Abstract
Objective:
The study aimed to prospectively investigate whether uterine leiomyoma greater than 10 cm in diameter could be treated with simple ultrasound-guided high-intensity focused ultrasound (USgHIFU) in one-time treatment.
Methods:
A total of 36 patients with 36 symptomatic uterine leiomyoma greater than 10 cm in diameter who underwent simple USgHIFU treatment alone were analysed. Enhanced MRI was performed before and after HIFU treatment, and all patients had follow-up for 6 months after treatment. Symptom severity scores, treatment time, treatment speed, ablation rate, energy effect ratio, uterine leiomyoma regression rate, adverse events, liver and kidney functions, coagulation function and routine blood count were included in the study endpoints.
Results:
The mean diameter of uterine leiomyoma was 11.2 ± 1.3 cm (10.0–14.3 cm). The median treatment time and treatment speed were 104.0 min (90.0–140.0 min) and 118.8 cm3 h−1 (86.2–247.1 cm3 h−1), respectively. The ablation rate of uterine leiomyoma was 71.9 ± 20.4% (32.1–100.0%), and the regression rate of uterine leiomyoma was 40.8 ± 7.5% (25.6–59.9%) at 6 months after treatment. The mean symptom severity scores decreased by an average of approximately 8.6 ± 2.3 (5–14) points. There were no significant changes in haemogram and blood chemical indexes of patients, except for the transient elevation of aspartate aminotransferase, total bilirubin and white blood cells after treatment. No serious adverse reactions occurred.
Conclusion:
According to our preliminary results, simple USgHIFU is a safe and effective single-treatment method of treating uterine leiomyoma greater than 10 cm in diameter and is an almost innocuous alternative therapeutic strategy.
Advances in knowledge:
The conclusions indicate simple USgHIFU is safe and effective as one-time treatment of uterine leiomyoma greater than 10 cm in diameter, it could be a promising therapeutic strategy.
Introduction
High-intensity focused ultrasound (HIFU) as a new non-invasive treatment for females with symptomatic uterine leiomyoma has been applied widely.1–3 It has the advantages of preserving the uterus, requiring no general anesthesia, requiring no hospital stay, having fewer complications and a quicker recovery, and it is cost effective.4 However, HIFU treatment protocols of previous studies5,6 were restricted by the regulatory agency, the size of uterine leiomyoma was restricted to 10 cm in diameter, and procedure duration was limited to 3 h or less. On the basis of past experience,7 leiomyoma that are larger than 10 cm in diameter may require a longer treatment time. Because the patient is in the prone position and motionless during HIFU treatment, especially during sonication, procedure time should be as short as possible to reduce patient discomfort and the risk of deep vein thrombosis. In previous studies,8,9 when treating leiomyoma greater than 10 cm in size, a gonadotropin-releasing hormone agonist (GnRHa) was used to shrink the leiomyoma before treatment. In a study by Smart et al8 patients with large leiomyoma greater than 10 cm in diameter were pretreated over 3 months with three courses of GnRHa injections prior to HIFU, and the outcome of HIFU treatment was improved significantly in treatment time allowed by treatment protocols. Another way to approach the problem is by performing a second procedure after 3 months, splitting the leiomyoma into a superior and an inferior region for each of the treatments.10 Recently, some scholars adopted improving technology to treat large uterine leiomyoma, such as volumetric MR-guided HIFU ablation with a one-layer strategy. Preliminary research results show that it might be a highly energy- and time-efficient method.11
Whether patients received the combination of HIFU and GnRHa treatments or two sessions of HIFU treatment, the treatment cycle was prolonged and the medical cost to patients was increased. However, volumetric MR-guided HIFU treatment is in a process of perfection and still waits for more efforts to update. At present, the safety and effectiveness of simple ultrasound-guided high-intensity focused ultrasound (USgHIFU) for large uterine leiomyoma (Diameter > 10 cm) is no correlated study. The aim of this study was to evaluate whether uterine leiomyoma greater than 10 cm in diameter could be treated with simple USgHIFU in one treatment.
methods and materials
Patients and enrollment
This prospective study was approved by the institutional review board of the First Affiliated Hospital of Xinxiang Medical University, and written informed consent for the USgHIFU procedure and for the future use of the data collected was obtained from all patients. A total of 36 patients with 36 symptomatic uterine leiomyoma greater than 10 cm in diameter who rejected operation and medication, and underwent simple USgHIFU treatment at the First Affiliated Hospital of Xinxiang Medical University from January 2013 to January 2016 were retrospectively analysed.
