Metastasis is the most common neoplasm in the adult liver in the United States. The liver is a principle metastatic site for GI malignancies.1 The most common primary sites for metastatic lesions to the liver are malignancies of the colon, stomach, pancreas, breast, and lung. Multiple liver metastases are common and often vary in size.
The pathologic appearance of metastatic deposits in the liver closely resembles the primary tumor, including the degree of vascularity. Most metastases are hypovascular, but some malignancies characteristically have hypervascular metastases.
Large metastases can outgrow their blood supply, leading to central necrosis, which appears hypoechoic to anechoic on US. Benign hepatic lesions are common2 and may be difficult to distinguish from metastatic lesions. This differentiation is important because it may significantly change a patient’s stage and treatment options.
Transabdominal US, CT scan, and magnetic resonance imaging (MRI) are the diagnostic tests of choice for the detection of hepatic lesions (Figs. 1 and 2).3 The detection of lesions less than 1 cm remains challenging.4 EUS is an important tool in the staging of esophageal, gastric, and pancreatic malignancy5 and is complementary to CT and MRI in the detection and sampling of metastatic lesions. EUS-FNA detected distant metastases in 5% to 20% of cases of pancreaticobiliary and upper-GI cancers, thus altering the treatment plan (Figs. 3 and 4).6, 7 EUS-FNA can be performed for pancreas and liver lesions along with lymphadenopathy at the same setting.8
Figure 1.
CT scan showing 2 well-demarcated hepatic cysts (green arrows).
Figure 2.
CT of the abdomen showing multiple low-attenuating metastatic lesions in both right and left hepatic lobes.
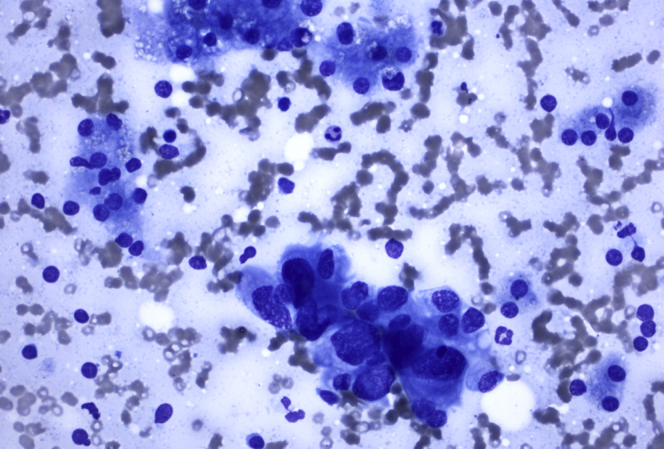
Figure 3.
FNA sample from liver lesion shows single and loose cohesive clusters of tumor cells with moderate amount of vacuolated cytoplasm, pleomorphic nuclei with irregular nuclear contours, and small nucleolus, characteristic of adenocarcinoma. A few small clusters of benign hepatocytes are present. Diff-Quik stain, orig. mag. × 400.
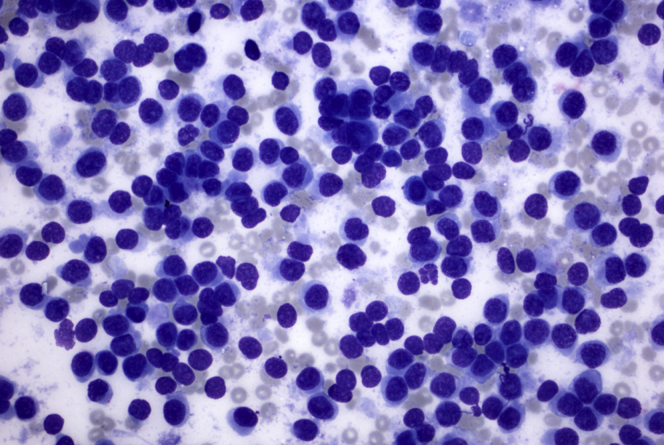
Figure 4.
FNA sample from pancreas mass showing loose cohesive clusters of tumor cells with moderate amount of vacuolated cytoplasm and pleomorphic nuclei with irregular nuclear contours and small nucleolus, consistent with adenocarcinoma. Single tumor cells are present. Diff-Quik stain, orig. mag. × 200.
Detailed visualization of various benign and malignant lesions can be achieved with dynamic transgastric and transduodenal imaging (Figure 5, Figure 6, Figure 7); however, visualization of the far right lobe of the liver, specifically segments 6 and 7, is limited. Dewitt et al9 evaluated the clinical impact of EUS-FNA of benign and malignant solid liver lesions and identified 82% to 94% sensitivity for the detection of malignant lesions. Anand et al10 reported EUS to be an effective method of diagnosing hepatobiliary malignancy, with sensitivity and specificity of 94% and 100%, respectively.11
Figure 5.
Endoscopic view of gastric pouch and anastomosis after gastric bypass surgery.
Figure 6.
CT scan of abdomen, coronal view, showing multiple hepatic metastases and small gastric pouch (green arrow).
Figure 7.
