Abstract
Recent results from the Franco-Swiss team of Institute Curie and Centre Hospitalier Universitaire Vaudois demonstrate a remarkable sparing of normal tissue after irradiation at ultra-high dose rate (>40 Gy s−1). The “FLASH” radiotherapy maintains tumour control level, suggesting that ultra-high dose rate can substantially enhance the therapeutic window in radiotherapy. The results have been obtained so far only with 4–6 MeV electrons in lung and brain mouse model. Nevertheless, they have attracted a great attention for the potential clinical applications. Oxygen depletion had been discussed many years ago as a possible mechanism for reduction of the damage after exposure to ultra-high dose rate. However, the mechanism underlying the effect observed in the FLASH radiotherapy remains to be elucidated.
FLASH radiotherapy is attracting great attention in the radiation oncology community following the report from the Franco-Swiss team from Institute Curie and Centre Hospitalier Universitaire Vaudois in a mouse model.1 The authors exposed the thorax of C57 black 6 mice to 4.5 MeV electrons or γ-rays. The two radiation qualities had similar effectiveness in lung fibrogenesis when delivered at the same conventional dose rate of 1.8 Gy min−1. Electrons and γ-rays also had the same effectiveness in controlling orthotopic lung tumours or human tumours (breast or head-and-neck cancers) xenografted in nude mouse. However, electrons delivered in a single short-pulse (<500 ms) at ultra-high dose rate (>40 Gy s−1 = 2400 Gy min−1) produced less pulmonary lesions than conventional dose-rate exposure (Figure 1). In a second study, the authors also found that spatial memory is preserved after whole-brain mouse FLASH irradiation at 10 Gy, whereas the same dose at conventional dose rate totally impairs spatial memory.2
Figure 1.
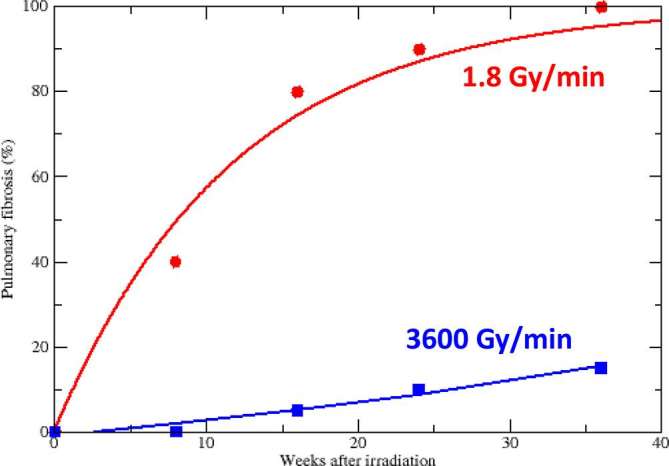
Time dependence of pulmonary fibrosis in C57BL/6J mice after thoracic irradiation at conventional (circles) or ultra-high dose rate (squares). Data points and all details in reference Favaudon et al1 lines are guides for the eye.
The reduction of the normal tissue complication probability (NTCP) associated to FLASH radiotherapy in these experiments1, 2 is very significant (Figure 1), and the potential clinical benefit very large. If tumour control probability (TCP) is unaffected by dose rate, FLASH radiotherapy would consistently widen the therapeutic window TCP-NTCP, thus allowing dose escalation in hypofractionation. Not surprisingly, these results attracted a great interest in the radiotherapy community (e.g. http://www.cancerresearchuk.org/about-us/cancer-news/news-report/2014-07-17-experimental-pulse-radiotherapy-kills-cancer-cells-while-sparing-healthy-tissue) and are stimulating a discussion about the role of dose rate in radiotherapy. Of course, caution is justified. At the moment the experiments come from a cooperation in between two groups using two similar prototypes linear accelerator; they are limited to the mouse model, and to side effects in two parallel organs (lung and brain); dosimetry at very high dose rate is notoriously complicated, but the researchers were very well aware of these problems and have compared four different techniques (ionization chambers, radiochromic films, TLD and alanine pellets);3–5 and, finally, there is not a simple mechanistic interpretation of the data.
