Abstract
We report a rare case of drug-induced intestinal lung disease (ILD) secondary to adalimumab, a tumour necrosis factor alpha-receptor blocker. A 52-year-old smoker with ankylosing spondylitis, treated with adalimumab, presented with progressive breathlessness. A high resolution CT chest demonstrated predominantly upper-zone patchy ground glass changes and small bilateral pleural effusions. Bronchoscopy and bronchoalveolar lavage showed no evidence of infection or malignant cells and an echocardiogram was normal. The working diagnosis was that of possible adalimumab-induced ILD. Adalimumab was subsequently stopped. The patient’s breathlessness and cough improved on cessation of the drug. A further CT chest several months later showed resolution of the ground glass changes. Adalimumab-induced ILD is rare. We review the literature surrounding this and discuss the diagnostic challenges. This case highlights the importance of considering the possibility of drug-induced lung disease in patients taking adalimumab.
Keywords: drugs: respiratory system, interstitial lung disease, biological agents
Background
Adalimumab is increasingly being used in clinical practice to treat many rheumatological conditions. However, clinicians should be aware of the possibility of adalimumab-induced intestinal lung disease (ILD), as if left untreated, can have significant impact on quality of life and is associated with high mortality rates.1 Adalimumab-induced ILD should be considered as a possible cause of progressive breathlessness in this patient group, as it is potentially reversible.
Case presentation
Patients with rheumatological diseases such as rheumatoid arthritis, ankylosing spondylitis and many connective tissue disorders are at risk of developing ILD as a potential extra-articular manifestation of the underlying disease process. They are frequently treated with drugs that may themselves cause ILD and predispose to pulmonary infections that may mimic ILD.1 2 When these patients present with respiratory symptoms, making the correct diagnosis can be difficult. CT chest findings may be non-specific. The possibility of drug-induced lung disease should be considered in order to avoid potentially fatal consequences.3 Biologic agents are increasingly being used in clinical practice to manage inflammatory conditions, including rheumatoid arthritis and Crohn’s disease.1 The direct causal relationship between biologic therapy and ILD remains a topic of ongoing research and discussion. We describe a rare case of adalimumab-induced lung disease in a patient with ankylosing spondylitis.
A 52-year-old man presented to the emergency department after having suffered multiple falls and worsening breathlessness that had been gradually worsening over a period of a few months. He had a background of ankylosing spondylitis and had recently been diagnosed with early Lewy-Body dementia. He was a smoker. His medication included adalimumab, which he had been taking for his ankylosing spondylitis since 2010, morphine, gabapentin, omeprazole, nortriptyline and domperidone. The patient had previously taken methotrexate 2 years prior to starting adalimumab for 6 months and leflunomide for 2 months following cessation of methotrexate; however, these were stopped due to intolerance secondary to gastrointestinal disturbances.
On examination, he was found to have bibasal crepitations on auscultation of his chest with tenderness over his right humerus. He was noted to have oxygen saturation levels of 94% on room air, with a respiratory rate of 28 breaths/min. He had a temperature of 36.9°C. Blood tests on admission revealed a white cell count of 7.5×109/L, neutrophil count of 5.09×109/L, eosinophil count of 0.35×109/L, normal renal function and a C-reactive protein of 91 mg/L. X-rays of his humerus revealed a right humeral head fracture as a consequence of his falls.
