Abstract
Objective
Successfully stopping or reducing treatment for patients with rheumatoid arthritis (RA) in low disease activity (LDA) may improve cost-effectiveness of care. We evaluated the multi-biomarker disease activity (MBDA) score as a predictor of disease relapse after discontinuation of TNF inhibitor (TNFi) treatment.
Methods
439 RA patients who were randomized to stop TNFi treatment in the POET study were analyzed post-hoc. Three indicators of disease relapse were assessed over 12 months: 1) restarting TNFi treatment, 2) escalation of any DMARD therapy and 3) physician-reported flare. MBDA score was assessed at baseline. Associations between MBDA score and disease relapse were examined using univariate analysis and multivariate logistic regression.
Results
At baseline, 50.1%, 35.3% and 14.6% of patients had low (<30), moderate (30−44) or high (>44) MBDA scores. Within 12 months, 49.9% of patients had restarted TNFi medication, 59.0% had escalation of any DMARD and 57.2% had ≥1 physician-reported flare. MBDA score was associated with each indicator of relapse. At least one indicator of relapse was observed in 59.5%, 68.4% and 81.3% of patients with low, moderate or high MBDA scores, respectively (P = 0.004). Adjusted for baseline DAS28-ESR, disease duration, BMI and erosions, high MBDA scores were associated with increased risk for restarting TNFi treatment (OR = 1.85, 95% CI 1.00–3.40), DMARD escalation (OR = 1.99, 95% CI 1.01–3.94) and physician-reported flare (OR = 2.00, 95% 1.06–3.77).
Conclusion
For RA patients with stable LDA who stopped TNFi, a high baseline MBDA score was independently predictive of disease relapse within 12 months. The MBDA score may be useful for identifying patients at risk of relapse after TNFi discontinuation.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that can cause joint damage and physical disability [1]. Early detection of RA and the availability of biologic agents have markedly improved outcomes in these patients [2]. Many studies have shown that the use of combinations of conventional synthetic DMARDs (csDMARDs) and biological DMARDs (bDMARDS) such as tumor necrosis factor inhibitors (TNFi) is effective for reaching and maintaining a state of low disease activity (LDA) or remission [3–5]. Once LDA or remission has been reached, patients often continue their combination therapy indefinitely. This practice may lead to overtreatment, as recent studies suggest that in some RA patients the more expensive TNFi can be tapered or stopped [6,7]. However, before implementing this therapeutic strategy in routine care, a validated predictor of disease relapse would be desirable [8].
Several studies have explored predictors of successful TNFi discontinuation. Results varied considerably, possibly due to differences in population, design and definitions of success, but most studies identified deep remission or lower disease activity at the time of discontinuation as a predictor [9–11]. Rheumatoid factor (RF) positivity, shorter disease duration, non-smoking and normal body mass index (BMI) may also be associated with better outcomes [10,12]. Although these studies all found that some patients could discontinue TNFi treatment without flaring, it remains a challenge to accurately predict which patients may successfully discontinue treatment and which are at higher risk of disease relapse [13].
Studies of strategies for reducing DMARD treatment have mainly evaluated the predictive value of conventional clinical measures of disease activity and traditional biomarkers such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA) [10,12,14]. However, new biomarkers with interesting potential have become available. The multi-biomarker disease activity (MBDA) blood test measures 12 serum proteins to produce a score that has been clinically validated as a measure of disease activity in patients with RA [15–17]. MBDA scores have been shown to reflect current clinical disease activity and changes in disease activity over time, including treatment responses in RA patients treated with TNFi [18]. The MBDA score assesses the activity of underlying biologic pathways rather than external signs and symptoms and may therefore provide information that is complementary to clinical assessment [16]. The MBDA score was a more accurate predictor of radiographic progression than the 28-joint Disease Activity Score with C-reactive protein (DAS28-CRP) or CRP, and it is often elevated in patients with low clinical disease activity or low CRP [18–22].
