee et al., 2017). Though the microbial composition of kimchi can vary during the initial stages of kimchi preparation, lactic acid bacteria (LAB) are the dominant microorganisms by the end of fermentation (Cheigh and Park, 1994; Ick, 2003; J.-S. Lee et al., 2005). LAB are known to enhance the shelf life, flavor, and nutritional properties of food through the production of organic acids, bacteriocins, vitamins, and flavor compounds (Cheigh and Park, 1994; H. Lee et al., 2011; Settanni and Corsetti, 2008; Turpin et al., 2011). It has been proposed that certain LAB can even act as probiotics to promote human health and microbiome stability (Ji et al., 2013; Ljungh and Wadström, 2006). Whether or not this perspective is accurate, the purported health benefits of kimchi have contributed to increased popularity and consumption in the United States.
Recent advances in high throughput sequencing technology have allowed researchers to examine the community structure of the kimchi microbiome using culture-independent techniques. The advent of 16S rRNA amplicon sequencing technology has made it possible to systematically analyze the kimchi microbiome before, during, and after fermentation. Lee et al. found that, at the end of fermentation, the bacterial community of kimchi is dominated by species of the genera Lactobacillus, Leuconostoc, Weissella, and Lactococcus (M. Lee et al., 2017). The same technology has been used to show that certain ingredients have a more significant effect on the bacterial community than others. Kimchi with a lower salt concentration has been correlated with more unique operational taxonomic units (OTUs) (M. Lee et al., 2017). Large amounts of garlic are associated with more kimchi-associated LAB in the final product (S. H. Lee et al., 2015). A greater proportion of the bacterial community is made up of Weissella spp. when red pepper powder is used as an ingredient (Jeong et al., 2013).
Rates of vegetarianism and veganism in the United States are on the rise; according to a 2012 Gallup poll, 5% of Americans identify as vegetarian, while 2% of Americans identify as vegan (Radnitz et al., 2015). These populations have historically been unable to consume kimchi, because traditional Korean kimchi is made with jeotgal, or fermented seafood, often in the form of fish sauce (Guan et al., 2011). This fermented component is high in glutamate-containing compounds, which contribute to the rich umami flavor characteristic of kimchi (Li et al., 2002). However, use of animal-derived ingredient prevents consumption of traditional kimchi by vegetarians and vegans. To accommodate this segment of consumers, American artisanal kimchi producers are swapping jeotgal for miso paste. Miso paste, made by fermenting soybeans and grains, offers a depth of flavor comparable to jeotgal but contains no animal products. While both jeotgal and miso paste are dominated by LAB, the taxonomic composition of each ingredient differs. Jeotgal contains genera Bacillus, Brevibacterium, Micrococcus, and Lactobacillus among others (Koo et al., 2016), while miso primarily consists of Tetragenococcus and Enterococcus (Onda et al., 2003). Since both fermented ingredients contain their own unique LAB, it is possible that these fermentative bacteria could contribute differently to the final microbial community. Currently, it is unknown if or how this substitution affects the microbial composition of vegan kimchi.
To profile the impacts of vegan versus non-vegan ingredients, we assessed how the microbial community of kimchi is established and how it changes over the course of rapid room temperature fermentation, which is a method commonly used for large-scale commercial preparation because of its faster fermentation time. We investigated the roles of raw ingredients and environmental conditions in establishing the initial bacterial community of kimchi and the changes in community structure throughout fermentation. We found that, despite initial differences in microbial composition between vegan and non-vegan kimchi, there was no notable difference in the final products. Ultimately, the microbial community of both vegan and non-vegan kimchi is dominated by Lactobacillaceae and Leuconostocaceae, and lacks the Enterobacteriaceae found in the fish sauce or miso paste.
2. Materials and Methods
2.1. Kimchi Preparation and Sampling Methods
Kimchi was prepared for commercial sale during June 2017 by a small-batch kimchi producer in a facility near Providence, Rhode Island. The facility produces approximately 20,000 pounds of kimchi per year and makes both vegan and non-vegan varieties. All samples were collected from one production run of both vegan and non-vegan kimchi. Napa cabbage was salted for three hours before being drained of excess water. Ginger, scallion, onion, garlic, red pepper, sugar, and radish were then added to the salted cabbage. The mixture was then separated into two batches: vegan and non-vegan, which were mixed with miso paste or fish sauce, respectively. The vegan formulation was prepared as a 600 lbs. batch, and the non-vegan formulation was prepared as a 900 lbs. batch. Salt concentration of representative batches was 2.25% ± 0.03% for non-vegan kimchi and 1.86% ± 0.07% for vegan kimchi. These salt concentrations are within the expected range for kimchi (M. Lee et al., 2017), and show that there is a small difference between the two preparations.