Inclusion criteria for USgHIFU therapy of uterine leiomyoma were as follows: (a) premenopausal female aged 18 to 50 years; (b) uterine leiomyoma greater than 10 cm and less than 15 cm in maximum diameter on T2-weighted MRI and no invasive treatment (e.g. any type of surgery, uterine artery embolization, cryoablation, microwave or radiofrequency ablation) before HIFU procedure; (c) not currently pregnant; (d) no contraindications to MRI and MR contrast agents; (e) no evidence of spontaneous coagulation necrosis in the uterine leiomyoma as shown on the contrast MRI; (f) could easily communicate with the physician during the HIFU procedure; (g) the location and scope of the leiomyoma and normal tissues around the leiomyoma were clearly visualized on the two-dimensional ultrasound images and (h) no uncontrolled critical basic diseases.
USgHIFU treatment
The procedure was performed with a HIFU tumor therapeutic system [JC, Chongqing Haifu (HIFU) Tech Co., Ltd., Chongqing, China] by the same operator, and the instrument was guided by B-mode ultrasound (Esaote MyLab 70, Italy). All patients only take one treatment session. The transducer was 20 cm in diameter, with an operating frequency of 0.8 MHz, range of power of 0–400 W, and a focal length of 15 cm; and the dimensions of the focal region were 1.5 × 1.5 × 10 mm.
All patients received careful bowel and skin preparation before treatment. Bowel preparation included liquid food for 3 days, 12 h fasting before treatment and a cleansing enema 2 h prior to procedure. Skin preparation included shaving the hair of the anterior abdominal from the umbilicus to the superior border of the symphysis pubis, degreasing, and degassing the skin of the anterior abdominal wall. During treatment, a urinary catheter was inserted into the bladder and degassed normal saline was filled in to regulate the bladder volume.
All the patients finished the treatment under intravenous conscious sedation (midazolam, 0.8–1 mg kg–1; fentanyl, 0.02–0.03 mg kg–1; repeat administration at 30 to 40 min intervals). The patients were treated in the prone position on the HIFU table. Treatment began from the dorsal of uterine leiomyoma to the ventral and the distances between the focus and the boundary of junctional and endometrium were at least 1 and 1.5 cm, respectively. The procedure was repeated section by section basis to achieve complete ablation of the planned, the thickness of one section was 5 mm. The treatment area was dynamically adjusted according to changes in gray scale on ultrasound imaging, which were the areas of coagulative necrosis. Once the gray scale covered the planned treatment leiomyoma volume, the treatment was ended (Figure 1). The treatment session was monitored by ultrasound throughout the treatment process.
Figure 1.
Ultrasound images that show the ablation volume during the real-time USgHIFU; (a) ultrasound image shows a uterine leiomyoma with hypoecho before treatment; (b) gray scale changes and hyperechoic area was observed during treatment; (c) hyperechoic area covered whole uterine leiomyoma after treatment. USgHIFU,ultrasound-guided high-intensity focused ultrasound.
After treatment, all females were required to lie on prone position for 2 h in observation room, receive oral prophylactic antibiotics for 3 days after treatment and take contraceptive measures within 6 months after treatment.
Pre-therapeutic and post-therapeutic assessment
A series of standard T1 weighted imaging (repetition time/echo time (TR/TE), 280/2.0; flip angle, 15°; slice thickness, 8 mm; matrix size, 180 × 390 mm), T2 weighted imaging (TR/TE, 3,500/100; slice thickness, 8 mm; matrix size, 220 × 320 mm) and enhanced T1 weighted imaging (TR/TE, 3,600/100; slice thickness, 6 mm; matrix size, 200 × 320 mm) were performed 1 week before and after treatment and 6 months after procedure by a 1.5 T MR unit (Signa Excite-II, General Electric Company, Milwaukee, WI) pre-operatively and post-operatively. The images were read by two experienced radiologists to evaluate the size of the uterine leiomyoma, when there was a discrepancy, we turned to chief doctor of department and chief’s decision to be final. The volume of leiomyoma and non-perfused volume of leiomyoma (i.e. the coagulative necrosis after treatment) were measured in three dimensions: longitudinal (d1), anteroposterior (d2) and transverse (d3); the formula was: V = 0.5233 × d1 × d2 × d3.
