Abstract
The use of fluoroquinolones differs dramatically between adult and pediatric patients. For adults, they are the leading class of antibiotics prescribed in ambulatory care visits whereas in children, they are the least frequently prescribed class. The reasons for this difference in practice likely relate to perceptions of their potential for harm. This highlights the impact of physicians' perception of direct patient harm in antibiotic decision making which has implications for antibiotic stewardship.
Keywords: antibacterial agents, drug resistance, physician's practice patterns.
Overuse and misuse of antibiotics has contributed to the public health crisis of antibiotic resistance and the specter of untreatable infections [1]. Although local and national campaigns have contributed to declines in antibiotic prescribing for viral conditions such as colds and acute bronchitis where they confer no benefit, overuse for these conditions remains unacceptably high [2]. Another dimension of the overuse problem has emerged: rampant overuse of antibiotic classes with a relatively broad-spectrum of activity (particularly macrolides and fluoroquinolones) instead of guideline-recommended, narrower-spectrum alternatives that are usually equally effective. Some strategies, including education and benchmarking, show promise in reducing overuse [3]. Comparing fluoroquinolone prescribing patterns for children to adults may provide further insight into how to be more effective antibiotic stewards.
WHAT DO PRESCRIBING PATTERNS FOR FLUOROQUINOLONES REVEAL ABOUT STRATEGIES TO IMPROVE ANTIBIOTIC USE?
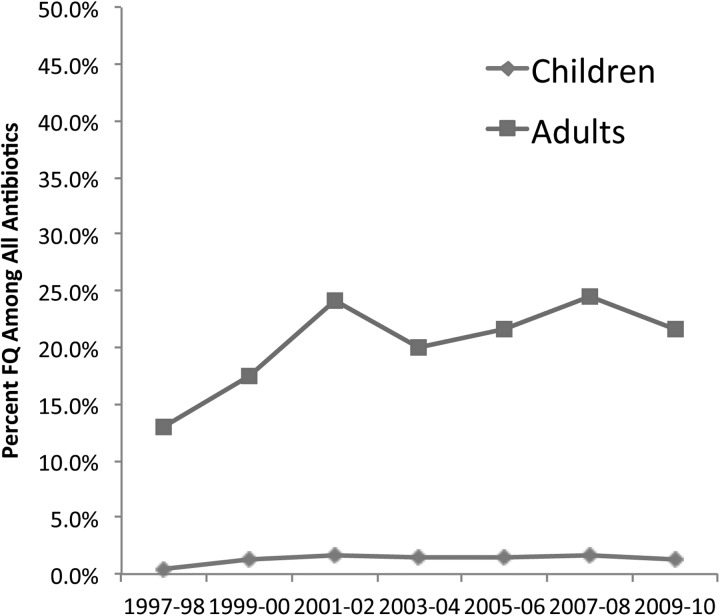
Data from the National Ambulatory and National Hospital Ambulatory Medical Care surveys indicate that fluoroquinolones are the leading class of antibiotics prescribed during adult ambulatory care visits, resulting in over 25 million prescriptions annually [4]. It is easy to understand why: they have convenient dosing regimens, high bioavailability, and the broad spectrum of activity targets the most important pathogens that cause common bacterial infections including community-acquired pneumonia and urinary tract infections. The use of fluoroquinolones is dramatically different among children. Although they are the most frequently prescribed antibiotics for adults, fluoroquinolones are the least frequently prescribed class for pediatric patients, accounting for under 2% of all antibiotics prescribed during ambulatory visits [4]. The extremely limited use in children has remained unchanged for over a decade (Figure 1). This low use of fluoroquinolones is despite the fact that the bacteriology of respiratory tract infections and urinary tract infections is very similar in adults and children.
Figure 1.
Annual percentage of all antibiotics prescribed accounted for by fluoroquinolones (FQs) during ambulatory care visits in the United States. Source: The National Ambulatory Care and Hospital Ambulatory Care Surveys.
WHY DON'T PHYSICIANS PRESCRIBE FLUOROQUINOLONES FOR CHILDREN?
The most likely explanation for why prescribing patterns for fluoroquinolones differ between children and adults is the perception of their potential for harm. A generation of physicians was trained to avoid prescribing fluoroquinolones to children. This practice was due to concerns about potential toxicity to developing cartilage, based largely on animal studies conducted in the 1970s that demonstrated damage to articular cartilage of weight-bearing joints in juvenile beagle dogs exposed to high doses [5]. In children, extensive review of safety data suggests that musculoskeletal adverse events, predominantly arthralgias, may occur slightly more often in children treated with fluoroquinolones. However, serious toxicity, including tendon rupture, is exceedingly rare and almost certainly substantially less likely than in adults. In a 2011 statement, the American Academy of Pediatrics concluded “fluoroquinolones are reasonably safe in children,” and it outlined conditions in which the use of fluoroquinolones was justified [5]. Nonetheless, the perception of risk remains high. It is not uncommon as pediatric infectious disease physicians consulting on patients with infections where fluoroquinolones are the only option to have the referring physician state “I thought we couldn't prescribe those drugs to children.” As a consequence of very limited use, fluoroquinolone resistance for several important organisms has remained very low in children compared with adults.