Both kimchi batches were placed into multiple plastic drums for a 72-hour fermentation period within the same fermentation room. The temperature of the fermentation environment was controlled at a range of 21.1–23.9°C. Successful fermentation is ensured by a pH measure of 4.6 or below at the end of fermentation, according to industry safety standards; representative samples of both types of kimchi showed a pH of 3.90 ± 0.02 for non-vegan kimchi and 3.80 ± 0.03 for vegan kimchi, indicating that both fermentations reached completion during the same period of time (Jung et al., 2011).
Samples were collected using Pasteur pipettes from fermenting vegan and non-vegan kimchi drums throughout fermentation at 24 hours intervals in triplicate: at day zero (0 hours), day one (24 hours), day two (48 hours), and day three (72 hours). Ingredient samples were collected in triplicate prior to the production run; approximately 0.5 g of each ingredient was placed into a 1.5mL Eppendorf tube containing 500 μL of nuclease-free water. This liquid collection protocol was conducted in accordance with previous work, and collects only the planktonic cells in the fermentation batch (Jung et al., 2011). It is important to acknowledge that some fermenting bacteria may be found in biofilms, and would be missed using this method.
To sample the environment of kimchi production, the surfaces of the production table, the industrial sink, and the floor within the production facility were swabbed in triplicate with flocked sterile swabs (Zymo Research, Irvine, CA, U.S; Cat: C1052), which were stored individually in Zymo Research DNA/RNA Shield Lysis Tubes (Zymo Research, Irvine, CA, U.S; Cat: R1103). To sample the air and dust in the production facility, empty Petri dishes were left uncovered in the facility from day zero through day three and subsequently swabbed in the manner described above (Adams et al., 2013). Aliquots of liquid from finished kimchi jars that were being stored in the facility walk-in refrigerator were collected in 1.5 mL Eppendorf tubes. All samples were immediately transported to the laboratory on ice after collection and stored at −80°C until processing.
2.2. DNA Extraction, 16S Library Preparation and Sequencing
DNA was extracted from kimchi, environmental, and ingredient samples using the ZymoBIOMICS DNA Microprep Kit (Zymo Research, Irvine, CA, U.S; Cat: D4305) according to the manufacturer’s instructions. The V4 hypervariable region of the bacterial 16S rRNA gene was targeted for PCR amplification according to the Earth Microbiome Project 16S Illumina Amplicon Protocol, with an 806Rb reverse primer (GGACTACCAGGGTATCTAATCC) and a barcoded 515F forward primer (CAGCAGCCGCGGTAAT) (Caporaso et al., 2010; 2012; Thompson et al., 2017; Walters et al., 2016). PCR was performed using Phusion High-Fidelity polymerase (New England BioLabs, Ipswich, MA, U.S.) under the following conditions: 98 °C for 3 minutes followed by 35 cycles of 98 °C for 45s, 50 °C for 60s, and 72 °C for 90s, with a final elongation step of 72 °C for ten minutes. A negative control was performed using the same reaction conditions using nuclease-free water instead of extracted DNA. The concentrations of resulting PCR amplicons were assessed using the using the Qubit 3.0 Fluorometer and the dsDNA-HS kit (Thermo Fisher Scientific, Waltham, MA, U.S.; Cat: Q32854) according to the manufacturer’s instructions. Equal masses of PCR amplicons from each sample were pooled, concentrated, and gel purified using the Machery-Nagel NucleoSpin Gel and PCR Clean-Up kit (Machery-Nagel, Düren, Germany; Cat: 740609) according to the manufacturer’s instructions. Samples were pooled and submitted to the Rhode Island Genomics and Sequencing Center at the University of Rhode Island (Kingston, RI, USA) for quality control and sequencing. Amplicons were paired-end sequenced (2 × 250 bp) on an Illumina MiSeq platform using a 500-cycle kit using standard protocols.
