Abstract
Objective:
To evaluate the accuracy of diffusion-weighted MRI with background suppression (DWIBS) in differentiating between malignant and benign mediastinal lymph-nodes.
Methods:
Consecutive patients with enlarged mediastinal lymph-nodes underwent MRI DWIBS within 10 days prior to mediastinoscopy. Relative contrast ratios (RCRs) were computed on b800 and apparent diffusion coefficient (ADC) maps by dividing the node signal with the chest muscle signal, using manually drawn regions of interest (ROIs) by radiologists, blinded to pathology. Unpaired Student's t-tests were used to compare RCR-b800 and ADC between malignant and benign nodes. Receiver operating characteristic curves analyses were also performed.
Results:
Six patients were excluded for poor image quality. Analysis was performed for 54 patients. Mean ADC values were significantly higher for benign (1740 ± 401 × 10−6 mm2 s–1) compared with malignant nodes (1266 ± 403 × 10−6 mm2 s–1, p = 0.0001). Mean RCR-b800 values were significantly lower for benign (2.64 ± 1.07) compared with malignant nodes (6.44 ± 3.47, p < 0.0001).
Receiver operating characteristic analysis for RCR-b800 (cut-off of 3.6), showed a sensitivity of 90.9%, a specificity 83% and an accuracy 85% for differentiating benign from malignant nodes. For ADC (cut-off of 1285), the sensitivity was 68.2%, the specificity 84.6% and the accuracy 80.4%.
Conclusion:
DWIBS can accurately differentiate malignant from benign states in enlarged mediastinal lymph-nodes and represents an alternative method in aetiological work-up of mediastinal lymphadenopathies.
Advances in knowledge:
DWIBS may represent a useful adjunctive imaging modality, particularly for diagnosis of benign mediastinal lymph node, and thus may reduce the frequency of futile mediastinoscopy, which remains an invasive procedure.
INTRODUCTION
The optimal method for enlarged mediastinal lymph node staging and distinction between malignant and benign states in the mediastinum is controversial. Nodal status assessment of mediastinal lymph nodes has relied a long time on anatomic features, most notably node diameter. However, this strategy has been limited by a relatively low sensitivity and specificity.1 Operative methods such as mediastinoscopy or thoracotomy are efficient but invasive and have potential complications. Positron emission tomography (PET) CT has been demonstrated to be a good modality, superior to CT alone,2 in diagnosing nodal involvement in lung cancer with a good sensitivity.3 Nevertheless, false-positive results are not uncommon in PET.4,5
With rapid technological developments, diffusion-weighted MRI (DW-MRI) has been linked to lesion aggressiveness and tumour response, although the biophysical basis for this is incompletely understood. The technique of DW-MRI indirectly measures the random (Brownian) motion of water molecules within the tissue. Because the random motion is hindered by boundaries, such as cell membranes, DW-MRI produces information on the viscosity and heterogeneity of the tissue being imaged. Parameters derived from DW-MRI, such as the value of the apparent diffusion coefficient (ADC), are appealing as imaging biomarkers because the acquisition is non-invasive, does not require any exogenous contrast agents, does not use ionizing radiation, yet is quantitative, widely accessible and easily incorporable into routine patient evaluations. However, these desirable features are offset by many challenges that face the validation of any imaging-based biomarker. It has been proposed as an alternative to PET-CT for non-invasive mediastinal lymph node characterization and several studies demonstrated the capabilities of DW-MRI in lymph node analysis, in the setting of lung cancer staging, with fewer false-positives than 18-fludeoxyglucose PET/CT.4,6,7
However, very little data are available on the potential of DW-MRI in differentiating the cause of enlarged lymph nodes as malignant or benign in patients with no diagnosed lung cancer, a common clinical problem.8,9 Furthermore, most DW-MRI studies, thorax acquisitions included, were performed at 1.5 T with a standard sequence.
