Abstract
Objective:
To compare the experience of four UK Centres in the use of intradermal microbubbles and contrast enhanced ultrasound (CEUS) to pre-operatively identify and biopsy sentinel lymph nodes (SLN) in patients with breast cancer.
Methods:
In all centres, breast cancer patients had a microbubble/CEUS SLN core biopsy prior to axillary surgery and patients in Centres 1 and 2 had a normal greyscale axillary ultrasound. Data were collected between 2010 and 2016; 1361 from Centre 1 (prospective, sequential), 376 from Centre 2 (retrospective, sequential), 121 from Centre 3 (retrospective, selected) and 48 from Centre 4 (prospective, selected).
Results:
SLN were successfully core biopsied in 80% (Centre 1), 79.6% (Centre 2), 77.5% (Centre 3) and 88% (Centre 4). The sensitivities to identify all SLN metastases were 46.9% [95% confidence intervals (CI) (39.4–55.1)], 52.5% [95% CI (39.1–65.7)], 46.4% [95% CI (27.5–66.1)] and 45.5% [95% CI (16.7–76.6)], respectively. The specificities were 99.7% [95% CI (I98.9–100)], 98.1% [95% CI (94.5–99.6)], 100% [95% CI (93.2–100%)] and 96.3% [95% CI (81–99.9)], respectively.The negative predictive values were 87.0% [95% CI (84.3–89.3)], 84.5% [95% CI (78.4–89.5)], 86.9% [95% CI (82.4–90.3)] and 86.2% [95% CI (78.4–91.5)], respectively. At Centres 1 and 2, 12/730 (1.6%) and 7/181 (4%), respectively, of patients with a benign microbubble/CEUS SLN core biopsy had two or more lymph node (LN) macrometastases found at the end of primary surgical treatment.
Conclusion:
The identification and biopsy of SLN using CEUS is a reproducible technique.
Advances in knowledge:
In the era of axillary conservation, microbubble/CEUS SLN core biopsy has the potential to succeed surgical staging of the axilla.
INTRODUCTION
Sentinel lymph node excision (SLNE) is the axillary staging method of choice for breast cancer patients with normal axillary lymph nodes (LN) on greyscale ultrasound or a benign biopsy of morphologically abnormal LN.1,2 Although SLNE has less reported morbidity than axillary lymph node dissection (ALND), it remains a surgical procedure performed under general anaesthesia with recognized immediate complications such as infection (11%) and long-term problems with sensory loss (11%) and arm lymphoedema (5%) at 12 months.3 The operation is also reliant upon two tracers (radioactive isotope and blue dye) to locate SLN and maintain a false negative rate of 6%.4 The blue dye carries a hazard of anaphylaxis (0.9%)5 and there are logistical challenges in obtaining medical grade radioisotopes.
Recently, the practice of removing all malignant axillary LN to achieve local control has been challenged by the results of a trial where patients with SLN metastases were randomized to a completion ALND or no further axillary surgery.6 The local recurrence rate in the axilla was low with no difference between the groups despite the fact that 27.3% of patients in the ALND arm had further lymph node metastases retrieved.6 These results emphasize the role of modern adjuvant treatment in preventing loco-regional disease recurrence and have led to the introduction of conservative surgical management of the axilla for patients with up to two SLN macrometastases.7
In patients with breast cancer, SLN can be identified and percutaneously biopsied in the clinic using intradermally injected microbubbles and contrast-enhanced ultrasound (CEUS).8–10The technique was originally described in a swine melanoma model11 and following a trial period, was incorporated into routine practice at Centre 1 for all invasive breast cancer patients with a normal greyscale axillary ultrasound12,13 to aid treatment planning such as the selection of appropriate axillary surgery,14 the initiation of neo-adjuvant systemic therapy15 and reconstructive decisions for patients who may consequently benefit from post-mastectomy radiotherapy.16The identification of SLN using microbubbles and CEUS was previously validated against the surgical detection of SLN using blue dye and radioisotope and concordance was found in 93% of cases that had a core biopsy.13Other Centres in the UK also adopted the microbubble/CEUS SLN biopsy technique for use in their own practice.
Previous work from Centre 1 has shown that patients with invasive breast cancer and a normal greyscale ultrasound and benign microbubble/CEUS SLN core biopsy are unlikely to have extensive metastatic axillary disease that is both greyscale ultrasound occult and missed on SLN core biopsy.14 These results suggest that complete radiological staging of the axilla might be feasible.
We therefore aimed to assess the reproducibility of the microbubble/CEUS SLN core biopsy procedure by comparing the test accuracy of Centre 1 with a smaller population of consecutive patients from Centre 2 as well as two other UK centres using patients that were selected on the basis of tumour clinicopathological features. We also examined the technical performance of individual radiologists at Centre 1. Lastly, we measured the volume of axillary metastases in patients from Centres 1 and 2 to determine the proportion of patients with a false negative microbubble/CEUS SLN core biopsy who had two or more axillary LN macrometastases at the end of primary surgical treatment.
