Abstract
Neonatal cardiogenic shock most commonly occurs due to critical congenital heart disease, sepsis, metabolic disorder or arrhythmias. In particular, enterovirus infections are common in the neonatal period, and patients can present with fulminant myocarditis. Early recognition is imperative due to its high morbidity and mortality without prompt and aggressive treatment. We present the successful treatment of fulminant neonatal enteroviral myocarditis in a pair of monochorionic diamniotic twins with cardiopulmonary support, intravenous immunoglobulin and pocapavir, an enteroviral capsid inhibitor. The twins took an almost exact parallel hospital course, including day of extracorporeal membrane oxygenation (ECMO) cannulation, day of ECMO decannulation, improvement of cardiac function, discharge and status at follow-up. While it was difficult to assess the relative contribution of each intervention, our case shows promise in the use of pocapavir for treatment of severe enteroviral infections. Remarkably, both twins demonstrated remarkable recovery within 2 weeks, underscoring that early aggressive cardiopulmonary support, and potentially pocapavir, contributed to their recovery.
Keywords: heart failure, cardiovascular system, infections, paediatric intensive care
Background
Neonatal cardiogenic shock may present secondary to a variety of causes. Most commonly, it is due to a critical congenital heart disease, sepsis (viral or bacterial), metabolic disorder and arrhythmias.1 Early identification of this condition is paramount, and failure to recognise cardiogenic shock is associated with increased risk of morbidity and mortality.2–4 To that end, we report a case of fulminant neonatal enteroviral myocarditis in a pair of twins who presented in cardiogenic shock. Both infants survived after treatment with mechanical circulatory support, intravenous immunoglobulin (IVIG) and the antiviral pocapavir, with marked improvement in cardiac dysfunction.
Case presentation
Both patients were monochorionic diamniotic twins born at 37 weeks’ gestation via induced vaginal delivery after an otherwise uncomplicated pregnancy. Their mother was positive for Group B Streptococcus bacteria but received ampicillin prior to delivery. Both infants had an uneventful delivery course, normal Apgar scores and were discharged home on day 2 of life from the newborn nursery. Aside from routine postnatal care, the infants had early healthcare facility exposures to monitor hyperbilirubinaemia which resolved prior to presentation. An older, but otherwise healthy, 3-year-old sibling had a febrile seizure 2 weeks prior to the twins’ delivery. Otherwise, there was no other history of maternal infection or infectious exposure.
The parents reported that the twins appeared sleepier and less active since birth compared with their previous child which they attributed to gestational age. Although they ate well initially, taking up to 1–1.5 ounces every 2–3 hours, their intake decreased to ¾ ounce per feed over the course of a week with increasing lethargy. There were no other reported symptoms, including fever, rhinorrhea, cough, respiratory distress or cyanosis. On day 12 of life, the twins were evaluated by their primary care paediatrician who noted poor weight gain since birth and core temperature of 35°C on examination. Appropriately concerned, the primary care physician referred them to our emergency department for further management.
In the emergency department, the twins were found to be in shock with hypotension, tachycardia and poor perfusion (table 1).
Table 1.
The twins presented in shock with hypothermia, hypotension and tachycardia
| Temperature (°C) | Blood pressure (mm Hg) | Heart rate (bpm) | Respiratory rate (per min) | SpO2 (%) | |
| Twin A | 33.7 | 54/36 | 148 | 25 | 95 |
| Twin B | 32.4 | 53/34 | 173 | 30 | 99 |
They were initially resuscitated with multiple normal saline boluses (total 60 cc/kg each) and initiation of broad-spectrum antimicrobial coverage with cefotaxime, ampicillin and acyclovir. However, both infants remained persistently hypotensive and thus were started on an epinephrine infusion. Initial laboratory tests were significant for bandaemia, transaminitis, lactic acidaemia, elevated brain natriuretic peptide (BNP) (twin A: 3281 pg/mL; twin B: >6127 pg/mL), and nasopharyngeal multiplex positive for rhinovirus or enterovirus (table 2).
Table 2.
Initial laboratory tests were significant for bandaemia, transaminitis, lactic academia and elevated BNP
| WBC (x109/L) |
Neutrophil bands (%) | AST (U/L) | ALT (U/L) | Lactic acid (mmol/dL) | BNP (pg/mL) |
|
| Twin A | 10.8 | 16 | 274 | 370 | 2.7 | 3281 |
| Twin B | 14 | 13 | 311 | 432 | 3.1 | >6127 |
ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BNP, brain natriuretic peptide; WCC, white cell count.