Primary study endpoints included treatment time (the time from the first to the last sonication), treatment speed (the immediate non-perfused volume divided by treatment time), ablation ratio (the non-perfused volume divided by the leiomyoma volume on enhanced images after HIFU treatment), and energy effect ratio (the joules of energy needed to ablate 1 mm3 of leiomyoma tissue), symptom severity scores (SSS, eight questions; scale of each item was 1–5 points), UFS-QOL (The Uterine Fibroid Symptom and Quality of Life) scores, changes and leiomyoma regression rate after 6 months, and serious adverse events included burns, nerve damage and intestinal necrosis. The degree of severity of adverse effects is based on the unified standardized Society of Interventional Radiology (SIR) grading system.
The secondary end points included, at 1, 3, and 7 days after treatment, the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), serum creatinine, blood urea, and total bilirubin (TB) in the serum samples, which were measured by autobiochemistry at baseline; And prothrombin times, which were evaluated by test tube method, white blood cells (WBC), haemoglobin and blood platelets, which were analysed by blood cell analyser.
Patients underwent plain and enhanced MRI scan immediately, 6 months after the operation, respectively, to evaluate postoperative ablation rate, leiomyoma regression rate; The occurrence of an adverse reaction was evaluated using the classification standard of SIR complications immediately and at 6 months after HIFU treatment; Symptom severity scores (SSS) and UFS-QOL scores were records before procedure and at 6 months after procedure.
The more common and milder adverse events were various pain conditions such as abdominal pain, sacrococcyx pain, hip pain, and radiating pain in the lower limbs. And they also belonged to secondary end points. The visual analog scale was used to assess the pain severity (0–10 points, 0: no pain, 10: the worst pain ever felt) immediately after treatment by doctor.
Statistical analysis
The data were statistically analyzed using SPSS19.0 software statistical bag (SPSS, IBM Company, Armonk, NY). Normally distributed data were reported as the mean ± standard deviation (SD); non-normally distributed data were reported as median and interquartile range. Pearson correlation test and paired t-test were used for statistical analyses after checking for normally distributed data; the Mann–Whitney U test was used after checking for non-normally distributed data. A p-value of less than 0.05 was considered statistically significant.
Results
Patients and leiomyoma
A total of 36 females were successfully treated with just a single session. The mean age of the study participants was 35.9 ± 8.7 years (range, 21–50 years). The median maximum diameter of leiomyoma was 11.2 ± 1.3 cm (range, 10.0–14.3 cm), and the median volume of leiomyoma was 373.9 cm3 (range, 303.3–595.9 cm3) (Table 1). The baseline levels of ALT, AST, serum creatinine, blood urea, total bilirubin, prothrombin time, leukocyte count, haemoglobin and blood platelets are provided in Table 2. All the indexes were in the normal range.
Table 1.
Baseline data of patients in USgHIFU
| Variable | Value |
| Cases | 36 |
| Age (Y) | 35.9 ± 8.7 (21–50) |
| BMI (kg m–2) | 21.3 ± 3.0 (15.6–28.2) |
| SSS | 30.5 ± 4.1 (25–38) |
| Leiomyoma location: AW/PW/LWa (n) | 13/13/9 |
| Leiomyoma type (IMF/SMF/SSF)b | 22/3/10 |
| Leiomyoma max diameter (cm) | 11.2 ± 1.3 (10.0–14.3) |
| Leiomyoma volume(cm3) | 373.9 (303.3–595.9) |
| UFS-QOLc | 115.5 ± 32.8 (92–138) |
BMI, body mass index; SSS, symptom severity scores; USgHIFU, ultrasound-guided high-intensity focused ultrasound.
aAW, anterior wall; PW, posterior wall; LW, lateral wall.
bIMF, intramural fibroid; SMF, submucous fibroid; SSF, subserous fibroid.
cThe Uterine Fibroid Symptom and Quality of Life.
Table 2.