2.3 Rarefaction and Sequencing Analysis
Raw paired-end FASTQ files were demultiplexed using idemp (https://github.com/yhwu/idemp/blob/master/idemp.cpp) and imported into QIIME2 (ver. 2017.9.0, https://qiime2.org/). Sequences were quality filtered, trimmed, de-noised, and merged using DADA2 (Callahan et al., 2016). Chimeric sequences were identified and removed via the consensus method in DADA2. Taxonomy was assigned to all ribosomal sequence variants in QIIME2 using a feature classifier trained with the SILVA 99% OTU database trimmed to the V4 region of the 16S rRNA gene (DeSantis et al., 2006). Contaminating mitochondrial and chloroplast sequences were filtered from the resulting OTU table. Representative sequences were aligned with MAFFT and used for phylogenetic reconstruction in FastTree (Katoh and Standley, 2013; Price et al., 2009). Diversity metrics were calculated using the core-diversity plugin within QIIME2.
One complication to the analysis of microbial communities from multiple sources is that some are of intrinsically low density. As a result, several ingredient and environmental samples yielded insufficient sequencing reads for certain forms of analysis. We excluded all samples with fewer than 1500 reads from analysis altogether and samples with fewer than 3398 reads from diversity analyses. This resulted in the exclusion of 17 of our original 74 samples from diversity analyses and 14 samples from our taxa bar plots. While this is computationally important for diversity analysis, we acknowledge it is a potential source of bias. In total, 13 of the 17 excluded samples belonged to the same five sources (three onion samples, three radish samples, three salt samples, two sugar samples, and two garlic samples). These consistently low counts likely result from a low abundance of bacteria in these samples.
2.4 Statistical Analysis
Shannon diversity was compared via unpaired t-test in Prism (version 7.0). To visualize differences in microbial community structure between and within sample groups, Bray-Curtis dissimilarity and weighted and unweighted UniFrac was plotted using principal coordinate analysis (PCoA) in QIIME2. Testing for differential abundance was performed by non-parametric negative binomial Wald test available within the DESeq2 package in R (version 3.3.2, https://www.r-project.org/) (Love et al., 2014). P values were adjusted to account for FDR using the default Benjamini-Hochberg method (Benjamini and Hochberg, 1995).
3. Results & Discussion
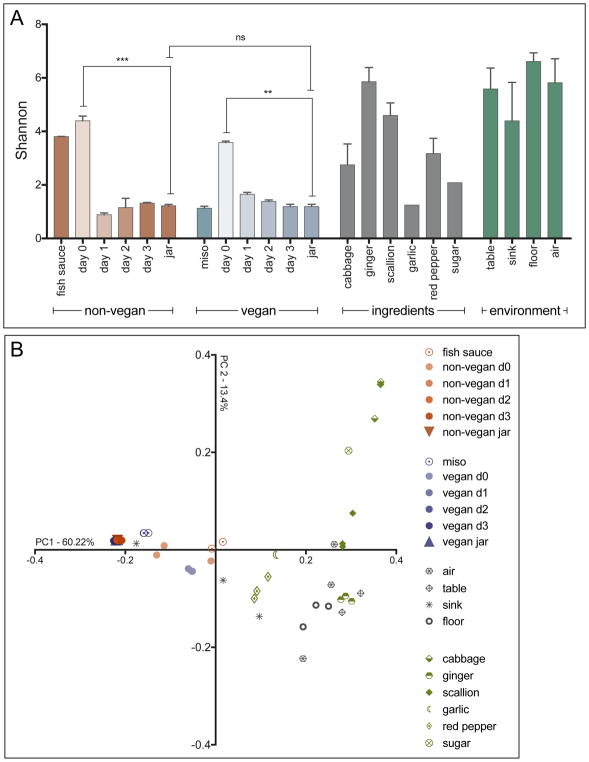
We observed a wide range of variation in the alpha diversity of constituent ingredients, assessed using the Shannon diversity index (Figure 1A). The mean Shannon diversity of ingredients ranged from as low as 1.2 (n = 1) for garlic to as high as 5.9 for ginger (±0.5 SD). Miso paste, the fermented food ingredient in vegan kimchi and a fermented vegetable product itself, had relatively low alpha diversity. The lack of diversity in the miso paste bacterial community is likely due to the dominance of the LAB that drive vegetable fermentation (Onda et al., 2003). However, fish sauce, the fermented food ingredient in non-vegan kimchi, had surprisingly high levels of alpha diversity. Finally, we found that the environmental samples taken from the facility—particularly the air samples—were among the most diverse communities sampled.
Figure 1. Comparison of diversity indices for kimchi ingredients, production environment, and non-vegan and vegan kimchis during 3-day fermentation.
