Abstract
Human adenovirus (HAdV) is one of the most feared infections among immunocompromised patients. In particular, in liver transplant patients, HAdV has been implicated in acute liver failure with resultant mortality. The development of current molecular techniques and surveillance testing protocols have provided tools for early detection of HAdV infection, prior to or at the early onset of HAdV disease. Although reduction in immune suppression is the mainstay of therapy, many researchers have also advocated for early administration of antiviral therapy. In multiple reports, cidofovir treatment has been associated with declines in HAdV viral loads or clinical improvement in solid organ and bone marrow transplant recipients. However, there have also been case reports that raise questions about the effectiveness of antiviral therapy in controlling systemic HAdV disease. We report a case of a 26-month-old male recipient of a liver transplantation for hepatoblastoma who developed adenoviremia with an associated hepatitis and gastroenteritis. He recovered with reduced immune suppression but without antiviral therapy, thus avoiding potential toxicities associated with cidofovir therapy. This case a contrast to previous reports, and it highlights the ambiguity regarding which patients should receive HAdV-specific antiviral therapy. Additional knowledge regarding specific pediatric host factors and HAdV factors that predict poor outcomes are needed. Such information would allow clinicians to better stratify patients by risk at the time of adenoviremia detection so that low-risk patients are not unnecessarily exposed to medications with potential toxicities.
Keywords: adenovirus, cidofovir, orthotopic liver transplant.
INTRODUCTION
Human adenovirus (HAdV) infections often result in self-limiting disease (including upper respiratory infections, conjunctivitis, hepatitis, and gastroenteritis) among immunocompetent hosts. However, HAdVs have emerged as significant viral pathogens in solid organ transplant (SOT) recipients and can cause morbidity and mortality and impact graft survival [1]. Michaels et al. [2, 3] retrospectively reviewed the posttransplant experience of 484 pediatric liver transplant recipients from whom surveillance viral polymerase chain reactions (PCRs) were obtained. Human adenovirus was the 3rd most common viral pathogen detected and was found in 10% of patients. The clinical experience of these patients ranged from asymptomatic shedding to self-limiting illness to adenoviral hepatitis or pneumonia resulting in death or the need for re-transplantation. Human adenovirus has been similarly implicated as a pathogen in other pediatric SOT recipients [4].
In the past 2 decades, diagnostic modalities such as PCR have improved the ability to readily identify the presence of HAdV in patient specimens before and after the onset of signs for disease. Unfortunately, there are limited data on the effectiveness of antiviral therapies such as cidofovir when HAdV is detected. Based primarily on data from hematopoietic stem cell transplant (HSCT) recipients, experts have endorsed the use of antiviral therapy when HAdV is detected in immunocompromised patients, suggesting the benefit of such an intervention compared with historical controls [5, 6]. In this report, we present a case of HAdV viremia with associated prolonged fever, hepatitis, and gastroenteritis in an immune-suppressed pediatric liver transplant patient who improved without antiviral therapy. We also discuss challenges in therapeutic decisions for HAdV in this setting.
Case Report
A 26-month-old white male with a history of hepatoblastoma, for which he received an orthotopic ABO compatible whole liver transplant at 25 months of age, presented with fever and nonbloody diarrhea. He was originally admitted to an outside institution at day 7 with an absolute neutrophil count (ANC) of 214 (cells/mm3), an absolute lymphocyte count (ALC) of 990 (cells/mm3), alanine aminotransferase (ALT) of 485 U/L (normal, 5–45 U/L), and an aspartate aminotransferase (AST) of 242 U/L (normal, 20–60 U/L). Blood, urine, and stool cultures were obtained, and he was empirically started on ceftazidime and then later switched to meropenem. A computed tomography (CT) scan of the abdomen revealed only a small fluid collection behind the left lobe of the liver, which is consistent with postsurgical changes. The patient remained clinically stable, but fevers persisted with diarrhea for 7 days despite negative stool cultures. He was then transferred to our institution for further investigation. His peritransplant hepatoblastoma chemotherapy regimen that was started 3 months before transplantation included vincristine, doxorubicin, 5-fluorouracil, and cisplatin. His last chemotherapy course was completed 2 days before admission to the outside hospital. His current immune suppressive therapy included tacrolimus, which had been increased from 2 mg to 2.5 mg every 12 hours 2 weeks before admission for a low tacrolimus level of 6.4 ng/mL (target of 9–12 ng/mL). The patient was also receiving 3 mg of prednisone daily, and his antimicrobial prophylaxis included acyclovir and trimethoprim-sulfamethoxazole.
