Abstract
A 41-year-old man with no medical history presented with 2 weeks of nausea, vomiting, a new palpable abdominal mass, constipation and a 14kgweight loss. On admission, CT abdomen and pelvis demonstrated a 6.9×3.7 cm soft-tissue abdominal mass deep to and invading the lower anterior abdominal wall with tethering of the urinary bladder and potential involvement of the urachus. Subsequently, a biopsy demonstrated a low-grade spindle cell neoplasm compatible with inflammatory myofibroblastic tumour with immunostain positive for smooth muscle actin and desmin and negative for CD21, CD117, DOG-1, TKE-1, mdm2, CD34 and ALK. One week following admission, he underwent en bloc excision of the mass including abdominal wall (umbilicus, portions of rectus sheath and muscle), bladder dome, right colon and a segment of small bowel. Final pathology of the mass confirmed an inflammatory myofibroblastic tumour, and his postoperative course was uneventful.
Keywords: small intestine, surgery, gastrointestinal surgery, general surgery, surgical oncology
Background
Inflammatory myofibroblastic tumours (IMTs) are uncommon tumours composed of myofibroblastic mesenchymal spindle cells with an inflammatory infiltrate of plasma cells, lymphocytes and eosinophils which are seemingly benign, as they rarely develop local recurrence or metastatic disease.1 IMT has a predilection for children and young adults, with reported median age of 9 years (range: 3 months to 47 years).2 3 IMT predominantly affects the lungs, with only 5% of IMTs occurring in extrapulmonary locations.2 4 Most commonly, extrapulmonary lesions have been described in the abdomen, head/neck, upper respiratory tract, trunk, extremities and uterus.5 Therefore, IMT involving multiple intra-abdominal organs causing bowel obstruction is quite unusual and even more unusual when occurring in a middle-aged man.
Case presentation
A 41-year-old male smoker, with no significant medical or surgical history presented with 2 weeks of nausea, vomiting, constipation, 14 kg weight loss and a new palpable midline abdominal mass. He denied any urinary or pulmonary symptoms. He has a prominent family history of cancer with 13 paternal aunts/uncles with lung, skin and pancreatic cancer. On physical examination, his abdomen was soft, mildly distended and tender to palpation in the periumbilical region with a palpable mass superior to the umbilicus.
Investigations
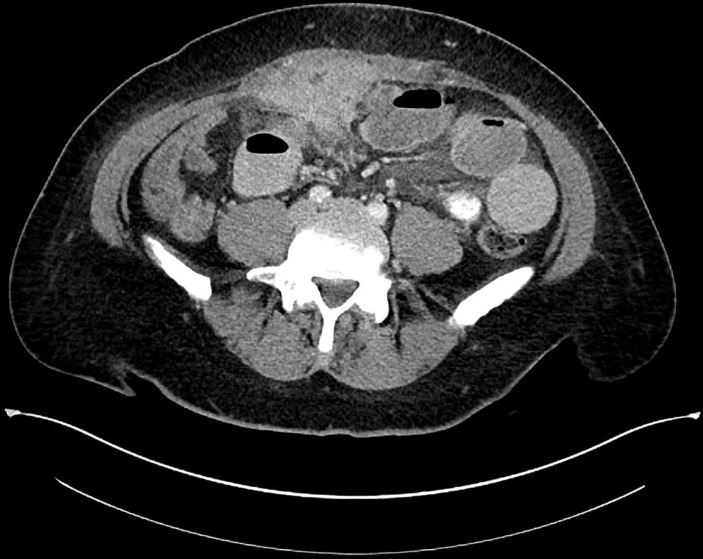
Given the palpable abdominal mass, a CT scan of the abdomen and pelvis with contrast was performed that was concerning for a small bowel obstruction and abdominal mass as the lead point. The 6.9×3.7 cm soft-tissue abdominal mass was noted to be deep to and invading the lower anterior abdominal wall with superoanterior tethering of the urinary bladder and potential involvement of the urachus (figure 1). Given the patient’s family history of lung cancer and current smoking, a CT scan of the chest with contrast was performed, which showed centrilobular emphysema and no evidence of intrathoracic primary or metastatic disease. A CT-guided core needle biopsy of the peritoneal mass was performed and demonstrated a low-grade spindle cell neoplasm compatible with IMT with immunostain positive for smooth muscle actin and desmin and negative for CD21, CD117, DOG-1, TKE-1, mdm2, CD34 and ALK.