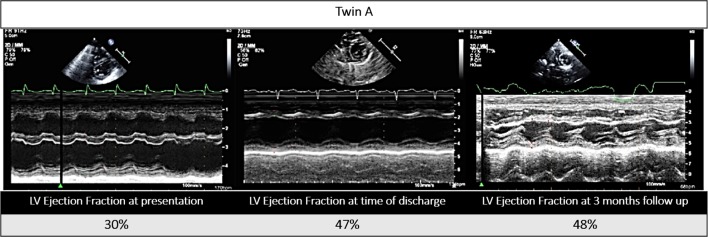
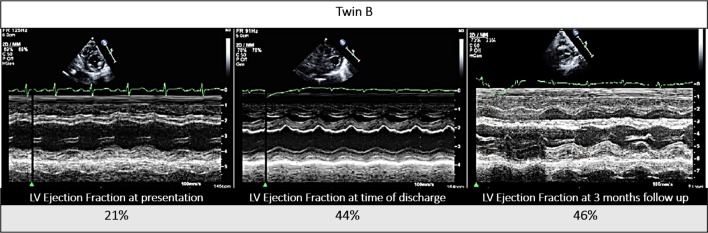
Echocardiogram showed moderate to severely depressed left ventricular systolic function in both infants (twin A: ejection fraction (EF) 30%; twin B: EF 21%; calculated by Simpson’s method) (figures 1 and 2).
Figure 1.
Twin A’s echocardiogram, parasternal short axis view (M-mode), at presentation, time of discharge and at 3-month follow-up. LV, left ventricular.
Figure 2.
Twin B’s echocardiogram, parasternal short axis view (M-mode), at presentation, time of discharge and at 3-month follow-up. LV, left ventricular.
The twins were admitted to our cardiac intensive unit for cardiogenic shock due to presumed enteroviral myocarditis. Both were placed on mechanical ventilatory support and multiple inotropes including milrinone, calcium and epinephrine infusions. Despite resuscitative efforts and escalation of medical support, their cardiopulmonary status remained marginal. Twin B required emergent cannulation for extracorporeal membrane oxygenation (ECMO) support due to declining clinical status. As for twin A, he developed an episode of wide complex tachycardia at a heart rate of 250 bpm (figure 3) for which adenosine (0.2 mg/kg/dose) was given. He subsequently developed complete heart block and received cardiopulmonary resuscitation (CPR) for 5 s. Soon thereafter, he had another episode of wide complex tachycardia at a similar heart rate. During this episode, he received a higher dose of adenosine (0.4 mg/kg/dose), after which the patient had another episode of complete heart block, significant bradycardia and pulse loss. He then received 14 min of CPR along with multiple rounds of epinephrine and calcium and was finally placed on ECMO.
Figure 3.
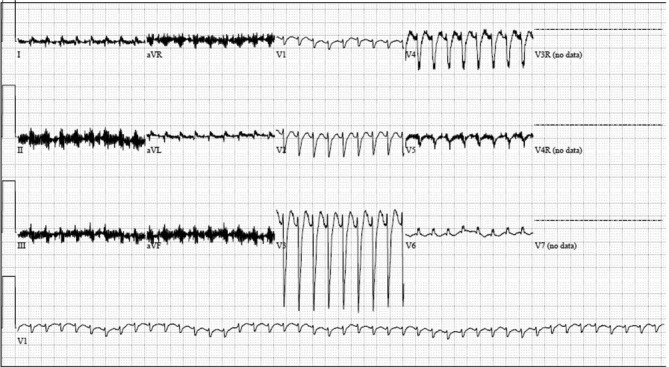
Twin A’s ECG showing wide complex tachycardia at a heart rate of 250 bpm, non-specific intraventricular conduction delay, non-specific ST and T wave abnormality, and low-voltage QRS complexes.