(A) Alpha diversity of microbial communities shown as Shannon values. Significance of change between samples determined by unpaired t-test (vegan d0 vs. subsequent days and vegan d0 vs. vegan jar: ** p< 0.01; non-vegan d0 vs. subsequent days and non-vegan d0 vs. non-vegan jar: *** p < 0.001; vegan jar – non-vegan jar: ns, not significant). Error bars depict the standard error of the mean. (B) Beta diversity of all kimchi, ingredient, and environmental samples as measured by weighted-UniFrac and visualized by PCoA.
The initial day zero mixtures of ingredients for both vegan and non-vegan preparations were found to have high diversity [Shannon 4.4 (±0.2 SD) for non-vegan kimchi and 3.6 (±0.1 SD) for vegan kimchi]. This diversity reflects the range of bacteria present in the constituent ingredients of kimchi. We found that non-vegan kimchi had a higher starting alpha diversity than its vegan counterpart. We attribute this to the high alpha diversity of fish sauce, the key differentiating ingredient in non-vegan kimchi.
Despite this difference, by day one, the alpha diversity of both preparations decreased significantly, converging to a similar level. Over the next two time points, the material maintained the low alpha diversity seen at day one (Figure 1A). The precipitous reduction in diversity likely reflects the selective pressure for LAB exerted by the fermentation process, and indicates proper fermentation progression. There appeared to be no significant difference in alpha diversity between the final vegan and non-vegan preparations (Figure 1A). This suggests that, despite the difference in initial diversity, both products achieved the same final low diversity state.
This finding is also supported by beta diversity metrics, which we assessed using weighted Unifrac and visualized using PCoA (Gower, 1966). As seen in Figure 1B, the initial bacterial compositions of both vegan and non-vegan kimchi clustered separately from each other. This is likely due to the difference in the bacterial communities present in miso paste and fish sauce, as these two ingredients are the only ones that differ between the two preparations. This also corresponds to the differences in alpha diversity observed between these two ingredients. However, by day one, the composition of both types of kimchi shifts and converges such that the two preparations are mostly indistinguishable. This is likely due to the selective pressure of the fermentative environment. They remain stable for the remainder of the fermentation period, through jarring and storage. By the time the product is ready for sale, there is no notable difference in the beta diversity of the microbial communities of the vegan and non-vegan kimchi varieties.
Figure 1B shows that many of the raw ingredients clustered with the kimchi samples at day zero, likely because the initial total mixture had not undergone fermentation and most of the bacteria in the day zero kimchi was a combination of the bacterial communities of the raw ingredients. Interestingly, the bacterial community of miso paste clustered closely with the final product of both types of kimchi, while fish sauce clustered separately from any of the other samples collected. This may be a result of miso paste being a fermented vegetable product, whereas fish sauce is made from fermenting seafood. These observations are in line with the trends in alpha diversity that we previously observed. The environmental samples collected from the production facility showed no clear pattern of clustering except within sample groups (Figure 1B, Figure S2).
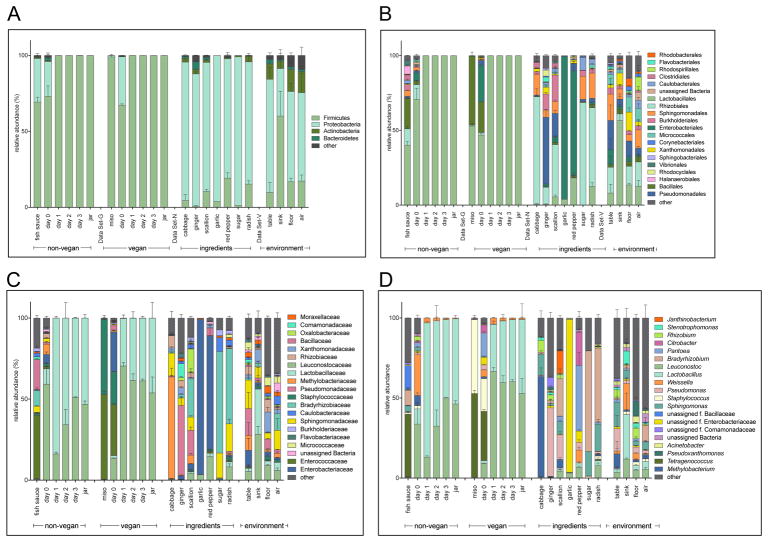
We next examined the taxonomic structure of the bacterial communities at the phylum, order, and family levels (Figure S1). At the phylum level, both vegan and non-vegan kimchi become very similar over time (Figure 2A). Though the bacterial taxa present in both types of kimchi vary at the beginning of fermentation, Firmicutes represent almost the entire bacterial community in both vegan and non-vegan kimchi by the time the product is ready to be jarred and sold. Most of the ingredients used to prepare both vegan and non-vegan kimchi had high levels of Proteobacteria and low abundance of Firmicutes. The two fermented ingredients, fish sauce or miso paste, are composed primarily of Firmicutes and thus likely contribute this phylum to the starting communities. Both the vegan and non-vegan kimchi at day zero reflect the characteristics of both these groupings. The bacterial community of vegan kimchi at day zero consists of mostly Firmicutes, with a smaller proportion of Proteobacteria. Non-vegan kimchi is similar, with a community dominated by Firmicutes, followed by Proteobacteria. Between the two, non-vegan kimchi has a greater proportion of Firmicutes as compared to the vegan kimchi at day zero. However, the bacterial populations are indistinguishable and dominated by Firmicutes by day one. This pattern persists through jarring of the final product. These results are in line with those previous published by Lee et al. 2017 (M. Lee et al., 2017).