Figure 1.
CT abdomen and pelvis with contrast of abdominal mass on admission.
Differential diagnosis
Prior to any work up of the patient and based on his presenting symptoms the differential diagnosis was quite broad including, but not limited to, malignancy, infectious, autoimmune, musculoskeletal and traumatic processes.
Following the CT scan of the abdomen and pelvis, there was a high index of suspicion for malignancy given the characteristics of the soft-tissue mass with no abdominopelvic lymphadenopathy noted. The differential diagnosis was then narrowed to different types of malignancy including sarcoma and urachal tumour. Given that the mass had possible urachal involvement and extension to the bladder dome urachal tumour was favoured, however given the patient’s lack of genitourinary symptoms, other malignancies such as colon adenocarcinoma and gastrointestinal stromal tumour were still considered.
Based on the results of the biopsy, there was a high index of suspicion for an IMT and a lower index of suspicion for urachal tumour as the results were not consistent with an adenocarcinoma. However, based on the known various patterns of IMT, other malignancies such as inflammatory malignant fibrous histiocytoma, leiomyosarcoma, inflammatory cell-rich gastrointestinal stromal tumours and solitary fibrous tumours were also considered. Following biopsy and immunostaining, a diagnosis of IMT was made.
Treatment
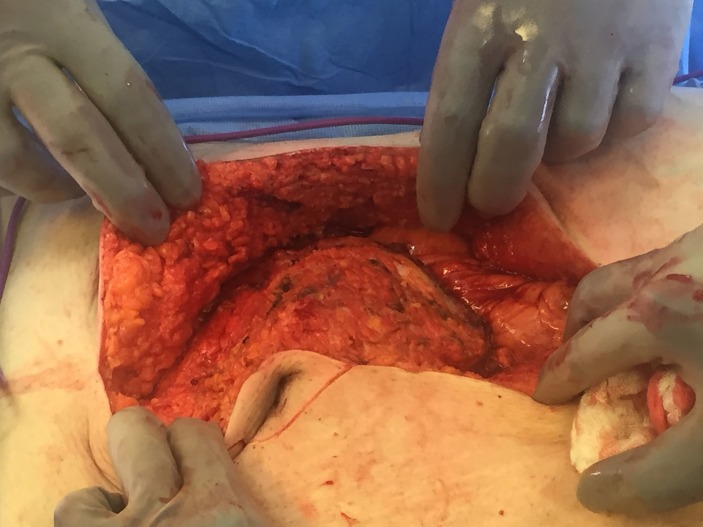
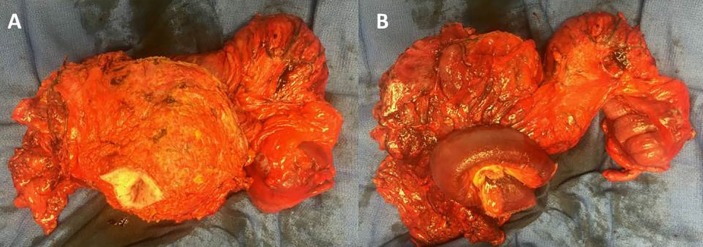
The patient underwent an exploratory laparotomy for excision of the abdominal wall mass with complex abdominal wall closure with plastic surgery. On entering the peritoneum (figure 2), a 10 cm mass in the abdominal wall which extended through the posterior rectus to the anterior rectus, with adherent right colon and small bowel, and inferior extension to the dome of the bladder was appreciated. The mass was excised (figure 3) with rectus sheath and muscle, the umbilicus, a segment of small bowel and right colon. Small bowel reconstruction was performed with primary anastomosis and colon with ileocolostomy. The bladder was repaired primarily. The abdominal wall was closed with bilateral muscle flap advancements using component separation, hernia repair with biological mesh and adjacent tissue transfer. Final pathology of the mass showed an IMT with central cavity abscess and negative margins.