USgHIFU treatment results of lesions
| Variable | Value |
| Treatment time (min) | 104.0 (90.0–140.0) |
| Treatment speed (cm3 h−1) | 118.8 (86.2–247.1) |
| Energy effect ratio (KJ cm–3) | 3.0 ± 1.4 (0.9–4.6) |
| Ablation rate (%) | 71.9 ± 20.4 (32.1–100.0) |
| Regression rate at 6 months after treatment (%) | 40.8 ± 7.5 (25.6–59.9) |
| Changes in SSS at 6 months | 8.6 ± 2.3 (5–14) |
| Changes in UFS-QOL at 6 months | 38.5 ± 16.1 (17–57) |
| Pain score (point) | 3.0 (2–3.75) |
| Incidences of adverse event (SIR-C) | 0 |
Post-procedure evaluation
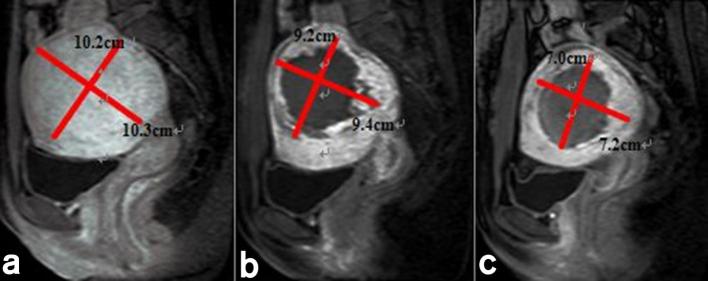
The acoustic sonication power was 400 W. The median treatment time and treatment speed were 104.0 min (range, 90.0–140.0 min) and 118.8 cm3 h–1 (range, 86.2–247.1 cm3 h–1). The mean ablation ratio for all leiomyoma was 71.9 ± 20.4% (range, 32.1–100.0%) (Figure 2). The mean energy efficiency ratio was 3.0 ± 1.4 KJ cm–3 (range, 0.9–4.6 KJ cm–3) in all leiomyoma. The average regression rate and decrease in SSS and UFS-QOL at 6 months after treatment were 40.8 ± 7.5% (range, 25.6–59.9%) and 8.6 ± 2.3 points (range, 5–14 points), 38.5 ± 16.1 (range,17–57 points) (Table 2). A non-perfusion area, which the treated area of leiomyoma seen on contrast MR images had no enhancement, continued in the leiomyoma more than 1 year and was gradually absorbed and metabolized by the body (Figure 2).
Figure 2.
Contrast-enhancement MRI before and after USgHIFU treatment: (a) uterine leiomyoma has evident enhancement before procedure; (b) the non-perfusion area is visible inside uterine leiomyoma on contrast-enhanced MRI immediately after procedure; (c) uterine leiomyoma clearly reduced in size at 6 months on contrast-enhanced MRI. USgHIFU, ultrasound-guided high-intensity focused ultrasound.
On day 1 after HIFU treatment, the levels of AST, TB and WBC were 24.0 ± 6.4 U l−1 (range, 16.7–40.8 U l−1), 12.9 ± 5.3 μmol l–1 (range, 6.3–23.0 μmol l–1) and 7.2 ± 2.0 109 l–1L (range, 4.8–11.0 109 l–1), which increased notably compared with the levels before treatment. However, there were no differences found before and after treatment in the other blood parameters (Table 3). On day 3 and day 7 after HIFU treatment, the levels of AST, TB, and WBC returned to baseline. There is no statistical difference in all blood parameters compared with those before treatment (Table 3).
Table 3.