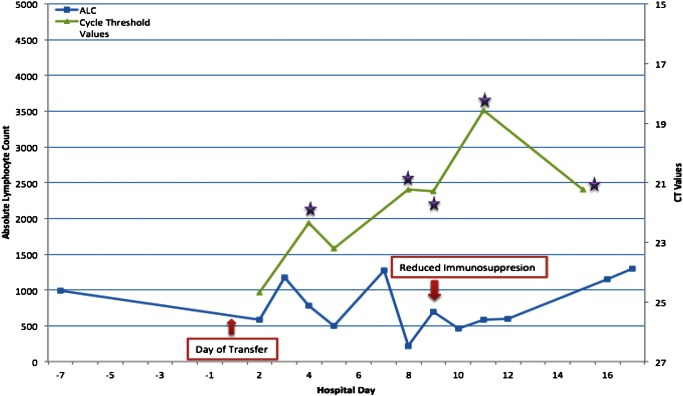
On day 1 (day of transfer), the patient developed nonbloody, nonbilious emesis along with persistent fevers, Tmax of 39.8°C, and diarrhea. He appeared to be ill and had an ANC of zero and an ALC of 588 cells/mm3 but was otherwise stable. Vancomycin, caspofungin, and granulocyte-colony stimulating factor were added for prolonged fever and neutropenia. Plasma PCR test for HAdV, CMV, human herpes virus 6 (HHV-6), and stool PCR test for norovirus were ordered. Of these, HHV-6 and HAdV blood PCR studies were positive. The patient's HHV-6 viral load was 326 copies/mL, and this measurement was considered insignificant. His HAdV DNA PCR cycle threshold (CT) value was 24.68, suggesting a significant blood viral load. The ALT and AST at the time of transfer had improved to 91 U/L and 90 U/L, respectively. Over the next 3 days, his fevers and diarrhea persisted and he remained neutropenic and thrombocytopenic. The patient's ALC remained low until day 15 of fever (Figure 1). Concomitant to his low ALC was an increase in blood HAdV viral load to a CT value of 18.58. In addition, his stool tested positive for HAdV by PCR with a CT value of 17.46, which suggests HAdV as the etiology of his gastroenteritis. Given the persistent fever, worsening HAdV viral load, and progressive lymphopenia, initiation of antiviral therapy with intravenous cidofovir was discussed among the liver transplant, oncology, and infectious disease teams. The care team was reassured by his stable clinical appearance and stable liver transaminases (ALT 74 U/L and AST 71 U/L), and thus they elected to continue symptomatic care and not to administer cidofovir. In addition, his tacrolimus dose was decreased to 1.5 mg every 12 hours on day 2 and then again to 0.5 mg every 12 hours on day 9 to achieve a target tacrolimus level of 5 ng/mL or less to reduce his immune suppression.
Figure 1.
Adenovirus plasma PCR cycle threshold (CT) values and abolsute lymphocyte counts by hospital day. *Denotes banked frozen plasma specimens that were successfully cultured and identified as adenovirus type C.
On day 6 of hospitalization, an abdominal CT scan and CT angiogram were performed to evaluate the possibility of fungal disease, given the prolonged fever and neutropenia, and to evaluate the status of the liver graft. These imaging studies were unremarkable. On day 12, his tacrolimus level had decreased to <1.5 ng/mL so the tacrolimus dose was increased to 0.75 mg every 12 hours. On day 16, the patient's AST and ALT slightly increased to 204 and 94, respectively, and he was discharged home on day 17 based on resolution of neutropenia, improvement of gastrointestinal symptoms, and a decreasing HAdV viral load. After 2 weeks at home, his fevers and diarrhea resolved and he returned to normal activity. Repeat plasma HAdV testing 6 months after clinical resolution of symptoms was negative. However, he continued to shed HAdV from his stool.
After the patient improved, his inpatient plasma and stool specimens were processed for further analysis and typing. Adenovirus was recovered from 5 of the plasma specimens and the 1 stool specimen. The isolates were subjected to restriction enzyme analysis [7] and molecular typing by PCR amplification and sequencing of hypervariable regions 1–7 of the hexon gene and the fiber gene. All isolates exhibited identical BamHI restriction profiles, identical HAdV-2-like hexon gene sequences spanning hypervariable regions 1–7 (GenBank accession number KC585031), and identical HAdV-2-like fiber gene sequences (GenBank accession number KC585032) supporting their identification as species C HAdV-2 (H2F2).