Figure 2.
Abdominal mass on entering the peritoneum.
Figure 3.
(A) Anterior view of excised abdominal mass, (B) Posterior view of excised abdominal mass.
Outcome and follow-up
The patient had an uneventful postoperative course. His pain was managed with a hydromorphone patient-controlled analgesia pump from postoperative day 1–8, and he was then transitioned to oral pain medications. Following his operation he had four drains, three placed by the plastic surgery team for the abdominal wall closure and one placed by the surgical oncology surgery team near the site where the tumour was excised off the bladder. The intraperitoneal drain was removed on postoperative day 4 and the patient was discharged with the three abdominal wall drains. His Foley was kept in place until postoperative day 9 following a X-ray cystogram performed by urology demonstrating no bladder leak. His nasogastric tube was removed on postoperative day 7 when he began passing flatus and his diet was advanced to clear liquids. On postoperative day 8, he was advanced to a regular diet and he was discharged on postoperative day 9. He followed up with surgical oncology and plastic surgery 10 days after surgery at which time his drains were removed. Nine months after resection, he has no evidence of disease and is recovering well.
Discussion
The clinical presentation of IMT is related to the location of the lesion and mass effect. However, a constitutional syndrome consisting of fever, weight loss and malaise has been described in 15%–30% of patients.6 Patients with intra-abdominal tumours present with intermittent abdominal pain and abdominal distension, weight loss, malaise, anorexia and vomiting.7 Palpable abdominal mass as the primary presenting symptom is unusual. Other atypical presentations of intra-abdominal IMT reported include intestinal intussusception and appendicitis.7 8 When a palpable abdominal mass is present with unintended weight loss and other obstructive symptoms, IMT should be included on the differential diagnosis and a biopsy may be performed.
In addition to non-specific clinical symptoms, a variety of microscopic patterns are seen in IMT including a myxoid/vascular pattern, a compact spindle cell pattern and a hypocellular fibrous pattern, which makes it difficult to differentiate IMTs from other tumours such as inflammatory malignant fibrous histiocytoma, leiomyosarcoma, inflammatory cell-rich gastrointestinal stromal tumours and solitary fibrous tumours.9 However, immunohistochemical analysis helps to differentiate IMTs from these other tumours. IMT tumour cells characteristically stain positive for vimentin, smooth muscle actin, desmin and S100 and negative for CD117 and CD34.8 In this case, the immunostain profile on biopsy increased our index of suspicion for IMT over a urachal cancer, demonstrating the potential contribution of immunohistochemical analysis in the work up of IMT.
Current treatment of choice for IMT is complete surgical resection, which additionally allows for a proper diagnosis. With negative margins, there are excellent outcomes.10 Wang et al report that complete resection is curative in most cases while chemotherapy, radiotherapy, immunomodulation with corticosteroids and non-steroidal anti-inflammatory drugs have had inconsistent effects.11 These alternatives can be considered in cases where there is a risk of an incomplete resection with microscopically or grossly positive margins. Following complete resection, tumour recurrence is unusual (<10%). When recurrence does occur, it is predominantly in cases where incomplete resection was performed and chemotherapy and radiotherapy were not administered.12 Close follow-up with appropriate imaging is needed for those who undergo surgical resection to monitor for local recurrence.11 12 For those cases that are unresectable for reasons such as anatomic location or comorbidities, the treatment remains controversial, however, chemotherapy combined with radiation therapy should be considered.12 Another prognostic indicator of outcomes is ALK reactivity on immunostain, as in the case of this patient. Positive ALK reactivity has been found to be a favourable prognostic indicator in IMT, while negative ALK reactivity has been associated with increasing age and death from disease or distant metastasis.1
Several previous cases of intra-abdominal IMT have been reported, with varying presentation.4 13 14 Two unresectable intra-abdominal IMTs in adults were reported.13 These spontaneously regressed thus supporting the use of conservative therapy with intense follow-up for unresectable IMT.13 Another report describes a mesenteric IMT in a young adult who presented with abdominal pain, weight loss and paraumbilical palpable mass who was treated with complete surgical resection.14 An additional report describes an adult man with a mesenteric IMT who presented with abdominal pain and weakness and was treated with complete surgical resection without recurrence.4 All reports agree that the primary treatment for IMT is complete surgical resection and highlight the importance of close, long-term follow-up to detect local recurrence.4 13 14
Learning points.