Investigations
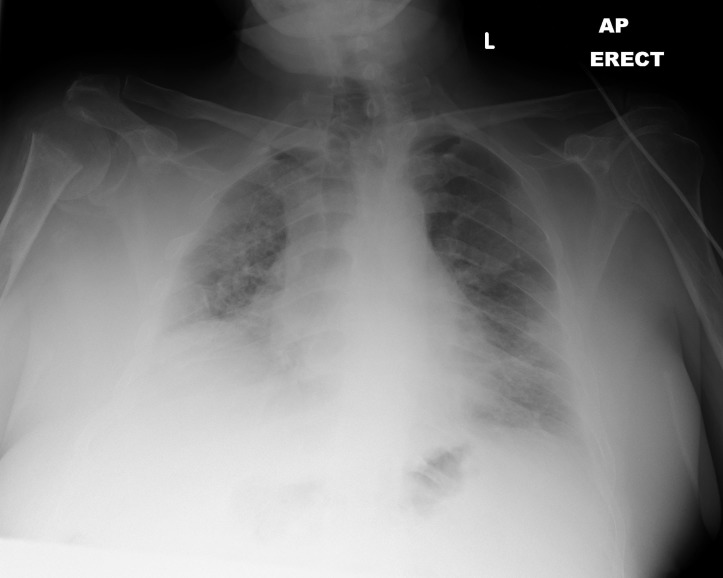
A chest X-ray showed patchy bilateral infiltrates with a chronically raised right hemidiaphragm (figure 1).
Figure 1.
Chest X-ray on admission showing bilateral patchy infiltrates and chronic raised right hemidiaphragm.
A high resolution CT (HRCT) chest showed extensive predominantly upper-zone ground glass opacification and consolidation. It was noted that the patient had an abnormal CT 7 months previously that showed some less prominent areas of ground glass and consolidation in both lungs suggesting a progressive subacute pathology (figures 2 and 3).
Figure 2.
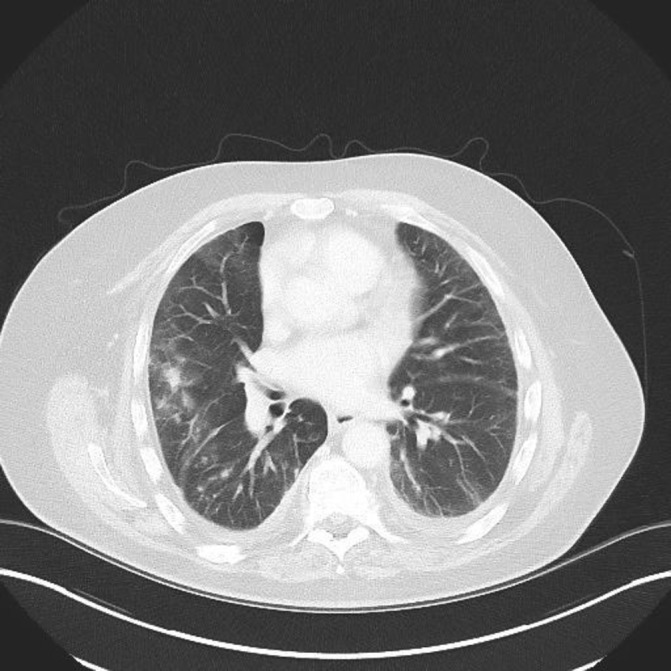
High resolution CT (March 2016) shows non-specific patchy changes bilaterally.
Figure 3.
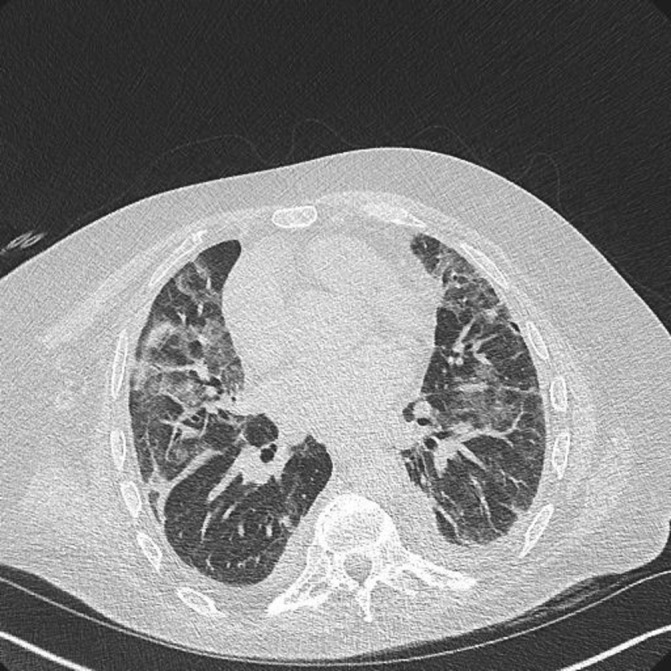
High resolution CT scan (October 2016) shows extensive predominantly upper-zone bilateral ground glass changes and small bilateral pleural effusions.