DISCUSSION
This case highlights a 26-month-old male recipient of a liver transplant who developed HAdV viremia with evidence of mild hepatitis and gastroenteritis and also clinically improved without initiating HAdV-specific antiviral therapy. HAdV disease, including fulminant hepatitis resulting in death, is a potential complication in pediatric immunocompromised patients [4, 8]. The results of case reports and case series have suggested the benefit of early initiation of antiviral therapy such as cidofovir to limit the progression of the HAdV infection [9–11]. Most recently, Cimsit et al. [11] reported on a 16-month-old liver transplant recipient who recovered from adenovirus hepatitis after initiation of cidofovir in conjunction with reduced immunosuppression. The results of other published reports have noted both resolution of asymptomatic adenoviremia without antiviral therapy but also fatal systemic HAdV disease despite antiviral therapy in pediatric SOT and HSCT recipients [12–14]. Our patient was symptomatic (fever and evidence of hepatitis) with his adenoviremia, but he improved with reduction of immune suppression and no antiviral therapy.
It is interesting to note that there are limited comparative studies to support the effectiveness or efficacy of cidofovir for HAdV infection or disease. The temporal improvement of some patients but lack of response in others, as depicted in the aforementioned case reports and case series, raise concerns about the true in vivo effectiveness of cidofovir for the treatment of HAdV. The current American Society of Transplantation guidelines support cidofovir as the drug of choice for disseminated adenovirus disease and reduced immune suppression. However, they note that there are limited data to guide the ideal time for initiation and dose to administer [15]. The guidelines comment that increases in viral load of 0.5 to 1.0 log may warrant HAdV therapy initiation. We chose not to follow the guidelines in this scenario because our patient had a reassuring clinical status and there was some concern regarding cidofovir toxicities such as nephrotoxicity. If we had administered cidofovir to our patient, the posttreatment perception would have been that cidofovir was effective. It is certainly plausible that in the absence of cidofovir, our patient could have had clinical worsening secondary to HAdV. This possibility is in fact the clinical dilemma: in an immunosuppressed patient with HAdV infection or disease, does the potential benefit from cidofovir outweigh the toxicity and, if so, when should it be initiated?
If other less toxic therapeutic options for the treatment of adenovirus existed, a clinician may be more likely to determine that the perceived benefits of starting therapy outweigh the risks. CMX-001 is a lipid conjugate of cidofovir that can be administered orally, has increased in vitro activity against adenovirus, and has decreased nephrotoxicity compared with cidofovir [15]. CMX-001 has rapid cellular uptake by target cells (peripheral blood mononuclear cells) where it is cleaved and phosphorylated to its active form, cidofovir diphosphate [16]. This results in low plasma concentrations of cidofovir [17]. This finding, along with the fact that CMX-001 is not a substrate of human organic anion transporters located in kidney tubules, limits the nephrotoxicity of CMX-001 [18]. CMX-001 is currently in clinical trials that include pediatric HSCT recipients. Assuming that it is found to be effective and safe, CMX-001 will be a welcome option for the treatment of HAdV. In addition, administration of adenovirus-specific cytotoxic T lymphocytes represents a novel option for the treatment of HAdV infection and disease. The American Society of Transplantation guidelines note that there are some convincing data for this intervention [15]. As more data are published supporting this approach, adenovirus specific T-lymphocyte therapy will likely become more readily available, but, at present, it is not an option for most clinicians.
In the interim, the updated American Society of Transplantation guidelines do offer clinicians expert opinion regarding testing and timing for treatment initiation of adenovirus in SOT recipients [15]. The guidelines support the institution of serial PCR testing to detect adenoviremia in otherwise asymptomatic patients. These PCR results should be reported as viral loads so that trends in viral burden can be monitored in correlation with patient characteristics such as degree of immune suppression and clinical status. Cycle thresholds were used in our patient as a proxy for viral load, but actual measurements of copies of virus per milliliter is preferred because it allows the clinician to trend viral burden more accurately.