Figure 2. Relative abundance (%) of taxa in kimchi, ingredient, and environment samples.
Error bars depict the standard error of the mean. Relative abundance of bacteria across all samples at the (A) phylum, (B) order, (C) family, and (D) genus levels. Low abundance taxa grouped as “other” and greyed out.
The environmental samples from the facility are similar to the samples of kimchi ingredients in that they are composed of mostly Firmicutes and Proteobacteria, with some Actinobacteria and Bacteroidetes present. The production table in the facility is particularly high in Firmicutes, possibly because most of the kimchi production process is conducted on or around this table. The large quantities of kimchi and constituent ingredients that pass through the premises on a regular basis likely contribute to the increased proportion of Firmicutes present in the environment.
At the order level, we observe new trends in addition to those evident at higher taxonomic levels. The bacterial compositions of vegan and non-vegan kimchi at day zero continue to differ from one another (Figure 2B). At this time point, non-vegan kimchi is composed of high proportions of Lactobacillales, with smaller proportions of Clostridiales, Enterobacteriales, Sphingomonadales, and Bacillales. Vegan kimchi shows a smaller proportion of Lactobacillales than the non-vegan kimchi, with more notable proportions of Bacillales, Enterobacteriales, and Pseudomonadales. The bacterial communities present in the ingredients continue to diverge at this level of analysis.
At the family level, we can ascertain the aforementioned trends at a higher level of taxonomic resolution (Figure 4C). We observe more notable differences in the bacterial composition of vegan and non-vegan kimchi at day zero; whereas non-vegan kimchi is predominantly composed of Lactobacillaceae and Leuconostocaceae at the beginning of fermentation, vegan kimchi contains small proportions of Lactobacillaceae and Leuconostocaceae with larger proportions of Enterococcaceae, Staphylococcaceae, and Enterobacteriaceae. Fish sauce and miso paste also appear more distinct, with miso paste consisting almost entirely of Enterococcaceae and Staphylococcaceae and fish sauce having a more diverse community. This might account for the differences between vegan and non-vegan kimchi at day zero. As previously observed, the bacterial communities of both types of kimchi at day zero seem to originate from the ingredients used to prepare them. Interestingly, there is a high proportion of Staphylococceaceae in miso paste, and some of this carries over into the day zero vegan kimchi, though it is ultimately outcompeted by LAB.
Over the first 24 hours of fermentation, there is a dramatic shift in the taxonomic composition at the family level of both vegan and non-vegan kimchi. By day one, both communities consist entirely of Lactobacillaceae and Leuconostocaceae (Figure 4C). On day one, non-vegan kimchi contains more Lactobacillaceae and vegan kimchi contains more Leuconostocaceae, but by day three they are indistinguishable in their respective bacterial communities.