Preliminary results from Stanford University in a mouse model irradiated in the abdomen with 20 MeV electrons support the initial results from Europe.6 It is therefore important to speculate on the mechanisms to understand what the impact of FLASH can be in the clinics. The dependence of the response from the dose rate is known since the initial studies of biological effects of radiation. In classical radiobiology, the dose-rate effects are seen when the repair time (from minutes to hours post-exposure) is comparable to the exposure time. At the conventional dose rate for radiobiological studies and therapy, around 1 Gy min−1, the energy is deposited in such a short time that it can be assumed that, at least up to moderate doses, the repair starts only after exposure. All lesions are present at once, and pairwise interaction of the lesions is possible. When the dose rate is reduced, the cell will be able to repair isolated lesions before fixation by pairwise lesion interaction, because the new damage is formed at later time. The intertrack effect, responsible for the quadratic term in the dose-response curve, is reduced while the intratrack damage remains the same. The biological effect is thus reduced. This reduction is mathematical expressed dividing the dose by the dose-rate effectiveness factor (DREF). The value of DREF depends on total dose, as this will change how the overall exposure time compares with the repair time. However, a schematic view of the variation of DREF with the dose rate is given in Figure 2. When the dose rate is low enough, the intertrack contribution is negligible and dose-response curves become all linear. A further reduction of the dose rate will not result in further sparing. Cytogenetic studies suggest that this threshold, or limiting low dose-rate, is around 0.1 Gy h−1 = 0.0017 Gy min−1,7 however at lower dose rates and therefore longer irradiation time other effects such as redistribution of cells through the cell cycle and cell repopulation can also modify the effect. In the clinic these effects are taken into account by using modelling to calculate the biological effective dose as a way of comparing the biological effect of different radiation schedules. Increasing the number of fractions has a sparing effect which is comparable to a reduced dose rate. On the other side of the spectrum, it can be argued that a limiting high-dose rate should also exist, because if all lesions are formed before repair begins, there will no further reduction in the DREF by increasing the dose rate.
Figure 2.
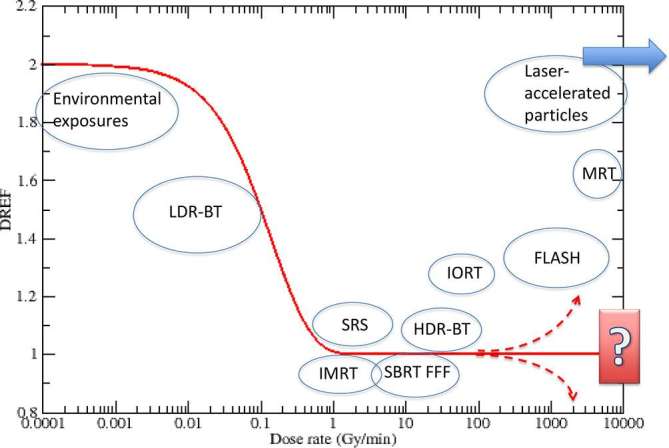
DREF as a function of the dose rate. Different exposure scenarios at different dose-rate levels are shown in the circles. DREF, dose-rate effectiveness factor; HDR-BT, high dose-rate brachytherapy; IMRT, intensity modulated radiotherapy; IORT, intraoperatory radiotherapy; LDR-BT, low dose-rate brachytherapy; MRT, microbeam radiotherapy; SBRT-FFF, stereotactic body radiotherapy flattening filter free; SRS, stereotactic radiosurgery.
In most of the clinical settings shown in Figure 1, no large dose-rate effects are detected. The dose rate in brachytherapy will be highly variable in the tumour volume, depending on the distance from the source, while it is quite constant in teletherapy. However, early experiments in the 1960s found a reduced damage at very high dose rate (around 108 Gy min−1).8, 9 The effect was attributed to oxygen depletion.10 At very high dose rate, a high total dose depletes oxygen too quickly for diffusion to maintain an adequate level of oxygenation, and the normal tissue will respond as a hypoxic tissue. If the total dose is delivered in pulses separated by less than 2–4 s, reoxygenation will not be effective during the exposure. The ultra-high dose rate will therefore deplete oxygen, mimic hypoxia, and therefore increase the radioresistance of the tissue. In a hypoxic (radioresistant) tumour is surrounded by an oxic normal tissue (radiosensitive), ultra-high dose rate will increase the radioresistance of the normal tissue with small impact on the already hypoxic tumour tissue. Hence the widening of the TCP-NTCP window observed in the FLASH experiment.