Recently, the MBDA score and ACPA were shown to be predictors of relapse for RA-patients in stable remission when treatments with conventional DMARDs and/or biological DMARDs were tapered, and for some patients, subsequently stopped. Prediction was strongest when MBDA score and ACPA were combined [23]. This finding, in a study of drug tapering, suggests that the MBDA score may be capable of predicting the outcome of complete TNFi discontinuation. The aim of the present study was to examine the prognostic value of the MBDA score for disease relapse after discontinuation of TNFi in RA patients with stable LDA.
Methods
Patient cohort
Data used for these analyses were from the Dutch POET trial (the Netherlands Trial Register, number NTR3112) [24]. The study was approved by the Ethical Review Boards of all participating hospitals. Ethical approval for the study was granted by the Committee on Research involving Human Subjects, region Arnhem-Nijmegen (Commissie Mensgebonden Onderzoek regio Arnhem-Nijmegen) and local feasibility by all regional Ethical Committees. Patients were included from March 2012 to March 2014 and written informed consent was obtained from all patients. In this pragmatic, multicenter, open-label, randomized clinical trial, RA patients with stable LDA were randomized 2:1 to either stop or continue TNFi treatment. Patients were included from March 2012 to March 2014 and written informed consent was obtained from all patients. All participating patients were ≥18 years old, had RA according to ACR 1987 criteria, and had received TNFi treatment for at least one year prior to inclusion. Patients had stable LDA for at least six months, defined as either two DAS28 with erythrocyte sedimentation rate (DAS28-ESR) measurements <3.2 or a rheumatologist clinical impression of remission or stable low disease activity and at least one CRP measurement <10 mg/L in the six months prior to inclusion. Nearly all patients were receiving concomitant csDMARDs. In the 6 months prior to inclusion, no dosage changes were allowed for csDMARDs or corticosteroids.
In total, 531 patients were randomized to stop TNFi treatment in POET and followed for 12 months [24]. Concomitant treatment with csDMARDs was continued. If RA flared (DAS28-ESR ≥3.2 with a change in DAS28-ESR >0.6) [25], TNFi could be restarted at the discretion of the treating rheumatologist. Because the current study focused on the value of the MBDA score as a predictor of disease relapse after discontinuation of TNFi treatment, only data from patients randomized to the stop group were used. For the current analyses, baseline serum samples were available to measure MBDA scores for 439 of the 531 patients in the group that stopped TNFi treatment.
Measurements
Patients were evaluated by their treating rheumatologist and rheumatology nurse at baseline and at least once every 3 months thereafter, for a period of one year. Baseline measures included: age, sex, weight, height, disease duration, medication use, rheumatoid factor (RF) and ACPA status, concomitant use of csDMARDs and, for this post hoc analysis, the MBDA score. Clinical measurements were performed at every scheduled or unscheduled visit and included the ESR (mm/h), CRP (mg/l), 28-joint tender joint count (TJC28), 28-joint swollen joint count (SJC28), and a patient-reported assessment of general health on a 100 mm visual analog scale (VAS-GH). These component measures were combined to calculate DAS28-ESR [26]. Physician-reported flares and all changes in medication were recorded throughout the study.
Serum biomarker measurement and MBDA score calculation
Serum samples were stored at −40°C from time of preparation until transfer to Crescendo Bioscience (South San Francisco, CA, USA), where they were stored at −70°C or lower until biomarker concentration testing was performed in the Crescendo clinical laboratory, which is certified under the CMS Clinical Laboratory Improvement Amendments and accredited by the College of American Pathologists for determination of Vectra® DA scores. Biomarker concentrations were measured by electrochemiluminescence-based multiplexed immunoassays (Meso Scale Discovery, Rockville, MD, USA). The MBDA algorithm combines the concentrations of 12 biomarkers (vascular cell adhesion molecule-1 [VCAM-1], epidermal growth factor [EGF], vascular endothelial growth factor-A [VEGF-A], interleukin [IL]-6, tumor necrosis factor-receptor type 1 [TNF-RI], matrix metalloproteinase [MMP]-1, MMP-3, cartilage glycoprotein 39 [YKL-40], leptin, resistin, serum amyloid A [SAA], and CRP)[15] to generate the MBDA score on a scale of 1 to 100, with previously validated categories for low (<30), moderate (30 to 44) and high (>44) disease activity [16].