Differential diagnosis
The patient was reviewed by a respiratory consultant who suggested differential diagnoses of atypical infection (due to immunosuppression with adalumimab), ILD as a possible extra-articular manifestation of the underlying ankylosing spondylitis, ILD due to adalumimab and heart failure.
The patient was further investigated with an echocardiogram which showed normal left ventricular systolic function. Lung function tests at the time showed a restrictive lung defect, with a forced expiratory volume of 2.75 L (56% predicted), forced vital capacity of 2 L (51% predicted). An autoimmune profile was also performed, which showed a positive antinuclear antibody and weakly positive ribonucleoprotein.
The patient commenced a course of intravenous Co-amoxiclav and a bronchoscopy was arranged. Bronchial washings from the right upper lobe showed no evidence of bacterial or viral infection. No acid fast bacilli were seen and culture for mycobacterium tuberculous complex was negative. Candida species was grown from the bronchial washings but this was not thought to be clinically significant. Cytological analysis of the bronchial washings did not reveal any abnormal/malignant cells. A QuantiFERON Gold test was also negative.
A radiologist with a specialist interest in ILD was asked to review the CT scans. The opinion given was that the evolving non-specific ground glass changes seen were compatible with diffuse alveolar damage which could be due to adalimumab and that adalimumab toxicity should be high on the differential diagnosis list.
ILD is a rare severe adverse event and has been noted to mostly occur within the first 20 weeks after initiation of therapy.4 No definitive causative relationship can be drawn from case reports and the diagnosis of drug-induced ILD is based on exclusion of other causes.
Treatment
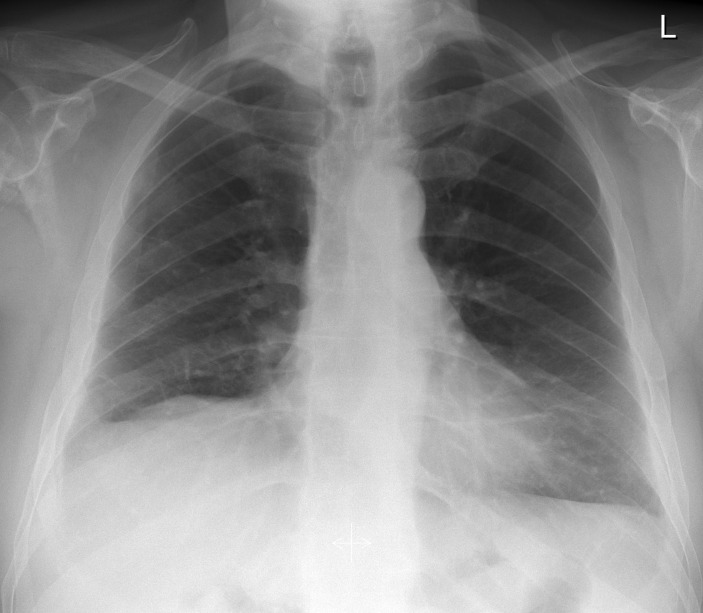
A working diagnosis of adalimumab-induced ILD was made and the drug was stopped. The patient was followed up in respiratory clinic on discharge. Two months after discharge, he denied any respiratory symptoms and subsequent chest X-rays and HRCT chest scans showed resolution of the ground glass changes (figures 4 and 5).