CT scan of abdomen showing a heterogenous mass occupying almost the entire right lobe of the liver.
Key learning points and endosonographic techniques
-
•
Hepatic lesions may be subtle and can be diagnosed endosonographically with repeated back-and-forth scanning through the liver by torqueing the EUS probe.
-
•
Recognition of a disrupted pattern of the normal liver parenchyma, vessels, and bile ducts can identify hepatic lesions.
-
•
Hepatic metastases may be hypoechoic or hyperechoic rounded structures.
-
•
When performing FNA or fine-needle biopsy (FNB), it is helpful to target lesions close to the EUS probe to minimize the amount of liver parenchyma traversed. The trajectory of the needle for distant lesions typically cannot be modified once the needle has been passed into the liver.
-
•
If possible, avoid targeting subcapsular lesions because of an increased risk of bleeding.
-
•
Hepatic metastases are typically very cellular. FNA with 25-gauge needles safely and effectively diagnoses malignancy. The high cellularity of hepatic metastases may provide a cell block with dedicated FNA or FNB sampling (Figs. 8 and 9).
Figure 8.
FNA sample from liver mass showing single and cohesive clusters of tumor cells in a background of necrosis. The tumor cells have dense cytoplasm, high nuclear-to-cytoplasmic ratios, and hyperchromatic nuclei with irregular nuclear contours, consistent with neuroendocrine tumor. Occasional mitoses are seen. Diff-Quik stain, orig. mag. × 200.
Figure 9.
FNA sample from liver showing single and dyscohesive clusters of tumor cells in a bloody background. The tumor cells are small and uniform, and have high nuclear-to-cytoplasmic ratios and eccentrically located oval nuclei with inconspicuous nucleolus, consistent with squamous cell carcinoma. Diff-Quik stain, orig. mag. × 400.
In the accompanying video (Video 1, available online at www.VideoGIE.org), we present several EUS cases demonstrating benign and malignant lesions within the liver.
Conclusion
EUS is an important tool for the evaluation of benign and malignant hepatic lesions.
Disclosure
All authors disclosed no financial relationships relevant to this publication.
Footnotes
Written transcript of the video audio is available online at www.VideoGIE.org.
Supplementary data
EUS evaluation of benign and malignant liver lesions, demonstrating EUS techniques for identification and sampling. The lesions here include liver cyst, hemangioma, portal vein thrombosis, cirrhosis, hepatocellular carcinoma, pancreatic adenocarcinoma, gallbladder mass, neuroendocrine tumor, and metastatic squamous cell carcinoma.
References
- 1.Sheth K.R., Clary B.M. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg. 2005;18:215–223. doi: 10.1055/s-2005-916282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliva M.R., Saini S. Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging. 2004;4:S42–S46. doi: 10.1102/1470-7330.2004.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martie A., Sporea I., Popescu A. Contrast enhanced ultrasound for the characterization of hepatocellular carcinoma. Med Ultrason. 2011;13:108–113. [PubMed] [Google Scholar]
- 4.Goletti O., Chiarugi M., Buccianti P. Subcutaneous implantation of liver metastasis after fine needle biopsy. Eur J Surg Oncol. 1992;18:636–637. [PubMed] [Google Scholar]
- 5.Parekh P.J., Majithia R., Diehl D.L. Endoscopic ultrasound-guided liver biopsy. Endosc Ultrasound. 2015;4:85–91. doi: 10.4103/2303-9027.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenssen C., Siebert C., Gottschalk U. The role of endoscopic ultrasound in m-staging of gastrointestinal and pancreaticobiliary cancer. Vid J Encyclo GI Endosc. 2013;1:105–109. [Google Scholar]
- 7.Agarwal B., Gogia S., Eloubeidi M.A. Malignant mediastinal lymphadenopathy detected by staging EUS in patients with pancreaticobiliary cancer. Gastrointest Endosc. 2005;61:849–853. doi: 10.1016/s0016-5107(05)00318-4. [DOI] [PubMed] [Google Scholar]
- 8.Oh D., Seo D.W., Hong S.M. Endoscopic ultrasound-guided fine-needle aspiration can target right liver mass. Endosc Ultrasound. 2017;6:109–115. doi: 10.4103/2303-9027.204813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt J., LeBlanc J., McHenry L. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976–1981. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 10.Anand D., Barroeta J.E., Gupta P.K. Endoscopic ultrasound guided fine needle aspiration of non-pancreatic lesions: an institutional experience. J Clin Pathol. 2007;60:1254–1262. doi: 10.1136/jcp.2006.045955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koduru P., Suzuki R., Lakhtakia S. Role of endoscopic ultrasound in diagnosis and management of hepatocellular carcinoma. J Hepatocell Carcinoma. 2015;2:143–149. doi: 10.2147/JHC.S60868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EUS evaluation of benign and malignant liver lesions, demonstrating EUS techniques for identification and sampling. The lesions here include liver cyst, hemangioma, portal vein thrombosis, cirrhosis, hepatocellular carcinoma, pancreatic adenocarcinoma, gallbladder mass, neuroendocrine tumor, and metastatic squamous cell carcinoma.