Statistical analysis
Baseline demographic and disease-related characteristics were compared between the 439 patients with a baseline MBDA assessment and the 92 patients without an MBDA assessment using independent samples t-tests and Mann-Whitney U tests for normally and non-normally distributed continuous variables, and Pearson χ2 tests for categorical variables. Disease relapse was defined three ways, using the criteria of: 1) restarting TNFi treatment, 2) any DMARD medication escalation and 3) physician-reported flare. DMARD medication escalation was defined as restarting TNFi treatment or starting or increasing the dosing of any bDMARD or csDMARD (including corticosteroids) [24]. Baseline characteristics of patients who did and did not meet the different criteria for relapse within the 12-month follow-up period were first compared using univariate logistic regression analyses. Patients who dropped out before 12 months of follow-up without meeting a criterion for relapse were counted in this analysis with those who continued to have a response. For each criterion for relapse, the proportions of patients in the low (<30), moderate (30–44) and high (>44) MBDA score groups who relapsed were compared by univariate Pearson χ2 tests with Bonferroni adjustment for the number of comparisons (P < (0.05 / 3 =) 0.017). Additional sensitivity analyses were performed limited to those patients that were included based on two available DAS28 scores <3.2 in the six months prior to inclusion, those who met the inclusion criteria for stable LDA but had moderate DAS28-ESR at baseline, and those who were in remission at baseline (DAS28 scores <2.6). One-year relapse-free survival was examined for the low, moderate, and high MBDA score groups using Kaplan-Meier survival curves; patients who dropped out early without disease relapse were censored at the time of withdrawal. Between-group differences in survival were tested by pairwise log-rank tests, again with Bonferroni adjustment for the number of comparisons (P < 0.017). Based on the results of the univariate χ2 tests and Kaplan-Meier survival analyses, baseline MBDA scores were dichotomized as high (>44) vs. moderate-to-low (≤44). Cox proportional hazard regressions were used to estimate the hazard ratio (HR), which may be interpreted as a relative risk, of high vs. moderate-to-low MBDA score for the time to relapse. Next, univariate and multivariable logistic regression analyses were performed to evaluate the association between disease relapse within 12 months and high baseline MBDA score in terms of unadjusted odds ratios (ORs), ORs adjusted for baseline DAS28-ESR score, and ORs further adjusted for all other variables that were significantly (P < 0.05) associated with a relapse criterion in the univariate logistic regression analyses. Final sensitivity analyses were performed in which all patients with a missing visit (missing DAS28 score at 3, 6, 9, or 12 months) were counted as a flare on all flare criteria. All analyses were performed using SPSS version 22.
Results
Demographic and clinical data at baseline
From the 531 patients who were randomized to stop TNFi treatment in POET, baseline serum samples were available for MBDA testing for 439 patients. Among these patients, 356 (81.1%) were included based on at least two available DAS28 scores <3.2, and 83 (18.9%) were included based on the rheumatologist clinical impression of remission or stable low disease activity in combination with at least one available CRP value <10 mg/L. Baseline demographic and clinical data were similar between patients with or without a baseline MBDA sample (Table 1). Patients were typically older Dutch Caucasian females, with longstanding RF-positive, erosive RA. Most patients were receiving their first TNFi, with 51.3% of 439 patients receiving adalimumab, 40.1% receiving etanercept and 8.6% receiving infliximab, certolizumab or golimumab. Clinical disease activity was generally low, in accordance with study inclusion criteria, and 349 (79.5%) patients were in remission (DAS28-ESR <2.6) at baseline. Seventeen (3.9%) patients dropped out during the first 12 months of follow-up because of their own decision to drop out (n = 13) or presence of a comorbidity (n = 4).