Figure 4.
Chest X-ray 2 months after stopping adalimumab showing resolution of infiltrative changes.
Figure 5.
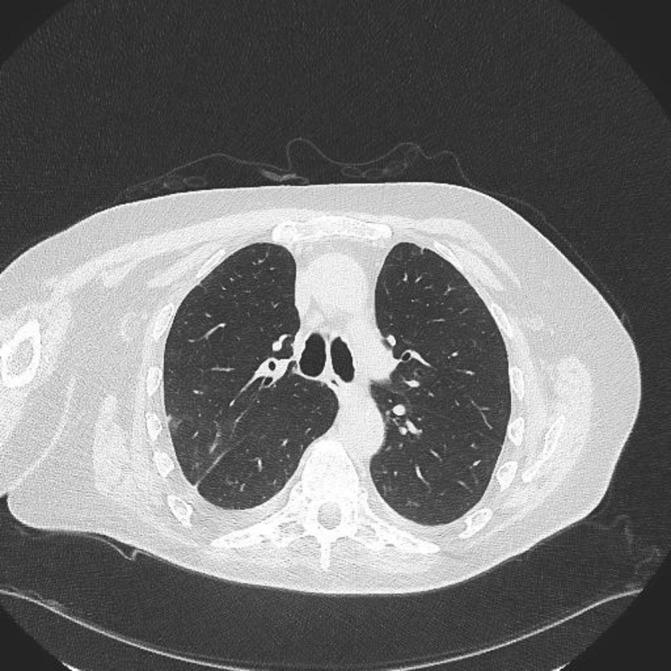
High resolution CT (January 2017) shows significant improvement in ground glass changes after cessation of adalimumab.
Outcome and follow-up
The patient has now stopped adalimumab and has complete resolution of his respiratory symptoms. The patient was followed up in respiratory outpatient clinic 6 months after discharge from hospital and it is clear that his quality of life has significantly improved, as he is no longer breathless.
Discussion
Tumour necrosis factor alpha (TNF-α) is a cytokine that plays a central role in establishing and maintaining the inflammatory response against infections. TNF antagonists are widely used in clinical practice in the management of inflammatory conditions. TNF antagonists are well recognised for causing lung toxicity, including bronchiolitis obliterans, pneumonitis, as well as worsening pre-existing ILD. This is thought to be due to a combination of pathophysiological factors, including blockade of macrophage and cytokine recruitment, thus leading to increased risk of infection and by triggering an inflammatory cascade leading to proliferation of pneumocytes, leading to fibrosis.4–6 Adalimumab is a recombinant human monoclonal antibody that blocks TNFα and is one of the most recent drugs in the anti-TNF class. Golimumab is also another novel anti-TNF agent; however, it is not currently licensed for use in the UK. Golimumab has a similar profile to adalimumab and has been noted to cause lung toxicity, the mechanism of which remains unclear.4 7
The prevalence of interstitial pneumonia among patients treated with anti-TNF agents ranges from 0.5% to 3%.8 We believe our patient had adalimumab-induced ILD because there was a subacute presentation with compatible radiological findings, other explanations such as infection and heart failure had been excluded, and there was a dramatic clinical and radiological improvement on ceasing the drug in the absence of another intervention that could explain the improvement. Only a few cases of adalimumab-induced ILD have been reported globally. In 2013, a case of a 78-year-old man with rheumatoid arthritis in Brazil who developed an acute lung injury immediately after an adalimumab infusion was reported. This study was the first reported case of acute ILD secondary to adalimumab.1 Another case report in 2015 describes a patient with Crohn’s disease who developed adalimumab-induced interstitial pneumonia.9 10
Disease-modifying antirheumatic drugs (DMARDS) are recognised for causing pulmonary toxicity, in particular, methotrexate.5 6 It has been previously suggested that the pathophysiology underlying methotrexate-induced lung disease is due to scattered expansion of the interstitium by mononuclear inflammatory cells, mild interstitial fibrosis and reactive hyperplastic type II pneumocytes.5 6
It is important to consider tuberculosis (TB) as a potential differential diagnosis in patients with respiratory symptoms on anti-TNF therapy, as this is also a recognised complication associated with this class of drug. TNF-α blockade will result in the interruption of TNF receptor-mediated functions, which comprises cell activation and proliferation, production of cytokines and the formation of granulomas. In the host immune response, the mycobacteria arrive in the alveoli and are engulfed by alveolar macrophages. This releases TNF-α and other cytokines to attract and activate CD4+, CD8+ cells and lymphocytes. These lymphocytes strengthen T cell adhesion and antigen presentation, which results in the proliferation and recruitment of more T and B cells. Thus, anti-TNF-α therapy disturbs the physiological TNF-α mediated immunoinflammatory responses and may cause TB reactivation or dissemination.11 12