The purpose of this study was to evaluate the accuracy of diffusion-weighted MRI with background suppression (DWIBS)10 at 3 T in diagnosing malignant and benign lymph nodes of the chest, as compared with pathological results following mediastinoscopy.
METHODS AND MATERIALS
Study subjects
64 consecutive patients presenting with previously diagnosed enlarged mediastinal lymph nodes and scheduled for a mediastinoscopy in our hospital (age 58 ± 14, range 27–80 years) were included in this study between June 2011 and December 2013, prior to any clinical treatment. One patient was excluded because of claustrophobia, one because of a cerebral metallic implant and two patients decided to leave the study. Thus, MRI was performed on the remaining 60 patients.
The Institutional Review Board of our hospital approved this prospective study and an informed consent was obtained from all the enrolled patients.
Surgical procedure
For all patients, surgery was performed within 10 days after the MRI examination by an experienced thoracic surgeon (FT) without knowledge of the imaging results. All visible lymph nodes at mediastinoscopy fields were removed for pathological examination and their anatomical position was recorded using the Mountain-Dresler modification of the American Thoracic Society (MD-ATS) map and used to identify the same nodes on the MR images.
All the excised lymph nodes were then referred to pathology. An experienced pathologist who was blinded to the imaging findings evaluated the specimen as to pathological type and degree of differentiation of the primary cancer or inflammatory disease.
MR technique
MRI examinations were performed at 3 T (Achieva, Philips HealthCare, Best, Netherlands), using a 16-element phased array surface coil (SENSE-XL-Torso, Philips HealthCare, Best, Netherlands). The MR protocol included a T1 weighted turbo spin echo sequence with breathhold (repetition time/echo time of 633/7.6 ms, field of view = 450 × 258 mm, pixel = 1.2 × 1.2 mm, 40 slices, slice thickness = 4 mm, gap = 1 mm, turbo spin echo factor = 8, 2 signal averages) and a free-breathing DW-MRI sequence (DWIBS) both in a transverse plane and covering the thorax from the superior thoracic inlet to the superior part of the left atrium in order to include all the lymph nodes accessible to the mediastinoscopy.
For the DWIBS sequences, the following parameters were used: single-shot spin echo echo-planar imaging read-out, three b-values of 0, 400 and 800 s mm–2, slice thickness = 5 mm, slice gap = 2 mm, number of slices 25, adapted FOV, acquired pixel size 2 × 2 mm, reconstructed to 1.4 × 1.4 mm, repetition time/echo time of 6674.1/44.7 ms, SENSE factor 1.9 in the phase direction, a short inversion time inversion-recovery pulse to suppress signal from fat using an inversion time of 260 ms, 2 signal averages.
Image post-processing and analysis
With the access to the location of resected lymph nodes but without knowledge of pathologic results, MR review was performed independently by two radiologists on the DWIBS images acquired at a b-value of 800, using GE Centricity PACS viewer (GEMS, GE Healthcare, United States).
The resected lymph nodes were identified based on their anatomic localization. Free-hand regions of interest (ROIs) were drawn in each node on the b800 images and the signal intensity (SI) of each ROI was measured. Similarly, a circular ROI was drawn in the back chest muscles. The relative contrast ratio (RCR) was calculated as follows: RCR-b800 = SIlesion/SImuscle, where SIlesion was the SI of the lymph node and SImuscle was calculated from muscle. Finally, the ROIs were automatically propagated to the ADC map and the mean ADC values of the lymph node were obtained using the same ROIs established for the SI measurements. Diameter of each lymph node, measured as the smallest diameter of the lymph node, was also recorded.
Statistical analysis
The data were expressed as mean ± standard deviation. For each lymph node, the mean value of RCR-b800 and ADC were calculated between the two observers and Student's unpaired t-tests were used for the comparison between RCR-b800 and ADC for the two histopathological groups as well as the difference in lymph node diameter. Receiver operating characteristic (ROC) analyses were performed for RCR-b800 and ADC maps. Areas under the two ROC curves were compared using a test of equality as part of the ROC analysis.