Nevertheless, the authors of the FLASH experiment have not yet examined the oxygen depletion hypothesis.1 High-dose rates can be achieved in radiotherapy with different techniques: flattening-filter-free (FFF) linear accelerators (up to 30 Gy min−1) are used in stereotactic body radiation therapy (SBRT);11 intraoperative radiation therapy (IORT) delivers a high electron dose during surgery at dose rates up to 75 Gy min−1;12 microbeam radiation therapy, which exploits spatially fractionated beams, uses dose rates greater than 100 kGy min−1 in preclinical studies;13 laser-driven particle accelerators are not yet technologically mature for clinical applications, but they have the potential to perform proton therapy at dose rates >1010 Gy min−1.14 However, most of the experiments designed to study the impact of increased dose rate with these techniques failed to find significant effects. Ideally, any in vitro studies should be performed using physiological relevant oxygen levels. From the theoretical point of view, the spatio-temporal proximity of the tracks must be very high to result in significant effects on the production of radiolytic species.15 Indirect damage to molecules such as DNA is dominated by hydroxyl radicals (.OH), however the highly reactive environment within the cell limits their lifetime to <10−8 s and therefore their diffusion distance to ∼6 to 9 nm.16 Even for laser-accelerated protons with ps pulses, doses exceeding 50 Gy would be necessary to see an impact on the radical yields.15 Therefore, it is difficult to imagine that the chemical stage can be modified by FLASH radiotherapy dose rates, even if most of these arguments apply to in vitro experiments, without simulations of the oxygen effect.
What alternative explanations are possible for the FLASH-effect? The authors of the FLASH experiment point to chromatin remodelling mediated by poly (adenosine diphosphate ribose) polymerase17 or to possible dependence of the inflammatory/anti-inflammatory cell signalling on the overall treatment time. In this line, it should be noted that the high dose rate reduces the fraction of circulating blood cells irradiated, and thus spares the immune system more than fractionated, conventional dose-rate exposure.18 Chromosomal aberrations in circulating blood lymphocytes are indeed strongly dependent on the irradiated volume19 and on the exposure time. In FLASH irradiation the reduction in time will spare many more circulating immune cells. In this case, the effect would be lost for fractionated radiotherapy. Since the mechanisms are not clear it would be interesting to test if the sparing of normal tissue observed for a single high dose-rat exposure are also observed for fractionated protocols, as this would open up the clinical situations where FLASH could be beneficial.
In conclusion, recent evidence of reduced normal tissue toxicity at very high dose rate potentially paves the way to a substantial improvement in radiotherapy. The experiments need to be confirmed, and presently no simple radiobiological explanation for these findings is available.
ACKNOWLEDGMENTS
Authors are grateful to Marie-Catherin Voenin and Michael Cornforth for useful discussions.
Contributor Information
Marco Durante, Email: Marco.Durante@tifpa.infn.it.
Elke Bräuer-Krisch, Email: brauer@esrf.fr.
Mark Hill, Email: mark.hill@oncology.ox.ac.uk.