METHODS AND MATERIALS
Study design
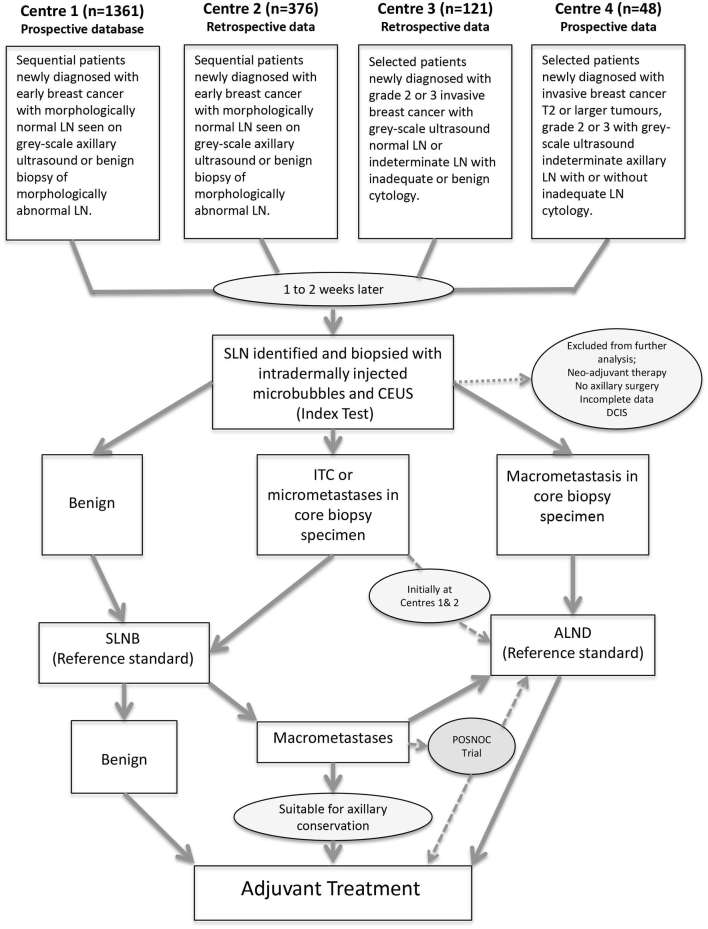
Data were collected from four Breast Units across the UK (Figure 1) . In Centres 1 and 2, patients were included with newly diagnosed breast cancer and an initial greyscale axillary ultrasound ± biopsy of indeterminate/abnormal LN17 and only those with normal axillary imaging/benign pathology proceeded to have a microbubble/CEUS SLN core biopsy. At Centre 3, patients with newly diagnosed Grade 2 or 3 invasive breast cancer and a normal greyscale axillary ultrasound or indeterminate LN that were inadequate (not enough cells to make a diagnosis) or benign on cytology were included. At Centre 4, patients with newly diagnosed T2 or larger tumours, Grade 2 or 3 invasive breast cancer with greyscale ultrasound indeterminate axillary LN were included. Centres 3 and 4 selected patients at the discretion of the multidisciplinary team. In all centres, pregnant patients and those with locally advanced/metastatic disease were excluded. At Centre 1, 1361 consecutive patients were identified from a prospective database of patients having microbubble/CEUS-guided SLN core biopsy between December 2010 and November 2016. A proportion of this data (570 patients) has been previously analysed and published.14 Retrospective data were collected on 376 consecutive patients from Centre 2 between July 2010 and July 2014. Retrospective data were collected on 121 selected patients from Centre 3 between July 2013 and December 2015. Prospective data were collected on 48 selected patients from Centre 4 between June 2013 and February 2015.
Figure 1.
Diagram showing the selection and flow of participants from each centre through the study. Axillary lymph node dissection (ALND), Contrast enhanced ultrasound (CEUS), Ductal Carcinoma in situ (DCIS), Isolated tumour cells (ITC), Lymph node (LN), Sentinel lymph node (SLN), Surgical sentinel lymph node biopsy (SLNB) and POSNOC (POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy. A randomized controlled trial of axillary treatment in females with early stage breast cancer who have metastases in one or two sentinel nodes).
Identification and biopsy of SLN using intradermal microbubbles and CEUS
Following a diagnosis of breast cancer, patients had an injection of ultrasound contrast agent (Sonovue, BRACCO Imaging S.p.A, Italy). 0.2 ml of ultrasound contrast agent was injected intradermally in the periareolar, upper outer quadrant position using a 26G needle with a 1 ml tuberculin syringe at Centre 1, Centre 2 and Centre 3. Centre 4 used a 24G needle with a 1 ml tuberculin syringe.
Lymphatic channels were visualized on contrast pulse sequencing and tracked into the axilla. Areas of contrast accumulation were also imaged with greyscale or live dual images (Figure 2). Further injections of contrast up to 1 ml and three consecutive injections together with injection site massage for 10–30 s were performed if the progress of contrast through the lymphatic vessels was slow/not immediately evident. Once identified, the SLN was biopsied using a core biopsy technique, Centres 1 and 2 (Achieve automatic biopsy device, Carefusion, San Diego, CA. 2−3 × 14–16G), Centre 3 (Trucut biopsy device, San Diego, CA. 3−4 × 14G) and Centre 4 (Trucut biopsy device, San Diego, CA. 3−4 × 18G).
Figure 2.
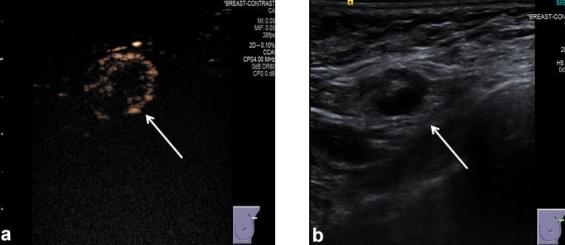
(a) Ultrasound contrast pulse sequencing image of an SLN (white arrow) after injection of intradermal microbubbles (between 0.2 and 1 ml injected using a 26G needle with 1 ml tuberculin syringe) into the UOQ periareolar area of the breast. (b) Greyscale ultrasound image of the same SLN as visualized in (a) Images provided by Centre 3. Sentinel lymph nodes (SLN).