Correlation between lymph node diameter and RCR-b800 and ADC were calculated using the Pearson's correlation coefficient.
Differences were considered significant when the p-value was less than 0.05. To determine the level of interreader agreement between the two readers, the intraclass correlation coefficients with their 95% confidence interval (CI) were calculated for RCR-b800 and ADC in lymph nodes. All statistical analyses were performed using Intercooled Stata 10.0 (StataCorp LP, College Station, TX).
RESULTS
Among the 60 patients who underwent the MRI, 6 patients were excluded because of poor image quality owing to strong motion and breathing artefacts. Thus, the analysis was performed on the remaining 54 patients. Among these 54 patients, 22 were referred for a staging of a known or highly suspected lung cancer, 7 for a suspicion of other metastatic lymph node [history of lymphoma (1), breast (3), prostatic (1) and pancreatic (1) cancer and lung nodules (1)], 1 for a suspicion of tuberculosis, 7 for a suspicion of sarcoidosis and 17 for isolated enlarged mediastinal lymph nodes found incidentally on CT performed for indications such as COPD follow-up, weight loss and chronic cough.
The average number of nodes studied per patient was five (range 1–10), and lymph node cross-sections assessed with histopathology was two (range 1–4 cross-sections). Pathology revealed that 22 excised lymph nodes excised from 12 patients were malignant: 6 adenocarcinomas, 4 small-cell and 2 squamous cell carcinoma; and 65 from 42 patients were benign: 17 sarcoidosis, 8 silicosis, 1 tuberculosis, 1 anthracosis and 15 normal pathological results.
Mean lymph node diameter ± standard deviation was 12.25 ± 4.63 mm (range 5–30.5) for benign and 15.23 ± 6.44 mm (range: 7–34.1) for malignant lymph nodes (p = 0.01). Excised lymph nodes were located in area 2L (4 cases), 2R (13 cases), 4L (9 cases), 4R (45 cases) and 7 (16 cases). A weak correlation was found between lymph node diameter and RCR-b800 values (r = 0.25) and ADC (r = 0.22).
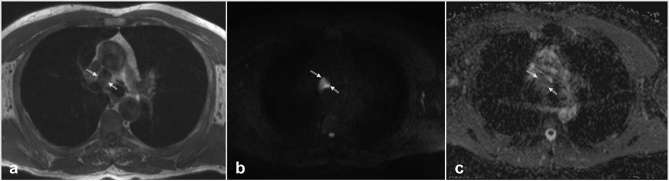
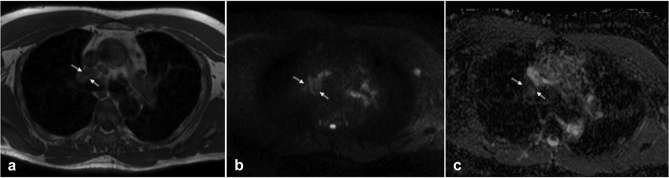
Figures 1 and 2 show two typical cases of pathologic and benign lymph nodes.
Figure 1.
Representative transverse images from left to right: T1 weighted turbo spin echo, diffusion-weighted MRI with background suppression-b800 and apparent diffusion coefficient map acquired in the thoracic region of a 62-year-old patient diagnosed with lung adenocarcinoma. The ROI was placed in the 4R node area (white arrows), mean relative contrast ratio-b800 = 5 and mean apparent diffusion coefficient = 2088 mm2 s–1.
Figure 2.
Representative transverse images from left to right: T1 w turbo spin echo, diffusion-weighted MRI with background suppression-b800 and apparent diffusion coefficient map acquired in the thoracic region of a 49-year-old patient diagnosed with sarcoidosis. The region of interest was placed in the R4 node area (white arrows), mean relative contrast ratio-b800 = 3.6 and mean apparent diffusion coefficient = 1487 mm2 s–1
The mean ADC values of the benign and malignant lymph nodes were, respectively, 1740 ± 401 × 10−6 mm2 s–1 (range 1046–2578 × 10−6 mm2 s–1) and 1266 ± 403 × 10−6 mm2 s–1 (range 718–2088 × 10−6 mm2 s–1), p = 0.0001.