REFERENCES
- 1.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6. doi: https://doi.org/10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 2.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond J-F, Petit B, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol 2017; 124: 365–9. doi: https://doi.org/10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Jaccard M, Petersson K, Buchillier T, Germond JF, Durán MT, Vozenin MC, et al. High dose-per-pulse electron beam dosimetry: usability and dose-rate independence of EBT3 Gafchromic films. Med Phys 2017; 44: 725–35. doi: https://doi.org/10.1002/mp.12066 [DOI] [PubMed] [Google Scholar]
- 4.Schüler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys 2017; 97: 195–203. doi: https://doi.org/10.1016/j.ijrobp.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 5.Petersson K, Jaccard M, Germond JF, Buchillier T, Bochud F, Bourhis J, et al. High dose-per-pulse electron beam dosimetry - a model to correct for the ion recombination in the advanced markus ionization chamber. Med Phys 2017; 44: 1157–67. doi: https://doi.org/10.1002/mp.12111 [DOI] [PubMed] [Google Scholar]
- 6.Loo BW, Schuler E, Lartey FM, Rafat M, King GJ, Trovati S, et al. (P003) Delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys 2017; 98. doi: https://doi.org/10.1016/j.ijrobp.2017.02.101 [Google Scholar]
- 7.Cornforth MN, Bailey SM, Goodwin EH. Dose responses for chromosome aberrations produced in noncycling primary human fibroblasts by alpha particles, and by gamma rays delivered at sublimiting low dose rates. Radiat Res 2002; 158: 43–53. doi: https://doi.org/10.1667/0033-7587(2002)158[0043:DRFCAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 8.Town CD. Radiobiology. Effect of high dose rates on survival of mammalian cells. Nature 1967; 215: 847–8. [DOI] [PubMed] [Google Scholar]
- 9.Berry RJ, Hall EJ, Forster DW, Storr TH, Goodman MJ. Survival of mammalian cells exposed to x rays at ultra-high dose-rates. Br J Radiol 1969; 42: 102–7. doi: https://doi.org/10.1259/0007-1285-42-494-102 [DOI] [PubMed] [Google Scholar]
- 10.Wilson P, Jones B, Yokoi T, Hill M, Vojnovic B. Revisiting the ultra-high dose rate effect: implications for charged particle radiotherapy using protons and light ions. Br J Radiol 2012; 85: e933–e939. doi: https://doi.org/10.1259/bjr/17827549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stieb S, Lang S, Linsenmeier C, Graydon S, Riesterer O. Safety of high-dose-rate stereotactic body radiotherapy. Radiat Oncol 2015; 10: 27. doi: https://doi.org/10.1186/s13014-014-0317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scampoli P, Carpentieri C, Giannelli M, Magaddino V, Manti L, Moriello C, et al. Radiobiological characterization of the very high dose rate and dose per pulse electron beams produced by an IORT (intra operative radiation therapy) dedicated linac. Transl Cancer Res 2017; 6: S761–S768. doi: https://doi.org/10.21037/tcr.2017.05.21 [Google Scholar]
- 13.Grotzer MA, Schültke E, Bräuer-Krisch E, Laissue JA. Microbeam radiation therapy: clinical perspectives. Phys Med 2015; 31: 564–7. doi: https://doi.org/10.1016/j.ejmp.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 14.Manti L, Perozziello FM, Borghesi M, Candiano G, Chaudhary P, Cirrone GAP, et al. The radiobiology of laser-driven particle beams: focus on sub-lethal responses of normal human cells. J Instrum 2017; 12: C03084–C03084. doi: https://doi.org/10.1088/1748-0221/12/03/C03084 [Google Scholar]
- 15.Kreipl MS, Friedland W, Paretzke HG. Interaction of ion tracks in spatial and temporal proximity. Radiat Environ Biophys 2009; 48: 349–59. doi: https://doi.org/10.1007/s00411-009-0234-z [DOI] [PubMed] [Google Scholar]
- 16.Roots R, Okada S. Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res 1975; 64: 306–20. doi: https://doi.org/10.2307/3574267 [PubMed] [Google Scholar]
- 17.Fernet M, Ponette V, Deniaud-Alexandre E, Ménissier-De Murcia J, De Murcia G, Giocanti N, et al. Poly(ADP-ribose) polymerase, a major determinant of early cell response to ionizing radiation. Int J Radiat Biol 2000; 76: 1621–9. [DOI] [PubMed] [Google Scholar]
- 18.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013; 31: 140–4. doi: https://doi.org/10.3109/07357907.2012.762780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durante M, Yamada S, Ando K, Furusawa Y, Kawata T, Majima H, et al. Measurements of the equivalent whole-body dose during radiation therapy by cytogenetic methods. Phys Med Biol 1999; 44: 1289–98. doi: https://doi.org/10.1088/0031-9155/44/5/314 [DOI] [PubMed] [Google Scholar]