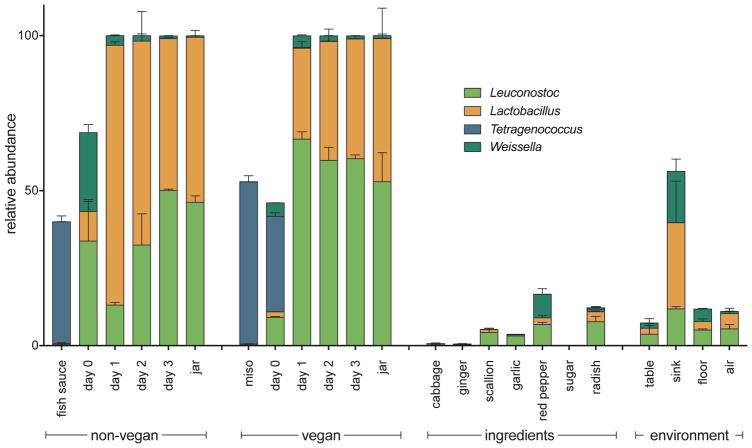
Traditionally, kimchi is fermented at cold temperatures, and that product can be referred to as cold-fermented kimchi. However, the popularization of kimchi around the world and the demand for large-scale commercial production has necessitated a more efficient, quicker way to produce this traditionally slow-fermented food. Thus, an increasing number of industrial producers have turned to room temperature fermentation for a shorter fermentation time. The kimchi we sampled was of the quick-fermented variety. In commercial cold-fermented kimchi analyzed by Lee et al. 2017, Proteobacteria were present in most kimchi samples but were quickly overtaken by Firmicutes, which remained dominant through the end of fermentation (M. Lee et al., 2017). Bacteria from genus Weissella were dominant at the beginning of fermentation, while Lactobacillaceae and Leuconostocaceae were common during the later periods of fermentation (Jeong et al., 2013; M. Kim and Chun, 2005; J. S. Lee, 2002; M. Lee et al., 2017). The quick-fermented kimchi we analyzed also initially contained Proteobacteria and then became dominated by Firmicutes (specifically, Lactobacillaceae and Leuconostocaceae). Weissella was detected at day zero and declined over the fermentation period, but at no point was this genus dominant in the microbial community of either kimchi preparation (Figure 3).
Figure 3. Relative abundance (%) of LAB genera across all sample types.
Error bars depict the standard error of the mean.
The initial ingredients contributed very little to the final composition of the fermented products. It appears that the two families that dominate the final community are present only in very small proportions in the day zero samples, ingredients, and the environment (Figure 2C). There are a few other minimally represented families of bacteria at day zero in both vegan and non-vegan kimchi, but for both preparations the major LAB families present at the end of fermentation are Lactobacillaceae and Leuconostocaceae (Figure 4C). The highest-abundance taxon common to both fermented ingredients is Enterococcaceae (Figure 4C). Thus, it seems that the fermentation conditions rapidly select for a bloom of low abundance taxa.
Since Lactobacillus, Leuconostoc, Tetragenococcus, and Weissella appeared to be the most prevalent LAB genera in the kimchi, ingredient, and environmental samples, we further examined the patterns exhibited by these genera and how their proportions within larger communities change over time (Figure 2D). These data are represented in Figure 3 and Figure S3. Lactobacillus is found at very low levels in most ingredients. Similarly, it is also found at low levels on day zero for both vegan and non-vegan kimchi. In both preparations of kimchi, this proportion increases over the first 24 hours of fermentation, and then stabilizes at around 50% by the end of fermentation. These trends are nearly identical for Leuconostoc. Tetragenococcus, the dominant LAB in miso paste and fish sauce, is initially found in high proportions in both ingredients. However, this prevalence in the fermented food ingredients does not persist through kimchi fermentation. This may be due to the differences in the characteristic salinity of fish sauce, miso paste, and kimchi. The salinity of fish sauce has been found to be around 25% (Nakano et al., 2017), and the salinity of commercial miso paste has been found to be around 10–12% (Watanabe et al., 1982). By contrast, the salinity of kimchi has been measured at about 1.62% (J.-A. Kim et al., 2012). Similarly, the salinity of the vegan and non-vegan kimchi we sampled was also much lower than the characteristic values of salt concentration for fish sauce and miso paste. Overall, these data show the dominant role that LAB play in the kimchi fermentation process—and how quickly they dominate the fermentation environment.
Interestingly, we found that the terminal LAB families in kimchi are also present in high abundance in the environmental samples from the production facility (Figure 3 and Figure S3). These results suggest the final kimchi product may contribute to the bacterial community of the factory. However, it may also be the case that the cycle of continuous fermentation in the production facility contributes LAB to each new batch of kimchi, acting as a form of starter culture for the fermentation process. The higher proportion of LAB in the environment than in the starting ingredients points to this as a possibility, although this observation would need additional confirmation in the future. Additionally, because both non-vegan and vegan kimchi were produced in the same facility, it is also possible that the high degree of similarity between the two communities resulted from introduction of LAB already present in the facility. This would present a form of inadvertent cross-contamination between the two samples. In sum, our analysis reveals that, despite the significant differences between the microbial communities of fish sauce and miso paste and between the starting communities of non-vegan and vegan kimchi on day zero of fermentation, there is no substantial difference in their microbial communities by the end of fermentation. This may be important for industrial production and consumer consumption of kimchi. For kimchi producers, it signifies more flexibility in the method of kimchi preparation employed in order to produce a very similar final product; for consumers, it means that people who adhere to vegetarian or vegan diets might be able to consume a food traditionally off-limits to them. Especially since fermented foods are gaining notoriety for their bacterial content, the lack of notable difference between the taxonomic composition of vegan and non-vegan kimchi may enhance the growing popularity of this traditional fermented food.