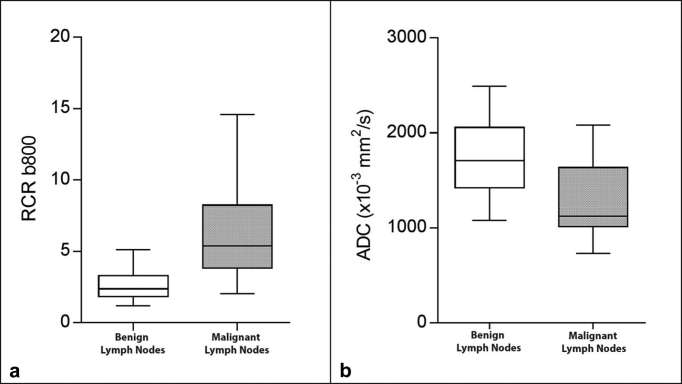
The mean RCR-b800 values of the benign and malignant lymph nodes were, respectively, 2.64 ± 1.07 (range 1.08–5.67) and 6.44 ± 3.47 (range 1.92–14.96), p < 0.0001 (Figure 3).
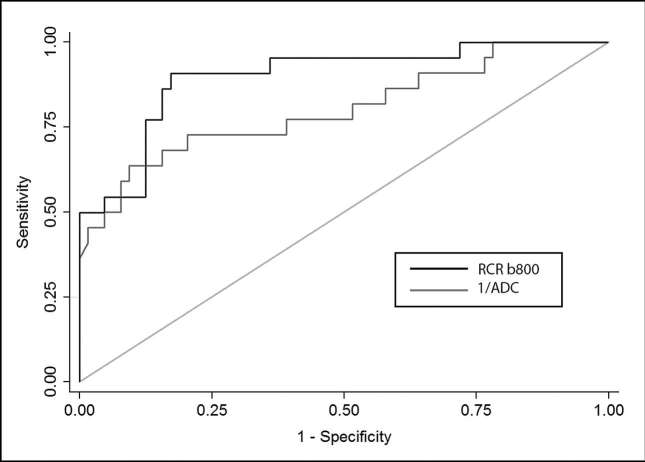
On the ROC analysis, with a cut-off value of 3.6 for the RCR-b800 parameter, we found a sensitivity of 90.9%, a specificity of 83%, a positive-predictive value of 64.5%, a negative-predictive value of 96.4% and an accuracy of 85% for differentiating benign from malignant lymph nodes. Similarly, for the ADC parameter, with a cut-off value of 1285, we found a sensitivity of 68.2%, a specificity of 84.6%, a positive-predictive value of 60%, a negative-predictive value of 88.7% and an accuracy of 80.4% for differentiating benign from malignant lymph nodes. No difference was found in the area under the ROC curves for RCR-b800 and ADC parameters (p = 0.11) (Figure 4). Regarding the interreader agreement, the intraclass correlation coefficient was 0.97 [95% CI (0.96–0.98)] for RCR-b800 and 0.93 [95% CI 0.89–0.95)] for ADC values.
DISCUSSION
Our study shows that DWIBS performed at 3 T has a sensitivity of 90.9% and a specificity of 83% for differentiating benign from malignant enlarged chest lymph nodes as compared with pathology as a gold standard.