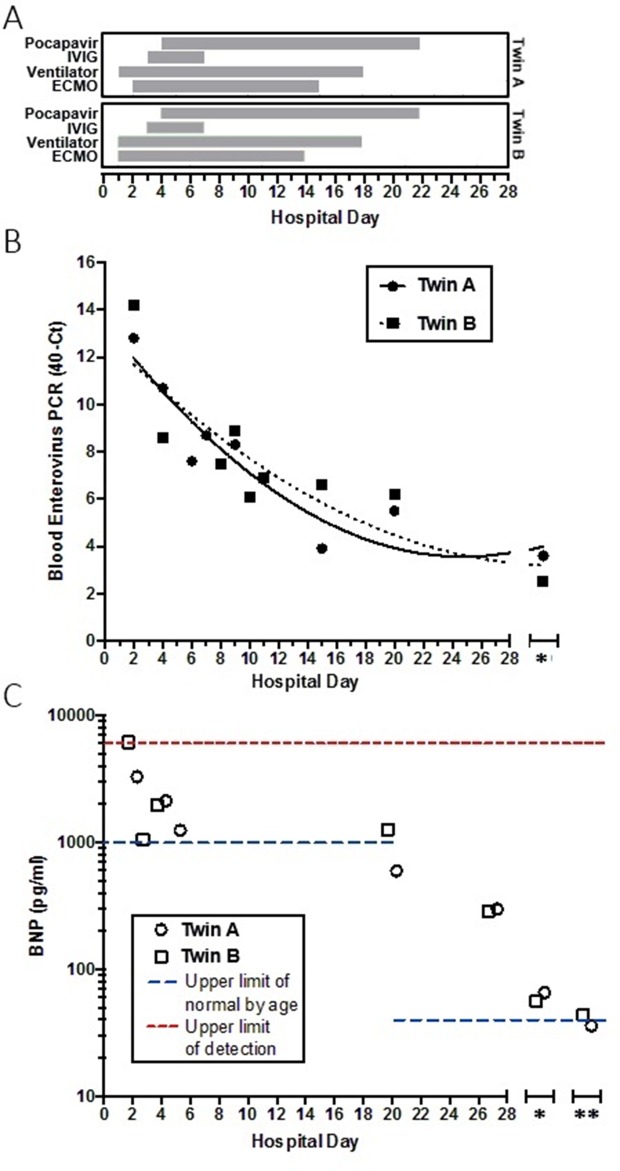
Blood enterovirus PCR returned positive on hospital day 2 for both twins. Nasopharyngeal swab, serum and stool samples from both patients then tested positive for coxsackie virus B5 at the Center for Disease Control (CDC) laboratory. Both patients completed a course of 4 doses of 0.5 g/kg/dose IVIG and 18 oral doses of 25 mg/kg/dose (62 mg/dose) of pocapavir, an enterovirus capsid inhibitor, under Emergency Investigational New Drug approval from the Food and Drug Administration. Pocapavir dosing was determined based on a previous adult study5 and a compassionate use study in paediatric patients (ViroDefense) showing tolerability and predicated activity of plasma levels achieved at the given dose. Dosing duration was continued for 18 days until no further decreases were observed in enterovirus (EV) polymerase chain reaction (PCR) levels. Duration was longer than an initially planned 14-day course as both patients continued to improve without any observable medication side effects. Subsequently, in vitro susceptibility testing of one viral isolate showed a half maximal effective concentration (EC50) >10 uM. After obtaining consent, both infants’ dried newborn blood spots from day 2 of life were tested and negative for enterovirus quantitative PCR using an experimental protocol at CDC.
Outcome and follow-up
Although the twins’ prognosis initially appeared poor, they slowly tolerated de-escalation of care with improvement of pulsatility and on minimal ECMO flows, after nearly a week and a half of support. On day 11 (twin A) and 12 (twin B) of ECMO support, echocardiograms with the cannulas clamped revealed normal biventricular function, after which both were successfully decannulated. Thereafter, the patients tolerated extubation and transition from milrinone to enalapril in the setting of mildly decreased left ventricular systolic function on repeat echocardiograms off mechanical support, although there was continued evidence of adequate end-organ tissue perfusion. Both twins received a metabolic cocktail including thiamine, levocarnitine, coenzyme Q10 after presentation until metabolic screening panels returned negative.
On the 27th hospital day, the twins were stable for discharge. Enterovirus blood PCR crossing thresholds were increasing which reflected lower viral levels, BNPs were trending down (twin A: 298 pg/mL; twin B: 286 pg/mL) (figure 4), and systolic functions on echocardiogram were slowly improving (twin A: EF 47%; twin B: EF 44%) (figures 1 and 2). Genetic cardiomyopathy panels were sent during their admission and later reported as negative. Both infants were discharged home with close follow-up with the heart failure team, on oral heart failure therapy consisting of enalapril optimised for weight. At their last follow-up 3 months after presentation, they were doing well, gaining weight appropriately and on 0.28 mg/kg/day of enalapril with improved systolic functions (twin A: EF 48%; twin B: EF 46%) (figures 1 and 2).
Figure 4.