Microbubble/CEUS-guided SLN core biopsies were performed by consultant breast radiologists: seven at Centre 1, four at Centre 2, two at Centre 3 and a single consultant breast radiologist at Centre 4. Three of the seven radiologists at Centre 1 also worked at Centre 2. Very rarely two SLN were visualized and both were biopsied at Centre 1, Centre 2 and Centre 3. At Centre 4, only the first enhancing SLN was biopsied. Centre 1 ultrasound examinations were performed with a Sequoia 512 Acuson (Siemens Medical Systems, Issaquah, Wash, Issaquah, WA) or LOGIQ 9 (GE Healthcare, Fairfield, CT) using a linear transducer operating at 4.5 to 15 MHz; Centre 2 ultrasound examinations were performed with a LOGIQ 9 using a linear transducer operating at 4.5 to 15 MHz; Centre 3 ultrasound examinations were performed with a Siemens Antaris or Acuson S1000 (Siemens Medical Systems, Wash, Issaquah, WA); and Centre 4 ultrasound examinations were performed with a Toshiba Aplio 500 (Toshiba Medical Systems Europe B.V., Zilverstraat 1, 2718RP, Zoetermeer, Netherlands) using a mid-range linear probe (PLT-704SBT Linear). All ultrasound machines provided conventional greyscale, pulse-inversion harmonic greyscale, contrast specific sonographic imaging with live dual images of tissue only and contrast agent images. In order to reduce microbubble destruction, low mechanical index values were applied (MI: 0.1–0.4). Each centre performed histological analysis on biopsy samples. Centres 1 and 2 also used pancytokeratin (MNF116) immunohistochemical staining for patients with a lobular phenotype.
Surgical management of the axilla
Initially, at Centres 1 and 2 if the microbubble/CEUS SLN core biopsy contained malignant cells, patients were advised to have an ipsilateral ALND. Later in the study period (Centres 1 and 2) and throughout the study period at Centres 3 and 4, to avoid surgical overtreatment, patients were recommended to have ALND only if a macrometastasis (>2 mm) was seen in the core biopsy specimen and those with core biopsy isolated tumour cells (ITC) or micrometastases (<2 mm) had SLNE. Patients also had SLNE if the core biopsy identified indeterminate cellular changes, normal lymphoid tissue, inadequate tissue sampling or the patient declined primary ALND. Before surgery, patients had an injection of radioactive isotope (Nanocoll, G.E. Healthcare, Chicago, IL), between 20 and 40 mbeq in the periareolar, upper outer quadrant position. Later that day or the following day, patients underwent surgical resection. After anaesthetic induction, patients received a 2 ml injection of blue dye (Bleu Patente V 2.5%, Guerbet, France) subdermally in the periareolar, upper outer quadrant position. A gamma probe (Navigator GPS, RMD Instruments, Watertown, MA) was used to identify SLN. All SLN within the axilla were excised and sent for local histological analysis including immunohistochemical staining for patients with a lobular phenotype at Centres 1 and 2. The total volume of axillary metastases at the end of surgical treatment was determined for each patient using the following scoring system: 1 (LN containing a macrometastasis), 0.5 (LN containing a micrometastasis) and 0.2 (LN containing isolated tumour cells).12
Statistics
The proportion of patients who had SLN identified was calculated. Using contingency tables, the sensitivity and specificity of a successful (LN tissue retrieved) CEUS-guided SLN core biopsy as the index test to identify SLN metastases in breast cancer patients with invasive disease was determined with axillary surgery (SLNE/ALND) as the reference standard. The estimated values were calculated along with corresponding 95% confidence intervals (CI) illustrating the uncertainty in the results. All CI were calculated using the exact binomial method. The prevalence of SLN metastases in each population was derived from the reference standard. For Centres 1 and 2, positive and negative predictive values were calculated directly, as they both employed consecutive enrolment so the study prevalence will be representative of the population of interest. For Centres 3 and 4, which only included a non-randomly selected subset of eligible patients, Bayesian methods were used to calculate positive and negative predictive values, using the prevalence from Centre 1 (the largest centre with consecutive enrolment). The Mann–Whitney test was used to compare the volume of axillary metastases between patients with a false negative and true positive microbubble/CEUS SLN biopsy. All analysis was completed using Stata software (Stata version 13.1; Stata Corp LP, College Station, TX).
Ethics
Kent Research Ethics Committee, UK approved the original trials at Centre 1 (reference numbers: 04/Q1801/25 and 11/H1101/1) and the Medicines and Healthcare Products Regulatory Agency sanctioned the use of microbubbles by intraparenchymal injection (Eudract Number: 2004-002423-41). All participating Centres were National Health Service Trusts and the New Procedures Committees at Centre 1, Centre 2 and Centre 3 and the Breast Services Committee at Centre 4 each approved the use of intradermal microbubbles and CEUS to identify and biopsy SLN in patients with breast cancer at their institution.
RESULTS
Visualization and targeted core biopsy of SLN using intradermal microbubbles and CEUS
A total of 1361 consecutive patients were identified from a prospectively maintained database at Centre 1. Complete data were not available for 12 patients and 1 patient had a fine needle aspiration biopsy rather than a core biopsy. Sentinel lymph nodes were clearly visualized by CEUS in 1216 of the 1348 patients (90%). In the 132 cases where the intradermally injected microbubbles failed to traffic through lymphatics to identify SLN, 25 (19%) had previous surgery on the breast/axilla, a post breast biopsy haematoma or malignant involvement of the nipple areolar complex. A successful SLN core biopsy was achieved in 1083 of the 1348 patients (80%). The performance statistics of the seven Centre 1 radiologists are presented in Table 1.
Table 1.
Performance statistics of seven consultant breast radiologists at Centre 1 (total 1349 of 1361 procedures) In 12 cases the data was incomplete and the name of the radiologist was not recorded. For radiologist no.1, one successful procedure was a fine needle aspiration biopsy rather than a core biopsy.
| Centre 1—consultant breast radiologists | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Total procedures | 276 | 37 | 501 | 81 | 116 | 207 | 131 |
| Procedures where core biopsynot attempted | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2. 5%) | 0 (0%) | 0 (0%) | 1 (0.8%) |
| Procedures with successful visualization of SLN | 269 (97.5%) | 33 (89.2%) | 457 (91.2%) | 59(72.8%) | 94 (81%) | 187 (90.3%) | 118 (90.1%) |
| Procedures without SLN tissue retrieved | 1 (0.4%) | 1 (2.7%) | 64 (12.8%) | 2 (2.5%) | 10 (8.6%) | 18 (8.7%) | 34 (26%) |
| Successful retrieval of lymphoid tissue in those with visualized SLN | 268 (99.6%) | 32 (97%) | 393 (86%) | 57 (96.6%) | 84 (89.4%) | 169 (90.4%) | 84 (71.2%) |
| Total successful visualization and core biopsy of SLN | 268 (97.1%) | 32 (86.5%) | 393 (78.4%) | 57 (70.4%) | 84 (72.4%) | 169 (81.6%) | 84 (64.1%) |
SLN, sentinel lymph nodes.