Multiple modalities have been applied for mediastinal lymph nodes assessment. Standard imaging modalities simply provide morphological criteria for detecting pathological nodes, criteria that cannot determine the type of underlying pathology. The gold-standard modality remains mediastinoscopy, especially in cancer staging, even if not all mediastinal lymph nodes can be accessed, particularly in the para-aortic region and the aorto-pulmonary window.11 Although it has a low mortality and morbidity rate, mediastinoscopy remains an invasive procedure. Thus, our study was performed on enlarged lymph nodes, in order to evaluate the sensitivity and specificity of physiological criteria obtained with a non-invasive imaging method to describe the underlying pathology.
Several non-invasive diagnostic procedures have been used (CT, PET/CT, MRI) for preoperative N-staging and diagnosis. Diffusion-weighted imaging by MRI has several advantages: accessibility, short time required for examination, no irradiation and it does not require contrast injection. DWI has proved particularly interesting as it can detect the differences in the motion of water molecules between oncological and other tissues.12, 13 Cell membranes represent boundaries that hinder the motion of water molecules, being measured by DWI. The resulting information, namely the diffusion-related parameters, such as ADC, are thus, a description of the heterogeneity and the viscosity of the tissue being imaged. We found significantly higher mean ADC values for benign compared with malignant lymph nodes and higher mean RCR-b800 values for malignant compared with benign lymph nodes. The difference in DWI parameters values between malignant and benign inflammatory lymph nodes is considered to be caused by the difference in cellularity and also the structural and microenvironmental features. Malignant lymph nodes have increased cellularity, larger nuclei, with more abundant macromolecular proteins, nuclear cytoplasmic ratios and less extracellular space than benign nodes.7, 14 DWI parameters are not only related to cell density, diffusion of the hydrogen around cells, but also to the microenvironment and the cohesion between cells. Malignant cells would be more cohesive, thus forming cellular aggregates, whereas inflammatory nodes present high level of non-cohesive free cells.6, 14–16
Despite an absence of difference in the area under the ROC curves between ADC and RCR-800, ADC demonstrated a lower sensitivity at the same level of specificity (68.2% vs 90.9% for a specificity of, respectively, 84.4 and 82.7% at maximum accuracy). This may be a result of a lack of accuracy of ADC maps, since the maps were computed using only three values of b (0, 400 and 800). Nevertheless, using additional values of b would increase the duration of examination, which is not always possible for patients in clinical practice and may result in increased motion artefacts. In addition, drawing an ROI was impaired by the lower signal to noise ratio on the ADC map compared with the native diffusion-weighted images at b800.
With standard DWI sequence, Wu and colleagues13 showed in a meta-analysis, an estimated pooled sensitivity of 72% and an estimated pooled specificity of 95% for DWI parameters. Compared with our study, the specificity was higher; nevertheless, the sensitivity was improved with our method. Higher sensitivity is a relevant parameter in clinical routine, particularly in cancer diagnosis. The lower specificity we found using DWIBS may be explained by the fact that we compared malignant and benign lymph nodes, while the meta-analysis compared lymph nodes of patients diagnosed with non-small-cell lung cancer with normal lymph nodes.
We demonstrated a positive-predictive value of 64.5% and an excellent negative-predictive value of 96.4%. Our results are in agreement with Hasegawa and colleagues17, who found a similar high negative-predictive value (97%) of DWIBS imaging for excluding mediastinal lymph node metastases from non-small- cell lung cancer.
Finally, regarding the DWIBS sequence itself, it was proven useful in discriminating metastatic malignant and non-metastatic mediastinal lymph nodes at 1.5 T.16, 18, 19 However, very few studies have reported applications in the thorax at 3 T, since susceptibility effects are stronger at this higher field strength which results in image distortions and signal loss.20 The advantages of the free-breathing DWIBS sequence were recently demonstrated at 3 T for an improved performance compared with conventional DWI in terms of signal-to-background, fat suppression and motion artefacts for assessing mediastinal lymph nodes.12
Our study had the following limitations: it was performed on a relatively small patient population. The quantitative analysis was only done on enlarged lymph nodes. However, this is explained by the fact that only the enlarged nodes were excised as they were the only ones accessible in mediastinoscopy fields. No analysis was performed on small lymph nodes, inaccessible to mediastinoscopy. The inclusion of patients scheduled for mediastinoscopy might represent a bias in the data for malignant cases. However, our objective was to evaluate the sensitivity and specificity of MRI in differentiating between malignant and benign states. MRI results were compared with pathology results, the gold standard for assessing node state. Absence of comparison with PET/CT could be considered a limitation. This was not possible because PET/CT was not available in all our patients owing to limited accessibility of the imaging system. Furthermore, our results were validated against a gold standard. As a matter of technical feasibility for patients, DWIBS was performed over a longer acquisition time, and therefore, the evaluation of tumour heterogeneity may be compromised by the inevitable bulk motion and volume averaging.