USgHIFU treatment results of lesions
| Variable | Baseline | 1st day | 3rd day | 7thday |
| ALT (U l–1) | 17.9 ± 6.2 (9.3–27.3) | 17.0 ± 7.1 (10.0–31.7) | 17.1 ± 5.8 (10.1–27.2) | 17.1 ± 5.7 (10.3–27.1) |
| AST (U l–1) | 18.9 ± 3.8a(13.0–25.0) | 24.0 ± 6.4a(16.7–40.8) | 20.1 ± 4.4 (14.6–30.7) | 19.1 ± 3.7 (14.4–27.8) |
| TB (umol l–1) | 9.5 ± 4.8b(4.5–19.9) | 12.9 ± 5.3b(6.3–23.0) | 11.0 ± 5.1 (5.1–22.0) | 10.4 ± 4.3 (4.9–20.5) |
| Serum creatinine (μmoll l–1) | 58.7 ± 6.5 (48.5–75.1) | 55.2 ± 8.3 (48.3–76.0) | 58.1 ± 5.9 (48.7–75.8) | 57.9 ± 5.6 (47.5–72.9) |
| Blood ureain (U l–1) | 4.2 ± 1.1 (2.6–6.4) | 3.8 ± 0.8 (2.9–5.7) | 4.1 ± 0.6 (2.8–6.1) | 4.0 ± 0.5 (3.0–5.9) |
| HB (g l–1) | 120.4 ± 21.5 (86–169) | 121.3 ± 19.0 (91–161) | 121.9 ± 18.3 (94–158) | 120.0 ± 18.9 (93-157) |
| WBC(109 l–1) | 5.8 ± 1.6c(3.5–8.6) | 7.2 ± 2.0c(4.8–11.0) | 6.5 ± 1.9 (5.1–7.4) | 6.0 ± 1.7 (4.0–8.3) |
| PLT(109 l–1) | 231.2 ± 49.6 (146–310) | 227.5 ± 47.6 (154–305) | 230.5 ± 45.5 (158–300) | 231.8 ± 47.1 (159–290) |
| PT (s) | 13.3 ± 0.9 (12.1–14.7) | 13.8 ± 0.7 (12.7–15.1) | 13.6 ± 0.5 (13.1–14.2) | 13.7 ± 1.2 (12.0–14.9) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HB, haemoglobin; PLT, platelet; PT, prothrombin times; SSS, symptom severity scores; TB, total bilirubin; UFS-QOL, The Uterine Fibroid Symptom and Quality of Life; USgHIFU, ultrasound-guided high-intensity focused ultrasound; WBC, white blood cells.
ap = 0.028.
bp = 0.032.
cp = 0.046.
Adverse events
The most frequent adverse events were lower abdominal pain, sacrococcyx pain, hip pain, and radiating pain in the lower limbs, which all occurred within 3 days after treatment. The median pain score was 3 points (range, 2–3.75 points), and no patient reported pain scores higher than 4 points. These adverse events were classified as grade A or B according to the SIR grading system. The other reported SIR-A adverse events were vaginal discharge and reddening of skin in the treatment area; no special management of adverse effects was needed. There were 3 cases of SIR-B degree adverse events. One cases showed skin bubbles, and required frequent local dressing changes for 1 week before recovery. One case exhibited numbness on one of the lower limbs between day 1–3 after operation. The pain eased after taking oral non-steroidal anti-inflammatory analgesics, vitamins and a neurotrophic drug for 7–30 days. One case showed perineum swelling and aching, and recovered after locally administered cold compress and analgesics for 1 week. No patient had higher than grade C complications.
Discussion
Although excellent clinical outcomes have been reported for HIFU in previous research,1–3 it had drawbacks in the treatment of large leiomyoma according to the traditional view. The primary drawbacks were seen as patient tolerance to the treatment and risk of deep vein thrombosis, because of the lengthy procedure time required and no motion allowed during HIFU ablation. Thus, the maximum diameter of leiomyoma was restricted to under 10 cm, and procedure duration was restricted to 3 h or less on the basis of HIFU treatment protocols.5,6 Doctors used to recommend myomectomy as an alternative treatment, and the injury to the body was worse than various minimally invasive treatments.
However, this situation will likely change with the improvement of this technology. In this study, our treatment time was significantly less than 3 h, and was comparable to that for studies using USgHIFU to ablate leiomyoma less than 10 cm in diameter. In previous studies,12,13 the treatment times, which were 106.1 min and 92 min, were significantly shorter than those in studies11,14 that used MRgHIFU equipment and adopted a new model-volumetric ablation strategy; treatment times were 166.2 min and 131.5 min with the latter treatment. At the same time, treatment speed was obviously improved and was equal to or faster than those for the latest treatment model, which were 110.6 cm3 h–1 and 81.8 cm3 h–1.11,14 We had better performance with power consumption compared with previous studies (3.0 KJ cm–3 vs 9.1 KJ cm–3),13 showing that the treatment protocol had higher ablation efficiency in contrast to previous studies using the same treatment device. The results of USgHIFU ablation were quite satisfactory and inspirational in this research; the NPV ratio was as high as 71.9%. Kim et al11 and Park et al14 reported that the immediate NPV ratios were 64.2 and 57.4%, which were inferior to our results. Nevertheless, the results of the study demonstrated that USgHIFU treatment resulted in a mean leiomyoma volume reduction of 40.8% at 6 months and continued to be significantly higher than Ikink et al's study (29%) using a volumetric ablation technique for treating uterine leiomyoma.15 As Leblang et al had described, a higher ablation ratio resulted in greater leiomyoma volume reduction and symptom improvement;16 a significant improvement of clinical symptoms and quality of life was seen in our study.