Supplementary Material
S1. Tabular comparison of the predominant LAB across all sample types. Predominant LAB genera include Tetragenococcus, Leuconostoc, Lactobacillus, and Weissella, expressed as a percentage of total OTUs.
S2. Simplified version of Fig. 1B PCoA depicting weighted-UniFrac distance of kimchi fermenting samples along with final product, fish sauce, and miso samples.
S3. Relative abundance (%) of bacterial genera in individual kimchi, ingredient, and environment sample. Relative abundances are from un-averaged triplicate samples.
Highlights.
The bacterial community of vegan kimchi closely mirrors the non-vegan final product
Lactobacillaceae and Leuconostocaceae dominate the final kimchi microbial community
LAB in fish sauce and miso paste do not carry over to the kimchi microbiome
Vegetable ingredient bacteria do not contribute markedly to the kimchi microbiome
Production facility is enriched with high-abundance kimchi LAB
Acknowledgments
The work published here was funded in part by a grant through the COBRE Center for Computational Biology of Human Disease (NIH P20 GM109035). Sequencing for this study was conducted at the Rhode Island Genomics and Sequencing Center, a Rhode Island NSF EPSCoR research facility supported in part by the NSF EPSCoR Cooperative Agreement #EPS-1004057. DJC was supported by the National Science Foundation Graduate Research Fellowship (Grant No. 1644760). MZ was supported in part by an Undergraduate Teaching and Research Award (UTRA) from Brown University.
Footnotes
Data Availability. Raw reads were deposited into the NCBI Sequence Read Archive (SRA) database under the BioProject ID number PRJNA418790.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RI, Miletto M, Taylor JW, Bruns TD. Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013;7:1262–1273. doi: 10.1038/ismej.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadsah LSF, Min SG, Han SK, Hong Y, Kim HY. Effect of Low Salt Concentrations on Microbial Changes During Kimchi Fermentation Monitored by PCR-DGGE and Their Sensory Acceptance. J Microbiol Biotechnol. 2015;25:2049–2057. doi: 10.4014/jmb.1506.06058. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. doi: 10.2307/2346101?ref=search-gateway:049f6d8d544726610c25b1c5fc04f248. [DOI] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheigh HS, Park KY. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products) Crit Rev Food Sci Nutr. 1994;34:175–203. doi: 10.1080/10408399409527656. [DOI] [PubMed] [Google Scholar]
- Cho J, Lee D, Yang C, Jeon J, Kim J, Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol Lett. 2006;257:262–267. doi: 10.1111/j.1574-6968.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower JC. Some Distance Properties of Latent Root and Vector Methods Used in Multivariate Analysis. Biometrika. 1966;53:325. doi: 10.2307/2333639. [DOI] [Google Scholar]
- Guan L, Cho KH, Lee JH. Analysis of the cultivable bacterial community in jeotgal, a Korean salted and fermented seafood, and identification of its dominant bacteria. Food Microbiol. 2011;28:101–113. doi: 10.1016/j.fm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Ick MT. Factors Affecting Kimchi Fermentation. Japanese Journal of Lactic Acid Bacteria. 2003;14:56–71. doi: 10.4109/jslab1997.14.56. [DOI] [Google Scholar]
- Jang DJ, Chung KR, Yang HJ, Kim KS, Kwon DY. Discussion on the origin of kimchi, representative of Korean unique fermented vegetables. Journal of Ethnic Foods. 2015;2:126–136. doi: 10.1016/j.jef.2015.08.005. [DOI] [Google Scholar]
- Jang JY, Lee ME, Lee HW, Lee JH, Park HW, Choi HJ, Pyun YR, Kim TW. Extending the shelf life of kimchi with Lactococcus lactis strain as a starter culture. Food Sci Biotechnol. 2015;24:1049–1053. doi: 10.1007/s10068-015-0134-8. [DOI] [Google Scholar]
- Jeong SH, Lee HJ, Jung JY, Lee SH, Seo HY, Park WS, Jeon CO. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. Int J Food Microbiol. 2013;160:252–259. doi: 10.1016/j.ijfoodmicro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Ji Y, Kim H, Park H, Lee J, Lee H, Shin H, Kim B, Franz CMAP, Holzapfel WH. Functionality and safety of lactic bacterial strains from Korean kimchi. Food Control. 2013;31:467–473. doi: 10.1016/j.foodcont.2012.10.034. [DOI] [Google Scholar]