In conclusion, the DWIBS may represent a useful adjunctive imaging modality, particularly for diagnosis of benign mediastinal lymph node, and thus may reduce the frequency of futile mediastinoscopy, which remains an invasive procedure. However, larger prospective studies are warranted to assess changes in patient management that may result from routine use of DW-MRI with DWIBS. Cost effectiveness analysis may further strengthen its role in the imaging algorithm of mediastinal lymph nodes assessment.
Figure 3.
Box and whisker plots of relative contrast ratio-B800 and apparent diffusion coefficient of the two histological groups of lymph nodes. The boxes show the 25th and 75th percentile (interquartile) ranges. Median values are shown as a horizontal black bar within each box. The whiskers show levels outside the 5th and 95th percentiles.
Figure 4.
Receiver operating characteristic curves for relative contrast ratio-B800 (black) and apparent diffusion coefficient (plotted as 1/apparent diffusion coefficient, grey). The isoline (reference, light grey) is also provided.
Contributor Information
Monica Sigovan, Email: monica.sigovan1@gmail.com.
Pia Akl, Email: piaakl@gmail.com.
Caroline Mesmann, Email: cmesmann@gmail.com.
Francois Tronc, Email: francois.tronc@chu-lyon.fr.
Salim Si-Mohamed, Email: salimaymeric@gmail.com.
Philippe Douek, Email: douekph@gmail.com.
Loic Boussel, Email: loic.boussel2@gmail.com.
REFERENCES
- 1.McLoud TC, Bourgouin PM, Greenberg RW, Kosiuk JP, Templeton PA, Shepard JA, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology 1992; 182: 319–23. doi: https://doi.org/10.1148/radiology.182.2.1732943 [DOI] [PubMed] [Google Scholar]
- 2.Steinert HC, Hauser M, Allemann F, Engel H, Berthold T, von Schulthess GK, et al. Non-small cell lung cancer: nodal staging with FDG PET versus CT with correlative lymph node mapping and sampling. Radiology 1997; 202: 441–6. doi: https://doi.org/10.1148/radiology.202.2.9015071 [DOI] [PubMed] [Google Scholar]
- 3.Roberts PF, Follette DM, von Haag D, Park JA, Valk PE, Pounds TR, et al. Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg 2000; 70: 1154–9. doi: https://doi.org/10.1016/S0003-4975(00)01769-0 [DOI] [PubMed] [Google Scholar]
- 4.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann Surg Oncol 2013; 20: 1676–83. doi: https://doi.org/10.1245/s10434-012-2799-z [DOI] [PubMed] [Google Scholar]
- 5.Nomori H, Mori T, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, et al. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J Thorac Cardiovasc Surg 2008; 135: 816–22. doi: https://doi.org/10.1016/j.jtcvs.2007.10.035 [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Liu M, Bao J, Xia Y, Zhang J, Zhang L, et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One 2013; 8: . doi: https://doi.org/10.1371/journal.pone.0079008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphries PD, Sebire NJ, Siegel MJ, Olsen ØE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 2007; 245: 848–54. doi: https://doi.org/10.1148/radiol.2452061535 [DOI] [PubMed] [Google Scholar]
- 8.Abdel Razek AA, Gaballa G, Elashry R, Elkhamary S. Diffusion-weighted MR imaging of mediastinal lymphadenopathy in children. Jpn J Radiol 2015; 33: 449–54. doi: https://doi.org/10.1007/s11604-015-0434-1 [DOI] [PubMed] [Google Scholar]