Among the reasons for the good results in our study, the adoption of the USgHIFU system, which uses ultrasound and is equipped with an acoustic lens transducer, provides good real-time performance and higher conversion efficiency compared with the MRgHIFU system, which uses MRI as monitoring apparatus and is equipped with a multi-element phased array transducer.17,18 Therefore, therapeutic efficacy was improved. Second, large leiomyoma are often accompanied by poor blood circulation, tissue denaturation, and sparing cellularity;19,20 the energy from this system is easy to deposit directly into the leiomyoma. The acoustic environment of leiomyoma tissue outside of the focus region would have changed with the rising temperature, expanding the area of necrosis in the target region. Laboratory tests showed that it takes more energy to ablate a mass lesion than that needed to ablate slice lesions.21 Thus, even if we used the same device and treatment protocol, leiomyoma greater than 10 cm in diameter were much easier to ablate.
In this study, after treatment, the levels of serum AST and TB increased markedly at day 1, but they were still in the normal range. Levels of WBC had similar changes, whereas the other detection indexes for patients had no significant change from pre-operation levels. Because the uterine leiomyoma consisted of great numbers of smooth muscle cells, necrotic smooth muscle cells could release ALT into the blood system in a short span of time after surgery, leading to the transient elevation of AST. At the same time, the damage to microvasculature and the red blood cell destruction in the treated areas could result in the transient elevation of TB. Yet, the transient elevation of WBC was related to coagulation necrosis of leiomyoma tissue. All aforementioned indexes returned to baseline without any treatment at day 3, and caused no discernable harm to any of the patients.
Concerning the safety of USgHIFU, only minor adverse events were observed during the procedure. Adverse events consisted primarily of various pains, which almost immediately disappeared after treatment, returning the pain scores to near pre-treatment levels.12 No serious adverse effects occurred, corresponding to the unified standardized SIR grading system.22
There were several limitations to our study. First, this was a small single-center retrospective trial without randomization, and there was no control group to assess the impact of the treatment. In addition, biases may be present in the patient selection; e.g. leiomyoma were less than 15 cm in diameter. Second, we only included the 6 month follow-up results; long-term outcomes need to be determined in future studies.
In conclusion, based on our preliminary results, simple USgHIFU is safe and effective as a single treatment of uterine leiomyoma larger than 10 cm in diameter and provides a non-surgical alternative therapeutic strategy.
ACKNOWLEDGMENTS
The authors thank Chongqing Medical University which provided technical support to this work.
Contributor Information
Ruijie Hou, Email: hourj1208@163.com.
Liwei Wang, Email: 75048914@qq.com.
Shaoping Li, Email: fuyibingqu@163.com.
Fengmin Rong, Email: rfm2008yeahnet@163.com.
Yuanyuan Wang, Email: wyy15973143013@163.com.
Xuena Qin, Email: 184180300@qq.com.
Shijin Wang, Email: 13569858153@163.com.