Interventions, enterovirus crossing threshold and BNP levels during hospitalisation and follow-up. (A) Hospital course, showing day of initiation and duration of interventions (shaded boxes) for each twin indicated. (B) Blood enterovirus qPCR crossing threshold. Left axis represents the number of cycles from maximum number of cycles (40) on each qPCR run. Lines are non-linear fit, second order polynomial. (C) BNP levels, with assay upper limit of detection (red) and upper limit of normal levels by age (blue) indicated. Note log scale in (C). *, indicates 1-month follow up from discharge (B and C); **, indicates 2-month follow-up from discharge (C). BNP, brain natriuretic peptide; ECMO, extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin; qPCR, quantitative PCR.
Discussion
We report an unusual case of coxsackie myocarditis in monochorionic, diamniotic twins, with only few previous reports existing in the literature.6 7 Both our patients presented with cardiogenic shock which is not an uncommon complication of neonatal enteroviral sepsis.8–10
Although obtaining blood and cerebrospinal fluid studies are important to determining the aetiology of neonatal shock, it is important not to delay treatment for the sake of obtaining studies in the setting of haemodynamic instability.11–13 Neonates who present in shock within the first 2 weeks of life should be screened for critical congenital heart disease by echocardiogram given the potential serious clinical outcomes if the diagnosis is missed.14 15 In addition, neonates should be started on broad spectrum antibacterial and antiviral therapy until complete evaluation can rule out infection. As described in our case report, the twins were noted to have relatively decreased alertness and activity since birth which prompted additional concern about metabolic disorders and screening. Therefore, they were started on a metabolic cocktail (thiamine, levocarnitine, coenzyme Q10) which has been shown to be beneficial in patients with metabolic disorders.16 17 We include all of these treatments and evaluations in the stepwise management of these patients at our institution.
There are several interesting aspects in the clinical course of both twins. Both twins decompensated at the same timeframe with a similar clinical presentation. Although neonatal enteroviral myocarditis typically presents in the first week of life and rarely past 2 weeks, both infants presented with haemodynamic instability on day 12 of life. Both patients followed near-similar hospital course including day of ECMO cannulation, day of ECMO decannulation, improvement of cardiac function and discharge, as well as their status at follow-up. It is difficult to say whether prenatal exposure to a potentially ill sibling close to birth was the source of the infection. Negative testing of newborn blood spot for enterovirus at the CDC suggests postnatal acquisition, although the sensitivity of this experimental protocol is unknown.
Furthermore, it is difficult to assess the contribution of pocapavir in the twins’ outcome which initially was thought to be grim. Pocapavir is a capsid inhibitor that blocks virus uncoating and viral RNA release into cells.18 Case reports using pocapavir have described favourable outcomes in neonates with enteroviral sepsis.19 20 Also, the twins received IVIG 1 day prior to initiation of pocapavir. The routine use of IVIG in the treatment of myocarditis varies due to the often conflicting evidence of efficacy and high cost.21 Unfortunately, the relative contribution of each intervention, in addition to physiological immune clearance and aggressive supportive care, is unclear. Given the poor prognosis of neonatal enteroviral myocarditis,13 22 our cases suggest that pocapavir may be associated with a favourable outcome in enteroviral myocarditis and was well tolerated in our patients. This is the first case report to describe this outcome and treatment regimen in neonatal twins who presented with fulminant enteroviral myocarditis.
Learning points.
Neonatal enteroviral myocarditis may have a fulminant course and poor prognosis in some patients.
Rapid ventricular dysfunction can lead to sudden haemodynamic instability, cardiovascular collapse and significant morbidity and mortality without expedient mechanical circulatory support.
In our case report, we describe the use of pocapavir in twins who presented in fulminant enteroviral myocarditis who took an almost exact parallel hospital course. While pocapavir, an enteroviral capsid inhibitor, shows some promise for severe enteroviral infections, further experience is needed to determine its full utility.
Both twins demonstrated remarkable recovery within 2 weeks, underscoring that early aggressive cardiopulmonary support, and potentially pocapavir, contributed to their recovery.
Acknowledgments
We thank M Collett and J Hincks at ViroDefense for guidance on pocapavir treatment. We thank WA Nix and MS Oberste at the CDC/OID/NCIRD for assistance with virological testing. We thank the patients and their family for consent to publish these cases.