In previous literature, the potential impact of both host and HAdV type-specific factors was discussed. Patients with more significant immune suppression or greater degree of T cell depletion are considered to be at a higher risk for negative consequences of HAdV infection [5]. Regarding HAdV-specific epidemiology, Michaels et al. [3] suggested that liver transplant patients with HAdV serotype 2 or 5 infections are more prone to fatal HAdV outcomes. Leruez-Ville et al. [11] have suggested that HAdV from species A, B, and C is more likely to cause severe adenovirus disease. In addition, they concluded that earliest initiation of treatment results in improved outcomes. Our patient defies a number of these conventions because he had clinical recovery despite immune suppression, lymphopenia, and a species C HAdV infection. Although it is unlikely that a prospective randomized clinical trial comparing cidofovir with placebo for HAdV infection or disease will ever be performed, further observational investigations in larger cohorts are possible and warranted. Such efforts should focus on establishing criteria that incorporate both host and HAdV factors (eg, species and fiber type), which can more accurately stratify patients based on their risk of morbidity and mortality secondary to HAdV infection.
Acknowledgments
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases at the National Institute of Health [HHSN2722011000040C]. B. T. F. received research funding from Pfizer Pharmaceuticals.
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hoffman JA. Adenovirus infections in solid organ transplant recipients. Curr Opin Organ Transplant. 2009;14:625–33. doi: 10.1097/MOT.0b013e3283324e1b. [DOI] [PubMed] [Google Scholar]
- 2.Ison MG, Green M the AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplant recipients. Am J Transplant. 2009;9 doi: 10.1111/j.1600-6143.2009.02907.x. S161–5. [DOI] [PubMed] [Google Scholar]
- 3.Michaels MG, Green M, Wald ER, Starzl TE. Adenovirus infection in pediatric liver transplant recipients. J Infect Dis. 1992;165:170–4. doi: 10.1093/infdis/165.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman JA. Adenoviral disease in pediatric solid organ transplant recipients. Pediatr Transplant. 2006;10:17–25. doi: 10.1111/j.1399-3046.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 5.Tebruegge M, Curtis N. Adenovirus: an overview for pediatric infectious diseases specialists. Pediatr Infect Dis J. 2012;31:626–7. doi: 10.1097/INF.0b013e318250b066. [DOI] [PubMed] [Google Scholar]
- 6.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–6. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 7.Kajon AE, Erdman DD. Assessment of genetic variability among subspecies b1 human adenoviruses for molecular epidemiology studies. Methods Mol Med. 2007;131:335–55. doi: 10.1007/978-1-59745-277-9_23. [DOI] [PubMed] [Google Scholar]
- 8.Hough R, Chetwood A, Sinfield R, et al. Fatal adenovirus hepatitis during standard chemotherapy for childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2005;27:67–72. doi: 10.1097/01.mph.0000153958.95486.6f. [DOI] [PubMed] [Google Scholar]
- 9.Mateos ME, López-Laso E, Pérez-Navero JL, et al. Successful response to cidofovir of adenovirus hepatitis during chemotherapy in a child with hepatoblastoma. J Pediatr Hematol Oncol. 2012;34 doi: 10.1097/MPH.0b013e318266ba72. e298–300. [DOI] [PubMed] [Google Scholar]
- 10.Leruez-Ville M, Minard V, Lacaille F, et al. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38:45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 11.Cimsit B, Tichy EM, Patel SB, et al. Treatment of adenovirus hepatitis with cidofovir in a pediatric liver transplant recipient. Pediatr Transplant. 2012;16 doi: 10.1111/j.1399-3046.2010.01443.x. E90–3. [DOI] [PubMed] [Google Scholar]
- 12.Walls T, Hawrami K, Ushiro-Lumb I, et al. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis. 2005;40:1244–9. doi: 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 13.Seidemann K, Heim A, Pfister ED, et al. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am J Transplant. 2004;4:2102–8. doi: 10.1111/j.1600-6143.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann G, Heim A, Greil J, et al. Adenovirus infection and treatment with cidofovir in children after liver transplantation. Pediatr Transplant. 2009;13:421–8. doi: 10.1111/j.1399-3046.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Florescu DF, Hoffman JA the AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplantation. Am J Transplant. 2013;13:206–11. doi: 10.1111/ajt.12112. [DOI] [PubMed] [Google Scholar]
- 16.Paolino K, Sande J, Perez E, et al. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J Clin Virol. 2011;50:167–70. doi: 10.1016/j.jcv.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Painter W, Robertson A, Trost LC, et al. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56:2726–34. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of poxvirus treatment. Viruses. 2010;2:2740–62. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]