A total of 376 patients were identified retrospectively at Centre 2. Complete data were not available for 15 patients, 3 patients had a fine needle aspiration biopsy and 15 patients did not have the microbubbles/CEUS procedure. Sentinel lymph nodes were clearly visualized by CEUS in 290 of 343 patients (84.5%). A successful core biopsy of SLN was achieved in 273 of 343 patients (79.6%).
At Centre 3, 121 patients were identified retrospectively. Complete data were not available for one patient. Sentinel lymph nodes were clearly visualized by CEUS in 109 of the 120 patients (90.8%). A successful core biopsy of SLN was achieved in 93 of 120 patients (77.5%). Prospective data were collected on 48 patients at Centre 4. There were technical problems with the initial five patients, as the lymphatics could not be visualized. However, once the settings on the ultrasound machine were optimized, SLN were clearly visualized and successfully core biopsied in 38 of 43 patients (88%). In all centres, there were no allergic reactions following the administration of contrast agent and only one significant bleeding complication (Centre 1) of a large haematoma after SLN core biopsy, which was evacuated 2 weeks later at the time of primary surgical treatment.
Identification of SLN metastases in patients with invasive breast cancer and a successful microbubble/CEUS SLN core biopsy that underwent primary surgical treatment
In patients with a successful microbubble/CEUS SLN core biopsy, the following were excluded from final analysis; those with pre-invasive disease (DCIS), those who had primary systemic or endocrine therapy and patients who did not proceed with axillary surgery because of choice or inability to tolerate a general anaesthetic. At Centres 1 and 2, patients with un-biopsied greyscale abnormal LN were excluded and six patients at Centre 1 were also excluded because of incomplete surgical data.
At Centre 1, 816 of the 1083 patients with a successful microbubble/CEUS SLN core biopsy went on to have primary surgical treatment. Evidence of a core biopsy tract was seen in the excised SLN of 656 patients (80%) , 127 (16%) did not have a core biopsy tract visualized in excised SLN, 2 (0.2%) patients had a core biopsy tract seen in non-SLN and data regarding the presence of a core biopsy tract was not recorded for 31 patients (3.8%). For the other centres, 215 of 273 patients (Centre 2), 80 of 93 patients (Centre 3) and 38 of 38 patients (Centre 4) with a successful microbubble/CEUS SLN core biopsy went on to have primary surgical treatment. Comprehensive data documenting evidence of previous biopsy in excised SLN were not available for Centres 2, 3 and 4. For each centre, the accuracy of a successful microbubble/CEUS SLN core biopsy to identify SLN metastases in patients with invasive breast cancer is presented in Table 2.
Table 2.
Test accuracy of CEUS-guided SLN biopsy using intradermally injected microbubbles in females with normal (Centres 1 and 2) or indeterminate results from previous greyscale ultrasound (with or without previous biopsy)
| Centre | Prevalence of LN metastases | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV |
| 1 | 22% | 84 | 2 | 635 | 95 | 46.9% (39.4–55.5%) | 99.7% (98.95–100%) | 97.7% (91.9–99.7%) | 87.0% (84.3–89.3% |
| 2 | 27% | 31 | 3 | 153 | 28 | 52.5% (39.1–65.7%) | 98.1% (94.5–99.6%) | 91.2% (76.3–98.1%) | 84.5% (78.4–89.5%) |
| 3 | 35% | 13 | 0 | 52 | 15 | 46.4% (27.5–66.1%) | 100% (93.2–100%) | 100% (75.3–100%) | 86.9% (82.4–90.3%) |
| 4 | 29% | 5 | 1 | 26 | 6 | 45.5% (16.7–76.6%) | 96.3% (81.0–99.9%) | 77.6% (31.3–96.3%) | 86.2% (78.4–91.5%) |
ALND, axillary lymphnode dissection; CEUS, contrast enhanced ultrasound; FP, falsepositive; LN, lymph nodes; NPV, negative predictive value; PPV, positivepredictive value; TN, true negative; TP, true positive; SLN, sentinel lymphnodes; SLNE, sentinel lymph node excision.
Reference standard is SLNE or ALND. PPV and NPV were calculated directly for Centres 1 and 2 thatemployed consecutive recruitment, and using Bayesian methods with 22% prevalenceat Centres 3 and 4 as these were not a consecutivelyor randomly selected group.
Volume of axillary metastases at the end of primary surgical treatment in patients with invasive breast cancer, radiologically normal axillary LN and a successful microbubble/CEUS SLN core biopsy in Centres 1 and 2.