REFERENCES
- 1.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009; 34: 584–9. doi: https://doi.org/10.1002/uog.7455 [DOI] [PubMed] [Google Scholar]
- 2.Qin J, Chen JY, Zhao WP, Hu L, Chen WZ, Wang ZB. Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet 2012; 117: 273–7. doi: https://doi.org/10.1016/j.ijgo.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Qin J, Wang L, Chen J, Chen W, Tang L. Effect of high-intensity focused ultrasound on sexual function in the treatment of uterine fibroids: comparison to conventional myomectomy. Arch Gynecol Obstet 2013; 288: 851–8. doi: https://doi.org/10.1007/s00404-013-2775-2 [DOI] [PubMed] [Google Scholar]
- 4.Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser 2015; 15: 1–86. [PMC free article] [PubMed] [Google Scholar]
- 5.Taran FA, Hesley GK, Gorny KR, Stewart EA. What factors currently limit magnetic resonance-guided focused ultrasound of leiomyomas? A survey conducted at the first international symposium devoted to clinical magnetic resonance-guided focused ultrasound. Fertil Steril 2010; 94: 331–4. doi: https://doi.org/10.1016/j.fertnstert.2009.02.083 [DOI] [PubMed] [Google Scholar]
- 6.Chen WZ, Tang LD, Yang WW, Zhang Y, Li J, Xia WX, et al. Study on the efficacy and safety of ultrasound ablation in treatment of uterine fibroids. Zhonghua Fu Chan Ke Za Zhi 2010; 45: 909–12. [PubMed] [Google Scholar]
- 7.Gorny KR, Woodrum DA, Brown DL, Henrichsen TL, Weaver AL, Amrami KK, et al. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol 2011; 22: 857–64. doi: https://doi.org/10.1016/j.jvir.2011.01.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smart OC, Hindley JT, Regan L, Gedroyc WG. Gonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomata. Obstet Gynecol 2006; 108: 49–54. doi: https://doi.org/10.1097/01.AOG.0000222381.94325.4f [DOI] [PubMed] [Google Scholar]
- 9.Smart OC, Hindley JT, Regan L, Gedroyc WM. Magnetic resonance guided focused ultrasound surgery of uterine fibroids-the tissue effects of GnRH agonist pre-treatment. Eur J Radiol 2006; 59: 163–7. doi: https://doi.org/10.1016/j.ejrad.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 10.Fennessy FM, Tempany CM, McDannold NJ, So MJ, Hesley G, Gostout B, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-results of different treatment protocols. Radiology 2007; 243: 885–93. doi: https://doi.org/10.1148/radiol.2433060267 [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Kim JH, Rhim H, Lim HK, Keserci B, Bae DS, et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: initial clinical outcomes. Radiology 2012; 263: 600–9. doi: https://doi.org/10.1148/radiol.12111707 [DOI] [PubMed] [Google Scholar]
- 12.Zhao WP, Chen JY, Zhang L, Li Q, Qin J, Peng S, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol 2013; 82: e43–9. doi: https://doi.org/10.1016/j.ejrad.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 13.Peng S, Zhang L, Hu L, Chen J, Ju J, Wang X, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine 2015; 94: e650. doi: https://doi.org/10.1097/MD.0000000000000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park MJ, Kim YS, Keserci B, Rhim H, Lim HK. Volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids: treatment speed and factors influencing speed. Eur Radiol 2013; 23: 943–50. doi: https://doi.org/10.1007/s00330-012-2665-1 [DOI] [PubMed] [Google Scholar]
- 15.Ikink ME, Voogt MJ, Verkooijen HM, Lohle PN, Schweitzer KJ, Franx A, et al. Mid-term clinical efficacy of a volumetric magnetic resonance-guided high-intensity focused ultrasound technique for treatment of symptomatic uterine fibroids. Eur Radiol 2013; 23: 3054–61. doi: https://doi.org/10.1007/s00330-013-2915-x [DOI] [PubMed] [Google Scholar]
- 16.LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol 2010; 194: 274–80. doi: https://doi.org/10.2214/AJR.09.2842 [DOI] [PubMed] [Google Scholar]
- 17.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005; 5: 321–7. doi: https://doi.org/10.1038/nrc1591 [DOI] [PubMed] [Google Scholar]
- 18.Ter Haar G. HIFU tissue ablation: concept and devices. Adv Exp Med Biol 2016; 880: 3–20. doi: https://doi.org/10.1007/978-3-319-22536-4_1 [DOI] [PubMed] [Google Scholar]
- 19.Ueda H, Togashi K, Konishi I, Kataoka ML, Koyama T, Fujiwara T, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics 1999; 19 Spec No: S131–S145. doi: https://doi.org/10.1148/radiographics.19.suppl_1.g99oc04s131 [DOI] [PubMed] [Google Scholar]
- 20.Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol 2015; 41: 423–31. doi: https://doi.org/10.1016/j.ultrasmedbio.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 21.Li F, Wang Z, Du Y, Ma P, Bai J, Wu F, et al. Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2006; 23: 839–43. [PubMed] [Google Scholar]
- 22.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009; 20(7 Suppl): S377–S390. doi: https://doi.org/10.1016/j.jvir.2009.04.011 [DOI] [PubMed] [Google Scholar]