The mechanism of lung injury due to adalimumab remains unclear; however, it is likely that the relationship between adalimumab and the development of ILD is underpinned by an inflammatory-mediated pathway. As there is no single definitive test, the diagnosis is based on clinical suspicion and exclusion of other potential causes such as infection and improvement on discontinuation of the drug in the absence of another explanation to account for the improvement.
Learning points.
Clinicians should be aware of the possibility of adalimumab-induced ILD, as if left untreated, can have significant impact on patient’s quality of life.
Adalimumab can potentially cause subacute ILD and if the drug is stopped, symptoms gradually resolve. (There is evidence of clinical and radiological improvement on cessation of the drug.)
Patients should be warned of this potential side effect when being counselled for taking adalimumab.
Footnotes
Contributors: SA and QJ have been the sole contributors to this article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kohli R, Namek K. Adalimumab (Humira) induced acute lung injury. Am J Case Rep 2013;14:77–81. 10.12659/AJCR.889200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum 2011;41:256–64. 10.1016/j.semarthrit.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Bhargava S, Perlman DM, Allen TL, et al. Adalimumab induced pulmonary sarcoid reaction. Respir Med Case Rep 2013;10:53–5. 10.1016/j.rmcr.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 2000;20:1245–59. 10.1148/radiographics.20.5.g00se081245 [DOI] [PubMed] [Google Scholar]
- 5.Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum 2014;43:613–26. 10.1016/j.semarthrit.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Atzeni F, Boiardi L, Sallì S, et al. Lung involvement and drug-induced lung disease in patients with rheumatoid arthritis. Expert Rev Clin Immunol 2013;9:649–57. 10.1586/1744666X.2013.811173 [DOI] [PubMed] [Google Scholar]
- 7.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. 10.1002/art.24403 [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman C, Parrot A, Wislez M, et al. Spectrum of CD4 to CD8 T-cell ratios in lymphocytic alveolitis associated with methotrexate-induced pneumonitis. Am J Respir Crit Care Med 2001;164:1186–91. 10.1164/ajrccm.164.7.2010120 [DOI] [PubMed] [Google Scholar]
- 9.Momeni M, Taylor N, Tehrani M, et al. Cardiopulmonary manifestations of ankylosing spondylitis. Int J Rheumatol 2011;2011:1–6. 10.1155/2011/728471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casanova MJ, Chaparro M, Valenzuela C, et al. Adalimumab-induced interstitial pneumonia in a patient with Crohn’s disease. World J Gastroenterol 2015;21:2260–2. 10.3748/wjg.v21.i7.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Li F, Chen JW, et al. Risk of tuberculosis infection in anti-TNF-α biological therapy: from bench to bedside. J Microbiol Immunol Infect 2014;47:268–74. 10.1016/j.jmii.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Fan W, Yang G, et al. Risk of tuberculosis in patients treated with TNF-α antagonists: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]