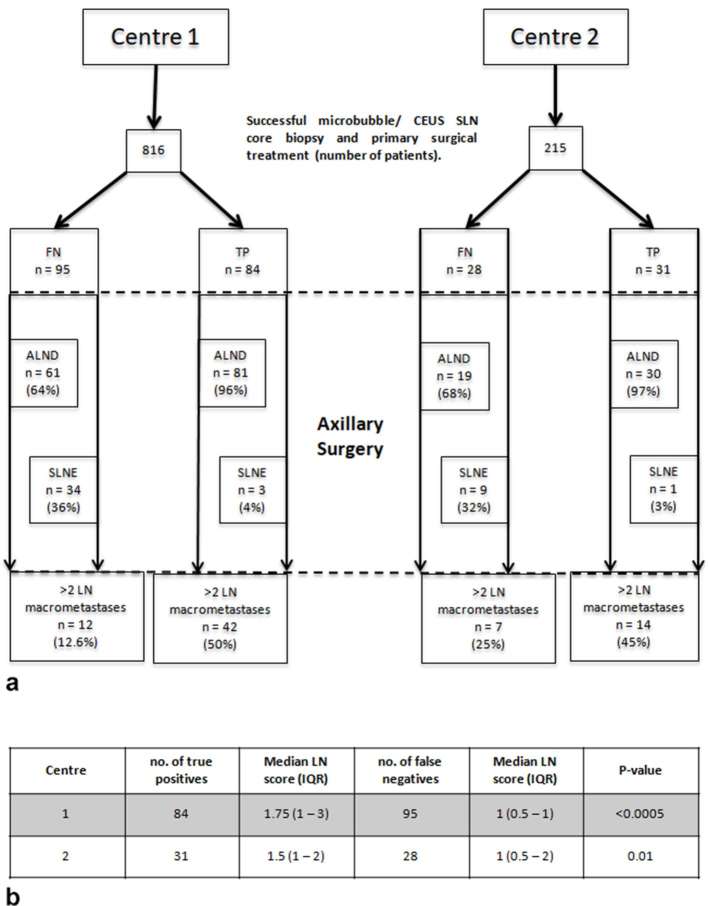
At Centre 1, 95 patients had a false negative microbubble/CEUS SLN core biopsy with metastases found in the excised SLN. Of these, evidence of a core biopsy tract was seen in the excised SLN of 69 patients (72.6%), 23 (24.2%) did not have a core biopsy tract visualized in excised SLN and data regarding the presence of a core biopsy tract were not recorded for 3 (3.2%). 61 patients (64%) with a false negative microbubble/CEUS SLN core biopsy result went on to have a completion ALND and 34 (36%) had no further axillary surgery (axillary conservation). At the end of primary surgical treatment, 12 patients (12.6%) patients were found to have two or more LN macrometastases and 82 patients (87.4%) had less than two LN macrometastases. Of the 730 patients with an initial benign microbubble/CEUS SLN core biopsy, only 12 (1.6%) had two or more LN macrometastases found at the end of primary surgical treatment (Table 3). 84 patients at Centre 1 had a true positive microbubble/CEUS SLN core biopsy and 81 (96%) had an ALND whereas 3 (4%) had an SLNE. Of these 84 patients, 42 (50%) had two or more axillary LN macrometastases at the end of primary surgical treatment.
Table 3.
Age and clinicopathological characteristics of all patients at Centre 1 (first column) with a successful microbubble/CEUS SLN core biopsy before primary surgical treatment
| Volume of disease at the end of surgical treatment (microbubble/CEUS false negative core biopsy) | ||||
| Centre 1 | All patients | Micrometastasis/ITC | Low (<2 LN macrometastases) | High (2 or more LN macrometastases) |
| Total number of patients | 816 | 37 | 46 | 12 |
| 15 (41%) ALND | 34 (74%) ALND | 12 (100%) ALND | ||
| Median age in years (range) | 61 (30–94) | 61 (42–89) | 55 (32–90) | 53 (36–69) |
| Receptor status | ||||
| ER positive | 707 (87%) | 32 (86%) | 40 (87%) | 11 (92%) |
| ER unknown | 4 (0.4%) | 0 | 0 | 0 |
| Her-2 positive | 79 (10%) | 3 (8%) | 2 (4%) | 1 (8%) |
| Her-2 not recorded | 13 (1.6%) | 0 | 2 (4%) | 0 |
| ER-/PR-/Her-2- | 72 (10%) | 4 (10.8%) | 4 (9%) | 0 |
| ER-/PR-/HER-2+ | 28 (3%) | 1 (2.7%) | 1 (2%) | 1 (8%) |
| Invasive tumour size | ||||
| DCIS + microinvasion | 5 (0.6%) | 0 | 0 | 0 |
| T1 | 472 (58%) | 19 (51%) | 17 (37%) | 4 (33%) |
| T2 | 199 (24%) | 10 (27%) | 18 (39%) | 5 (42%) |
| T3 | 29 (4%) | 5 (14%) | 3 (7%) | 1 (8%) |
| Multifocal | 111 (14%) | 3 (8%) | 8 (17%) | 2 (17%) |
| Unknown | 0 | 0 | 0 | 0 |
| Tumour grade | ||||
| Grade 1 | 180 (22%) | 6 (16%) | 7 (15%) | 4 (33%) |
| Grade 2 | 392 (48%) | 20 (54%) | 21 (46%) | 6 (50%) |
| Grade 3 | 209 (26%) | 11 (30%) | 14 (30%) | 1 (8%) |
| Mixed grade | 29 (4%) | 0 | 4 (9%) | 1 (8%) |
| Unknown | 1 (0.1%) | 0 | 0 | 0 |
| Tumour type | ||||
| IDC | 660 (81%) | 23 (62%) | 38 (83%) | 11 (92%) |
| ILC | 103 (13%) | 11 (30%) | 6 (13%) | 1 (8%) |
| Other | 29 (4%) | 1 (3%) | 1 (2%) | 0 |
| Mixed | 17 (2%) | 2 (5%) | 1 (2%) | 0 |
| Unknown | 2 (0.2%) | 0 | 0 | 0 |
ALND, axillary lymph node dissection; CEUS,contrast enhanced ultrasound; DCIS, ductal carcinoma in situ; ER, estrogenreceptor; Her2, human epidermal growth factor receptor 2; IDC, invasive ductalcarcinoma; ILC, invasive lobular carcinoma; ITC, isolated LN, lymph nodes; PR, progesteronereceptor.
Centre 1 patients with false negative microbubble/CEUS SLN core biopsies subdivided into micrometastases (<2 mm)/ITC, low volume metastases and high volume metastases identified at the end of primary surgical treatment. Data are expressed as n (%).