Footnotes
Contributors: SMA, HSK, AO, AOJ, Ahmed Said and Kathleen Simpson were involved in the conception of this manuscript, review of the first draft, revisions and formation of the final draft of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None decalred.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lees MH, King DH. Cardiogenic shock in the neonate. Pediatr Rev 1988;9:258–66. 10.1542/pir.9-8-258 [DOI] [PubMed] [Google Scholar]
- 2.Ramachandra G, Shields L, Brown K, et al. The challenges of prompt identification and resuscitation in children with acute fulminant myocarditis: case series and review of the literature. J Paediatr Child Health 2010;46:579–82. 10.1111/j.1440-1754.2010.01799.x [DOI] [PubMed] [Google Scholar]
- 3.Teele SA, Allan CK, Laussen PC, et al. Management and outcomes in pediatric patients presenting with acute fulminant myocarditis. J Pediatr 2011;158:638–43. 10.1016/j.jpeds.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 4.Amabile N, Fraisse A, Bouvenot J, et al. Outcome of acute fulminant myocarditis in children. Heart 2006;92:1269–73. 10.1136/hrt.2005.078402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collett MS, Hincks JR, Benschop K, et al. Antiviral Activity of Pocapavir in a Randomized, Blinded, Placebo-Controlled Human Oral Poliovirus Vaccine Challenge Model. J Infect Dis 2017;215:335–43. 10.1093/infdis/jiw542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissel SJ, Winkler CC, DelTondo J, et al. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathology 2014;34:429–37. 10.1111/neup.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozdemir O, Hizli S, Abaci A. Myocarditis Diagnosed in Twins. Iranian Journal of Pediatrics 2009;19:413–6. [Google Scholar]
- 8.Inwald D, Franklin O, Cubitt D, et al. Enterovirus myocarditis as a cause of neonatal collapse. Arch Dis Child Fetal Neonatal Ed 2004;89:F461–F462. 10.1136/adc.2003.034439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freund MW, Kleinveld G, Krediet TG, et al. Prognosis for neonates with enterovirus myocarditis. Arch Dis Child Fetal Neonatal Ed 2010;95:F206–F212. 10.1136/adc.2009.165183 [DOI] [PubMed] [Google Scholar]
- 10.Woodruff JF. Viral myocarditis. A review. Am J Pathol 1980;101:425. [PMC free article] [PubMed] [Google Scholar]
- 11.Sawyer MH. Enterovirus infections: diagnosis and treatment. Pediatr Infect Dis J 1999;18:1033–40. 10.1097/00006454-199912000-00002 [DOI] [PubMed] [Google Scholar]
- 12.Dancea AB. Myocarditis in infants and children: a review for the paediatrician. Paediatr Child Health 2001;6:543–5. 10.1093/pch/6.8.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebruegge M, Curtis N. Enterovirus infections in neonates-seminars: fetal and neonatal medicine: Elsevier, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009;37:666–88. 10.1097/CCM.0b013e31819323c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JG, Smith SL. Shock in the critically ill neonate. J Perinat Neonatal Nurs 2009;23:346–54. 10.1097/JPN.0b013e3181ba5842 [DOI] [PubMed] [Google Scholar]
- 16.Santa KM. Treatment options for mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome. Pharmacotherapy 2010;30:1179–96. 10.1592/phco.30.11.1179 [DOI] [PubMed] [Google Scholar]
- 17.Parikh S, Saneto R, Falk MJ, et al. A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol 2009;11:414–30. 10.1007/s11940-009-0046-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberste MS, Moore D, Anderson B, et al. In vitro antiviral activity of V-073 against polioviruses. Antimicrob Agents Chemother 2009;53:4501–3. 10.1128/AAC.00671-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Torres S, Myers AL, Klatte JM, et al. First use of investigational antiviral drug pocapavir (v-073) for treating neonatal enteroviral sepsis. Pediatr Infect Dis J 2015;34:52–4. 10.1097/INF.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 20.Wittekind SG, Allen CC, Jefferies JL, et al. Neonatal Enterovirus Myocarditis With Severe Dystrophic Calcification: Novel Treatment With Pocapavir. J Investig Med High Impact Case Rep 2017;5:23 10.1177/2324709617729393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson JL, Hartling L, Crumley E, et al. A systematic review of intravenous gamma globulin for therapy of acute myocarditis. BMC Cardiovasc Disord 2005;5:12 10.1186/1471-2261-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden K, Thiagarajan RR, Rycus PT, et al. Survival of neonates with enteroviral myocarditis requiring extracorporeal membrane oxygenation. Pediatr Crit Care Med 2011;12:314–8. 10.1097/PCC.0b013e3181e8b44b [DOI] [PubMed] [Google Scholar]