- 9.Usuda K, Maeda S, Motono N, Ueno M, Tanaka M, Machida Y, et al. Diagnostic performance of diffusion-weighted imaging for multiple hilar and mediastinal lymph nodes with FDG accumulation. Asian Pac J Cancer Prev 2015; 16: 6401–6. doi: https://doi.org/10.7314/APJCP.2015.16.15.6401 [DOI] [PubMed] [Google Scholar]
- 10.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22: 275–82. [PubMed] [Google Scholar]
- 11.Gonzalez-Stawinski GV, Lemaire A, Merchant F, O'Halloran E, Coleman RE, Harpole DH, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg 2003; 126: 1900–4. doi: https://doi.org/10.1016/S0022-5223(03)01036-5 [DOI] [PubMed] [Google Scholar]
- 12.Mesmann C, Sigovan M, Berner LP, Abergel A, Tronc F, Berthezène Y, et al. Evaluation of image quality of DWIBS versus DWI sequences in thoracic MRI at 3T. Magn Reson Imaging 2014; 32: 1237–41. doi: https://doi.org/10.1016/j.mri.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 13.Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhang W, et al. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res 2012; 178: 304–14. doi: https://doi.org/10.1016/j.jss.2012.03.074 [DOI] [PubMed] [Google Scholar]
- 14.Matsushima N, Maeda M, Takamura M, Takeda K. Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 2007; 34: 183–9. doi: https://doi.org/10.1016/j.neurad.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Liu H, Bai X, Ye Z, Sun H, Bai R, et al. Differentiation of metastatic from non-metastatic lymph nodes in patients with uterine cervical cancer using diffusion-weighted imaging. Gynecol Oncol 2011; 122: 19–24. doi: https://doi.org/10.1016/j.ygyno.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 16.Yu SP, He L, Liu B, Zhuang XZ, Liu MJ, Hu XS. Differential diagnosis of metastasis from non-metastatic lymph nodes in cervical cancers: pilot study of diffusion weighted imaging with background suppression at 3T magnetic resonance. Chin Med J 2010; 123: 2820–4. [PubMed] [Google Scholar]
- 17.Hasegawa I, Boiselle PM, Kuwabara K, Sawafuji M, Sugiura H. Mediastinal lymph nodes in patients with non-small cell lung cancer: preliminary experience with diffusion-weighted MR imaging. J Thorac Imaging 2008; 23: 157–61. doi: https://doi.org/10.1097/RTI.0b013e318166d2f5 [DOI] [PubMed] [Google Scholar]
- 18.Junping W, Tongguo S, Yunting Z, Chunshui Y, Renju B. Discrimination of axillary metastatic from nonmetastatic lymph nodes with PROPELLER diffusion-weighted MR imaging in a metastatic breast cancer model and its correlation with cellularity. J Magn Reson Imaging 2012; 36: 624–31. doi: https://doi.org/10.1002/jmri.23695 [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Tian J, Liu Y, Li C. Accuracy of diffusion-weighted (DW) MRI with background signal suppression (MR-DWIBS) in diagnosis of mediastinal lymph node metastasis of nonsmall-cell lung cancer (NSCLC. J Magn Reson Imaging 2014; 40: 200–5. doi: https://doi.org/10.1002/jmri.24343 [DOI] [PubMed] [Google Scholar]
- 20.Dietrich O, Reiser MF, Schoenberg SO. Artifacts in 3-T MRI: physical background and reduction strategies. Eur J Radiol 2008; 65: 29–35. doi: https://doi.org/10.1016/j.ejrad.2007.11.005 [DOI] [PubMed] [Google Scholar]