At Centre 2, 28 patients had a false negative microbubble/CEUS SLN core biopsy with metastases found in the excised SLN. 19 patients (68%) went on to have a completion ALND and 9 (32%) had axillary conservation. At the end of primary surgical treatment, 7 patients (25%) were found to have two or more axillary macrometastases and 22 patients (75%) had a malignant axillary LN score less than two or had ITC in multiple LN (one patient with ITC in 13 LN). Of the 181 patients with an initial benign microbubble/CEUS SLN core biopsy, 7 (4%) had two or more axillary macrometastases found at the end of primary surgical treatment (Table 4). 31 patients at Centre 2 had a true positive microbubble/CEUS SLN core biopsy and 30 (97%) had an ALND whereas 1 (3%) had an SLNE. Of these 31 patients, 14 (45%) had two or more axillary LN macrometastases at the end of primary surgical treatment.
Table 4.
Age and clinicopathological characteristics of all patients at Centre 2 (first column) with a successful microbubble/CEUS SLN core biopsy before primary surgical treatment and Centre 2 patients with false negative microbubble/CEUS SLN core biopsies subdivided into micrometastases (<2 mm)/ITC, low volume metastases and high volume metastases identified at the end of primary surgical treatment.
| Volume of disease at the end of surgical treatment (microbubble/CEUS false negative core biopsy) | ||||
| Centre 2 | All patients | Micrometastasis/ITC | Low (<2 LN macrometastases) | High (2 or more LN macrometastases) |
| Total number of patients | 215 | 10 | 11 | 7 |
| 2 (20%) ALND | 10 (91%) ALND | 7 (100%) ALND | ||
| Median age in years (range) | 64 (31–93) | 63.5 (37–93) | 62 (38–91) | 55 (47–72) |
| Receptor status | ||||
| ER positive | 176 (82%) | 10 (100%) | 8 (73%) | 6 (86%) |
| ER unknown | 6 (3%) | 0 | 1 (9%) | 1 (14%) |
| Her-2 positive | 29 (13%) | 1 (10%) | 0 | 1 (14%) |
| Her-2 not recorded | 8 (4%) | 0 | 1 (9%) | 1 (14%) |
| ER-/PR-/Her-2- | 23 (11%) | 0 | 1 (9%) | 0 |
| ER-/PR-/HER-2+ | 10 (5%) | 0 | 0 | 0 |
| Invasive tumour size | ||||
| DCIS + microinvasion | 0 | 0 | 0 | 0 |
| T1 | 118 (55%) | 2 (20%) | 6 (55%) | 2 (29%) |
| T2 | 71 (33%) | 6 (60%) | 4 (36%) | 5 (71%) |
| T3 | 9 (4%) | 0 | 0 | 0 |
| Multifocal | 17 (8%) | 2 (20%) | 0 | 0 |
| Unknown | 0 | 0 | 1 (9%) | 0 |
| Tumour grade | ||||
| Grade 1 | 35 (16%) | 1 (10%) | 0 | 2 (29%) |
| Grade 2 | 102 (47%) | 6 (60%) | 5 (45.5%) | 1 (14%) |
| Grade 3 | 68 (32%) | 2 (20%) | 4 (36%) | 4 (57%) |
| Mixed grade | 4 (2%) | 0 | 0 | 0 |
| Unknown | 6 (3%) | 0 | 2 (18%) | 0 |
| Tumour type | ||||
| IDC | 160 (74%) | 7 (70%) | 8 (73%) | 5 (71%) |
| ILC | 24 (11%) | 2 (20%) | 1 (9%) | 0 |
| Other | 16 (7%) | 0 | 0 | 0 |
| Mixed | 13 (6%) | 1 (10%) | 1 (9%) | 2 (29%) |
| Unknown | 2 (1%) | 0 | 1 (9%) | 0 |
ALND, axillary lymph node dissection; CEUS, contrast enhanced ultrasound; DCIS, ductal carcinoma in situ; ER, estrogen receptor; Her2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LN, lymph nodes; PR, progesterone receptor; SLN sentinel lymph nodes.
Data are expressed as n (%). For both centres, at the end of surgical treatment the difference between the final malignant LN score of false negative vs true positive microbubble/CEUS SLN core biopsies was statistically significant (Figure 3).
For both centres, at the end of surgical treatment the difference between the final malignant LN score of false negative true positive microbubble/CEUS SLN core biopsies was statistically significant (Figure 3).
Figure 3.
(a) Volume of axillary disease at the end of primary surgical treatment for individual patients at Centres 1 and 2 who had a false negative (FN) or true positive (TP) microbubble/CEUS core biopsy of SLN. ALND and SLNE. (b) The total volume of axillary metastases at the end of surgical treatment was determined using a scoring system (isolated tumour cells = 0.2, each LN micrometastasis = 0.5 and each LN macrometastasis = 1). The Mann–Whitney test was used to compare the volume of axillary metastases between patients with a false negative and true positive microbubble/CEUS SLN biopsy in both centres.
DISCUSSION
Despite the use of different ultrasound machines and variations in the methods and patient selection, the identification and core biopsy of SLN using intradermal microbubbles and CEUS in patients with breast cancer is a reproducible technique across multiple centres. Overall, the visualization of SLN across the four centres ranged from 84.5 to 90.8% and a successful core biopsy from 77.5 to 88%. Factors that appeared to adversely affect the visualization of SLN at Centre 1 included previous surgery and disease involvement of the nipple areolar complex.
There is undoubtedly a learning curve associated with the procedure, and familiarity with the equipment is important as demonstrated by the data from Centre 4 where they experienced technical problems with the first five patients. The performance of Centre 1 radiologists also highlight the distinct competencies of the two components of the procedure, namely SLN identification and SLN core biopsy. Although identical ultrasound equipment and methods were used, the percentage of procedures with successful visualization of SLN varied from 97 to 73%, which suggests either that not all radiologists at Centre 1 received adequate procedural training before performing the test or some found it difficult to visualize microbubbles trafficking through lymphatic channels. Six of the seven radiologists were fairly consistent in their ability to successfully core biopsy visualized SLN, but one obviously struggled and only successfully retrieved lymphoid tissue in 70% of cases. Anecdotally, most radiologists accustomed to the procedure recommend that novices observe three cases, then perform 10 cases supervised before undertaking 30 independent procedures with an audit of their results. Once proficient, the whole procedural time is 15 to 30 min.