- Jung JY, Lee SH, Kim JM, Park MS, Bae JW, Hahn Y, Madsen EL, Jeon CO. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl Environ Microbiol. 2011;77:2264–2274. doi: 10.1128/AEM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Kim YH, Ann MY, Lee YK. Measurements of Salinity and Salt Content by Menu Types Served at Industry Foodservice Operations in Daegu. Korean J Community Nutr. 2012;17:637. doi: 10.5720/kjcn.2012.17.5.637. [DOI] [Google Scholar]
- Kim M, Chun J. Bacterial community structure in kimchi, a Korean fermented vegetable food, as revealed by 16S rRNA gene analysis. Int J Food Microbiol. 2005;103:91–96. doi: 10.1016/j.ijfoodmicro.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Koo OK, Lee SJ, Chung KR, Jang DJ, Yang HJ, Kwon DY. Korean traditional fermented fish products: jeotgal. Journal of Ethnic Foods. 2016;3:107–116. doi: 10.1016/j.jef.2016.06.004. [DOI] [Google Scholar]
- Lee H, Yoon H, Ji Y, Kim H, Park H, Lee J, Shin H, Holzapfel W. Functional properties of Lactobacillus strains isolated from kimchi. Int J Food Microbiol. 2011;145:155–161. doi: 10.1016/j.ijfoodmicro.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo GY, Lee JW, Oh YJ, Park JA, Park YH, Pyun YR, Ahn JS. Analysis of kimchi microflora using denaturing gradient gel electrophoresis. Int J Food Microbiol. 2005;102:143–150. doi: 10.1016/j.ijfoodmicro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lee JS. Weissella koreensis sp nov., isolated from kimchi. International Journal of Systematic and Evolutionary Microbiology. 2002;52:1257–1261. doi: 10.1099/ijs.0.02074-0. [DOI] [PubMed] [Google Scholar]
- Lee M, Song JH, Jung MY, Lee SH, Chang JY. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 2017;66:173–183. doi: 10.1016/j.fm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jung JY, Jeon CO. Source Tracking and Succession of Kimchi Lactic Acid Bacteria during Fermentation. J Food Sci. 2015;80:M1871–7. doi: 10.1111/1750-3841.12948. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A, Wadström T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol. 2006;7:73–89. [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Sagane Y, Koizumi R, Nakazawa Y, Yamazaki M, Watanabe T, Takano K, Sato H. Data on the chemical properties of commercial fish sauce products. Data in Brief. 2017;15:658–664. doi: 10.1016/j.dib.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Yanagida F, Uchimura T, Tsuji M, Ogino S, Shinohara T, Yokotsuka K. Analysis of Lactic Acid Bacterial Flora during Miso Fermentation. FSTR. 2003;9:17–24. doi: 10.3136/fstr.9.17. [DOI] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnitz C, Beezhold B, DiMatteo J. Investigation of lifestyle choices of individuals following a vegan diet for health and ethical reasons. Appetite. 2015;90:31–36. doi: 10.1016/j.appet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Settanni L, Corsetti A. Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol. 2008;121:123–138. doi: 10.1016/j.ijfoodmicro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, Gonzalez A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R Earth Microbiome Project Consortium. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017;104:11436–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin W, Humblot C, Guyot JP. Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Appl Environ Microbiol. 2011;77:8722–8734. doi: 10.1128/AEM.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Caporaso JG, Fuhrman JA, Apprill A, Knight R. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems. 2016;1:e00009–15. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Miyasaka M, Koizumi A, Ikeda M. Regional difference in sodium chloride content in home-made and store-bought preparations of miso paste. The Tohoku Journal of Experimental Medicine. 1982;137:305–313. doi: 10.1620/tjem.137.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Tabular comparison of the predominant LAB across all sample types. Predominant LAB genera include Tetragenococcus, Leuconostoc, Lactobacillus, and Weissella, expressed as a percentage of total OTUs.
S2. Simplified version of Fig. 1B PCoA depicting weighted-UniFrac distance of kimchi fermenting samples along with final product, fish sauce, and miso samples.
S3. Relative abundance (%) of bacterial genera in individual kimchi, ingredient, and environment sample. Relative abundances are from un-averaged triplicate samples.