Inflammatory myofibroblastic tumours can occur in adults and do not exclusively occur in the paediatric population.
Immunohistochemical analysis is crucial when differentiating inflammatory myofibroblastic tumours from other cancers on the differential diagnosis.
Inflammatory myofibroblastic tumours can be extrapulmonary and present as a new palpable abdominal mass.
The primary treatment for inflammatory myofibroblastic tumour is complete surgical resection including en bloc resection of other involved structures.
Patients with inflammatory myofibroblastic tumour should have long-term follow-up due to the risk for local recurrence.
Footnotes
Contributors: MED cared for the patient described in the case and provided the included photographs. MED, EWB and EC prepared the case report, analysed the literature, provided critical revision and review, and approved the final version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509520 10.1097/01.pas.0000213393.57322.c7 [DOI] [PubMed] [Google Scholar]
- 2.Karnak I, Senocak ME, Ciftci AO, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 2001;36:908–12. 10.1053/jpsu.2001.23970 [DOI] [PubMed] [Google Scholar]
- 3.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–72. 10.1097/00000478-199508000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Tao YL, Wang ZJ, Han JG, et al. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol 2012;18:7100–3. 10.3748/wjg.v18.i47.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koyuncuer A. Inflammatory myofibroblastic tumor of the small-bowel mesentery: A case report of nonspecific clinical presentation and a review of the literature. Int J Surg Case Rep 2014;5:1214–7. 10.1016/j.ijscr.2014.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol 2008;61:428–37. 10.1136/jcp.2007.049387 [DOI] [PubMed] [Google Scholar]
- 7.Oeconomopoulou A, de Verney Y, Kanavaki K, et al. Inflammatory myofibroblastic tumor of the small intestine mimicking acute appendicitis: a case report and review of the literature. J Med Case Rep 2016;10:100 10.1186/s13256-016-0880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appak YÇ, Sahin GE, Ayhan S, et al. Inflammatory myofibroblastic tumor of the colon with an unusual presentation of intestinal intussusception. European J Pediatr Surg Rep 2014;2:54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagae I, Hamasaki Y, Tsuchida A, et al. Primary omental-mesenteric myxoid hamartoma of the mesoappendix incidentally detected after abdominal trauma in a child: report of a case. Surg Today 2005;35:792–5. 10.1007/s00595-005-3006-7 [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Kim JS, Choi YS, et al. Treatment of inflammatory myofibroblastic tumor of the chest: the extent of resection. Ann Thorac Surg 2007;84:221–4. 10.1016/j.athoracsur.2007.03.037 [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Zhao X, Li K, et al. Analysis of clinical features and outcomes for inflammatory myofibroblastic tumors in China: 11 years of experience at a single center. Pediatr Surg Int 2016;32:239–43. 10.1007/s00383-015-3840-7 [DOI] [PubMed] [Google Scholar]
- 12.Kovach SJ, Fischer AC, Katzman PJ, et al. Inflammatory myofibroblastic tumors. J Surg Oncol 2006;94:385–91. 10.1002/jso.20516 [DOI] [PubMed] [Google Scholar]
- 13.Zhao JJ, Ling JQ, Fang Y, et al. Intra-abdominal inflammatory myofibroblastic tumor: spontaneous regression. World J Gastroenterol 2014;20:13625–31. 10.3748/wjg.v20.i37.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groenveld RL, Raber MH, Oosterhof-Berktas R, et al. Abdominal inflammatory myofibroblastic tumor. Case Rep Gastroenterol 2014;8:67–71. 10.1159/000360843 [DOI] [PMC free article] [PubMed] [Google Scholar]