There is scope to improve the technology of CEUS. In swine models, LN metastases can be identified as areas devoid of contrast agent11 and in a recent study of breast cancer patients, the sensitivity of CEUS as a test to identify SLN metastases using only enhancement patterns (no biopsy) was 81.8%.18 Innovations such as ultrafast ultrasound,19 super resolution imaging20 and improved lymphatic microbubble transit21 may improve the ability of clinicians to visualize SLN and achieve a reliable standard.
The sensitivity of a microbubble/CEUS core biopsy as a test to identify SLN metastases in patients with invasive breast cancer and a normal greyscale axillary ultrasound/benign axillary LN biopsy is consistently around 50% with Centres 2, 3 and 4 within the 95% confidence intervals of Centre 1. As greyscale axillary ultrasound can usefully identify approximately 50% of LN metastases,22 the addition of a microbubble/CEUS SLN core biopsy substantially increases the overall detection rate for metastatic axillary LN. Consequently, the negative predictive value of the test is high and <5% of patients (Centres 1 and 2) with a normal greyscale ultrasound and benign microbubble/CEUS SLN core biopsy had two or more LN macrometastases detected by axillary surgery.
We have previously speculated that the technique has a high false negative rate because the core biopsy fails to pick up small metastatic deposits in SLN13 and this may be the reason why very few patients with a false negative microbubble/CEUS core biopsy at Centres 1 and 2 had two or more axillary LN macrometastases found at the end of primary surgical treatment. Yet, retrieving more LN tissue with a vacuum-assisted biopsy technique does not appear to appreciably increase the sensitivity of microbubbles and CEUS.23
Alternatively, it is usual for only one SLN to be visualized and biopsied with CEUS but the median number of SLN retrieved with a surgical excision is two.3 n this series, 80% of surgically excised LN at Centre 1 showed evidence of a previous core biopsy and this proportion dropped to 72.6% in patients with a false negative benign microbubble/CEUS SLN core biopsy. This raises the possibility that in the false negative cases, the second or subsequent SLN contained the metastases and perhaps more than one SLN should be actively sought with the microbubbles/CEUS procedure.
When compared to Centre 1, a higher proportion of Centre 2 patients with a benign microbubble/CEUS SLN core biopsy had two or more axillary macrometastases found at the end of surgical treatment (1.8 vs 4%). This may be related to the smaller patient sample size in Centre 2 or the higher prevalence of LN metastases in patients with a successful microbubble/CEUS SLN core biopsy in Centre 2 (27 vs 22% at Centre 1). As the patient and clinicopathological features of the tumours were similar in both centres, the greater prevalence of LN metastases at Centre 2 may be a consequence of a lower initial metastatic LN detection rate with grey-scale axillary ultrasound.
The difference in the volume of axillary disease between patients with a false negative and true positive microbubble/CEUS SLN core biopsy at the end of primary surgical treatment was statistically significant in Centres 1 and 2. When compared to the false negative groups in Centres 1 and 2, more patients with a true positive microbubble/CEUS SLN core biopsy had complete axillary surgery (ALND) rather than axillary conservation (SLNE). The retrieval of a greater number of LN in the true positive groups from Centres 1 and 2 may therefore have increased the total number of axillary LN metastases found at the end of surgical treatment and influenced the final metastatic score. However, in a previous publication from Centre 114 comparing only patients with complete axillary surgery (ALND), the difference in the volume of axillary disease between those with a false negative and true positive microbubble/CEUS SLN core biopsy remained statistically significant.
An argument against using CEUS to biopsy SLN in routine practice is that patients with a biopsy containing malignant cells are committed to a primary ALND for what may be a low burden of axillary disease. This can be mitigated against by offering SLNE to patients with micrometastases in the core biopsy specimen. It should also be noted that 50% of patients at Centre 1 and 45% of patients at Centre 2 with a true positive microbubble/CEUS SLN core biopsy had two or more axillary macrometastases found at the end of surgical treatment and therefore using the test for patients who are not eligible for axillary conservation7 is beneficial.
CONCLUSION
The results of the American College of Surgeons Oncology Group Z0011 trial6 have changed practice by showing that loco-regional control of axillary metastases is not solely dependant upon surgical excision and residual disease can be treated with adjuvant therapy. In addition, anatomic staging of breast cancer is likely to become less relevant to treatment decisions as tumour genomic and molecular assays are better understood.24 Based on recent information obtained from Centre 1, an SLNE costs 3.6x the cost of a microbubble/CEUS SLN core biopsy (£671.63 vs £189, respectively). Omitting axillary surgery will improve theatre utilization (potentially allowing more cases to be added to a list) as well as reducing the anaesthetic time for each patient. In the era of axillary conservation, molecular medicine and dwindling resources, the combination of grey-scale axillary ultrasound and microbubble/CEUS SLN core biopsy has the potential to succeed surgical staging of the axilla. Further work now needs to be undertaken to refine the procedure with protocols, standard setting and training.
ACKNOWLEDGMENTS
We would like to acknowledge the support of patients and staff at all four centres.
Contributor Information
Karina Cox, Email: karina.cox@nhs.net.
Sian Taylor-Phillips, Email: S.Taylor-Phillips@warwick.ac.uk.
Nisha Sharma, Email: nisha.sharma2@nhs.net.
Jennifer Weeks, Email: Jennifer.weeks@nhs.net.
Philippa Mills, Email: pippa.mills@nhs.net.
Ali Sever, Email: arsever@hotmail.com.
Adrian Lim, Email: a.lim@imperial.ac.uk.
Isobel Haigh, Email: isobel.haigh@nhs.net.
Mohamed Hashem, Email: m.hashem@nhs.net.
Tania de Silva, Email: tania.desilva@gmail.com.
Keshthra Satchithananda, Email: k.satchithananda@nhs.net.
Mengxing Tang, Email: mengxing.tang@imperial.ac.uk.