CHANGE THE MESSAGE: FOCUS ON PREVENTING HARM AND ADVERSE EVENTS
Over the last decade there has been, in effect, a natural experiment of strategies to control fluoroquinolone prescribing. In adults, conventional messaging through guidelines and educational efforts has emphasized the risk of resistance and the limited benefit of antibiotics for many common conditions. The impact on behavior has been modest. In children, the message has been that fluoroquinolones can harm children and the risks may outweigh the benefit. The impact has been striking. Maybe this should come as no surprise. In 1 study, concern about promoting antibiotic resistance was rated as the least important factor contributing to physician decision making about whether to prescribe an antibiotic [6].
Perhaps, as demonstrated by the experience with fluoroquinolones, efforts to curtail antibiotic overuse should more broadly incorporate a stronger focus on reducing direct patient harm, rather than the less tangible (although critically important) issue of antibiotic resistance. There is plenty of harm to consider. Adverse reactions to antibiotics are costly, including causing over 600 000 visits to offices and emergency departments annually [7]. Extended-spectrum β-lactams and cephalosporins, often used unnecessarily for respiratory tract infections, are strongly linked to increased risk of Clostridium difficile infection (CDI), and the rates and severity of CDI are increasing including community-onset cases [8, 9]. Multiple antibiotic classes can cause serious allergic reactions including Stevens Johnson syndrome [10], and macrolides are linked to an increased risk of sudden cardiac death [11]. There is more. As our understanding of the human microbiome advances, it is becoming evident that early and often unnecessary exposure to antibiotic therapy may be linked to an increasing number of chronic diseases such as inflammatory bowel disease [12], asthma [13], and even obesity [14]. The evolving evidence base for the direct patient harm of antibiotic exposure is compelling.
To develop new messages related to antibiotic-associated adverse events, the Centers for Disease Control and Prevention's Get Smart: Know When Antibiotics Work program conducted in-depth interviews with primary care physicians. Physicians reported not discussing the potential for adverse events or side-effects with parents or patients unless asked and perceived that the risk of antibiotic-associated adverse events was low. These findings are reinforced by evidence that physicians sometimes prescribe antibiotics for placebo effect [15].
CONCLUSION
Physicians are taught to weigh risks and benefits, and they are more likely to consider the immediate risk and benefit to the patient in front of them as opposed to future risk, or the more intangible public health threat of antimicrobial resistance. In the case of fluoroquinolone use in children, the true risk may have been lower than the perceived risk, but the impact on behavior is telling. If we better communicate the true risks and benefits of antibiotic use and overuse to patients and physicians, they are likely to make better decisions, especially when treating conditions where the benefits of treatment are modest or nonexistent. Most antibiotics prescriptions for children are for upper respiratory tract infections, the majority of which are viral. Although both physicians and patients have concerns about “missing” a bacterial infection, even for diagnoses in which antibiotics are usually indicated (eg, sinusitis or otitis media), the major benefits are limited to symptom resolution, not prevention of serious sequelae [16]. Sometimes “therapies,” such as chicken soup for colds, are worth trying because they “can't hurt.” Unlike chicken soup, antibiotics can hurt. The lesson of the fluoroquinolone experience—physicians treating children are responsive to concerns about direct patient harms that result from antibiotic use—can be harnessed and broadly applied as a core stewardship strategy.
Acknowledgments
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. The authors report receiving funding from the following sources: Centers for Disease Control and Prevention (CDC) Grant number 1U18IP000491 (to A. L. H., A. T. P.); National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant number 1R01AI089489-01 (to A. T. P.); CDC Contract number 11IPA110369 (to A. L. H.); Primary Children's Foundation (to A. L. H.); Pfizer, Inc (to A. L. H., A. T. P., J. S. G.); Agency for Healthcare Research and Quality Grant number 1R01HS20921-01 (to J. S. G.); and Patient-Centered Outcomes Research Institute Grant number CE-1304-7279 (to J. S. G.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/ . Accessed May 14, 2014. [Google Scholar]
- 2.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory. f58–66. [DOI] [PMC free article] [PubMed]
- 3.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345–52. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69:234–40. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JS, Jackson MA. The use of systemic and topical fluoroquinolones. Pediatrics. 2011;128:e1034–45. doi: 10.1542/peds.2011-1496. [DOI] [PubMed] [Google Scholar]
- 6.Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. 2002;17:87–94. doi: 10.1046/j.1525-1497.2002.10711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901–10. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;(55 Suppl 2):S65–70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics. 2014;33:651–8. doi: 10.1542/peds.2013-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman JL, Jackson MA, Herigon JC, et al. Trends in adverse reactions to trimethoprim-sulfamethoxazole. Pediatrics. 2013;131:e103–8. doi: 10.1542/peds.2012-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–90. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130:e794–803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stensballe LG, Simonsen J, Jensen SM, et al. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–8. doi: 10.1016/j.jpeds.2012.09.049. e3. [DOI] [PubMed] [Google Scholar]
- 14.Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilburt JC, Emanuel EJ, Kaptchuk TJ, et al. Prescribing “placebo treatments”: results of national survey of US internists and rheumatologists. BMJ. 2008;337 doi: 10.1136/bmj.a1938. a1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh AL, Jackson MA, Hicks LA. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132:1146–54. doi: 10.1542/peds.2013-3260. [DOI] [PubMed] [Google Scholar]