Matthew Wallis, Email: matthew.wallis1@nhs.net.
Disclosure
Dr Adrian Lim receives some funding from Toshiba. A proportion of this data was presented at the 102nd Scientific Assembly and Annual Meeting of the Radiological Society of North America 2016 (abstract SSA02-06).
REFERENCES
- 1.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003; 349: 546–53. doi: https://doi.org/10.1056/NEJMoa012782 [DOI] [PubMed] [Google Scholar]
- 2. NICE. CG80 Early and locally advanced breast cancer: full guideline. UK: The British Institute of Radiology.; 2009. [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98: 599–609. doi: https://doi.org/10.1093/jnci/djj158 [DOI] [PubMed] [Google Scholar]
- 4.Pesek S, Ashikaga T, Krag LE, Krag D. The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg 2012; 36: 2239–51. doi: https://doi.org/10.1007/s00268-012-1623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthelmes L, Goyal A, Newcombe RG, McNeill F, Mansel RE. Adverse reactions to patent blue V dye - The NEW START and ALMANAC experience. Eur J Surg Oncol 2010; 36: 399–403. doi: https://doi.org/10.1016/j.ejso.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011; 305: 569–75. doi: https://doi.org/10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Association of Breast Surgery Consensus Statement Management of the Malignant Axilla in Early Breast Cancer. 2015. Available from: http://www.associationofbreastsurgery.org.uk/media/50934/axilla_abs_consensus_statement_16_3_15.pdf [Google Scholar]
- 8.Omoto K, Matsunaga H, Take N, Hozumi Y, Takehara M, Omoto Y, et al. Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol 2009; 35: 1249–56. doi: https://doi.org/10.1016/j.ultrasmedbio.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg 2009; 96: 1295–9. doi: https://doi.org/10.1002/bjs.6725 [DOI] [PubMed] [Google Scholar]
- 10.Rautiainen S, Sudah M, Joukainen S, Sironen R, Vanninen R, Sutela A. Contrast-enhanced ultrasound -guided axillary lymph node core biopsy: diagnostic accuracy in preoperative staging of invasive breast cancer. Eur J Radiol 2015; 84: 2130–6. doi: https://doi.org/10.1016/j.ejrad.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Goldberg BB, Merton DA, Liu JB, Thakur M, Murphy GF, Needleman L, et al. Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 2004; 230: 727–34. doi: https://doi.org/10.1148/radiol.2303021440 [DOI] [PubMed] [Google Scholar]
- 12.Sever A, Broillet A, Schneider M, Cox K, Jones S, Weeks J, et al. Dynamic visualization of lymphatic channels and sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in a swine model and patients with breast cancer. J Ultrasound Med 2010; 29: 1699–704. doi: https://doi.org/10.7863/jum.2010.29.12.1699 [DOI] [PubMed] [Google Scholar]
- 13.Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H, et al. Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound. Eur J Surg Oncol 2013; 39: 760–5. doi: https://doi.org/10.1016/j.ejso.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 14.Cox K, Weeks J, Mills P, Chalmers R, Devalia H, Fish D, et al. Contrast-enhanced ultrasound biopsy of sentinel lymph nodes in patients with breast cancer: implications for axillary metastases and conservation. Ann Surg Oncol 2016; 23: 58–64. doi: https://doi.org/10.1245/s10434-015-4606-0 [DOI] [PubMed] [Google Scholar]
- 15.Kilbride KE, Lee MC, Nees AV, Cimmino VM, Diehl KM, Sabel MS, et al. Axillary staging prior to neoadjuvant chemotherapy for breast cancer: predictors of recurrence. Ann Surg Oncol 2008; 15: 3252–8. doi: https://doi.org/10.1245/s10434-008-0136-3 [DOI] [PubMed] [Google Scholar]
- 16.Su YL, Li SH, Chen YY, Chen HC, Tang Y, Huang CH, et al. Post-mastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1-3 axillary lymph node(s) metastasis. Radiol Oncol 2014; 48: 314–22. doi: https://doi.org/10.2478/raon-2013-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WT, Metreweli C, Lam PK, Chang J. Benign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power Doppler US. Radiology 2001; 220: 795–802. doi: https://doi.org/10.1148/radiol.2203001545 [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Zhang D, Cheng L, Yu L, Yang L, Tong F, et al. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J Surg Oncol 2015; 13: . doi: https://doi.org/10.1186/s12957-015-0736-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanter M, Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2014; 61: 102–19. doi: https://doi.org/10.1109/TUFFC.2014.2882 [DOI] [PubMed] [Google Scholar]
- 20.Christensen-Jeffries K, Browning RJ, Tang MX, Dunsby C, Eckersley RJ. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans Med Imaging 2015; 34: 433–40. doi: https://doi.org/10.1109/TMI.2014.2359650 [DOI] [PubMed] [Google Scholar]
- 21.Gorce JM, Arditi M, Schneider M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: a study of SonoVue. Invest Radiol 2000; 35: 661–71. doi: https://doi.org/10.1097/00004424-200011000-00003 [DOI] [PubMed] [Google Scholar]
- 22.Diepstraten SC, Sever AR, Buckens CF, Veldhuis WB, van Dalen T, van den Bosch MA, et al. Value of preoperative ultrasound-guided axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann SurgOncol 2014; 21: 51–9. [DOI] [PubMed] [Google Scholar]
- 23.Britton P, Willsher P, Taylor K, Kilburn-Toppin F, Provenzano E, Forouhi P, et al. Microbubble detection and ultrasound-guided vacuum-assisted biopsy of axillary lymph nodes in patients with breast cancer. Clin Radiol 2017; 72: 772–9. doi: https://doi.org/10.1016/j.crad.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 24.Donovan CA, Giuliano AE. Evolution of the staging system in breast cancer. Ann Surg Oncol 2017; 24: 3469–70. doi: https://doi.org/10.1245/s10434-017-6035-8 [DOI] [PubMed] [Google Scholar]