Table 1. Baseline characteristics of POET patients grouped according to sample availability for MBDA testing.
| Characteristic (N = 531) | MBDA sample (n = 439) | No MBDA sample (n = 92) | P |
|---|---|---|---|
| Female, n (%) | 296 (67.4%) | 66 (71.7%) | 0.419 |
| Age (yrs.), mean (SD) | 59.8 (10.8) | 61.7 (10.6) | 0.137 |
| Disease duration (yrs.), median (IQR) | 10 (6–17) | 9 (6–16) | 0.535 |
| BMI, mean (SD) | 25.9 (4.3) | 25.9 (4.0) | 0.854 |
| RF positive, n (%) | 270 (67.3%) | 58 (68.2%) | 0.872 |
| ACPA positive, n (%) | 277 (69.1%) | 55 (64.7%) | 0.431 |
| Erosive disease, n (%) | 252 (62.8%) | 53 (62.4%) | 0.932 |
| ESR, median (IQR) | 9.0 (5–17) | 9.5 (5–18) | 0.638 |
| CRP, median (IQR) | 2 (1–5) | 3 (1–5.8) | 0.388 |
| TJC28, median (IQR) | 0 (0–1) | 0 (0–0) | 0.043 |
| SJC28, median (IQR) | 0 (0–0) | 0 (0–1) | 0.328 |
| PGA, median (IQR) | 20.7 (9.0–28.1) | 20.4 (5.0–23.4) | 0.455 |
| DAS28-ESR | 2.0 (0.8) | 1.9 (0.7) | 0.549 |
| MBDA score, mean (SD) | 30.2 (12.6) | - | - |
| Number of TNFi, n (%) | 0.819 | ||
| 1st | 379 (86.5%) | 80 (87.0%) | |
| 2nd | 50 (11.4%) | 11 (12.0%) | |
| 3rd | 9 (2.1%) | 1 (1.1%) | |
| csDMARD, n (%) | 0.581 | ||
| Methotrexate | 382 (87.0%) | 77 (83.7) | |
| Other csDMARD | 35 (8.0%) | 8 (8.7%) | |
| No DMARD | 22 (5.0%) | 7 (7.6%) |
TNFi = tumor necrosis factor-alpha inhibitors; DAS28 = disease activity score in 28 joints; BMI = body mass index; RF = rheumatoid factor; ACPA = anti-cyclic citrullinated peptide antibodies; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; TJC28 = 28-joint tender joint count; SJC28 = 28-joint swollen joint count; PGA = patient global assessment; MBDA = multi-biomarker disease activity; csDMARD = conventional synthetic disease modifying anti-rheumatic drug; SD (standard deviation); IQR (interquartile range).
Association between baseline MBDA score and disease relapse
Baseline MBDA scores were low (<30) in 220 (50.1%) patients, moderate (30−44) in 155 (35.3%) patients, and high (>44) in 64 (14.6%). Clinical disease activity at baseline, as measured with the DAS28-ESR, was low in 413 (94.1%) patients and moderate in 26 (5.9%) patients. Within 12 months, 219 patients (49.9%) had restarted TNFi treatment, 259 (59.0%) patients had escalated any DMARD, and 251 (57.2%) had experienced at least one physician-reported flare. There was no significant difference in the proportion of patients experiencing a relapse between those who were included based on available DAS28 scores and those who were included based on the rheumatologist clinical impression and the CRP value (TNFi restart: 51.4% vs. 43.4%, P = 0.188; medication escalation: 60.1% vs. 54.2%, P = 0.325; physician-reported flare: 57.3% vs. 56.6%, P = 0.911).
There was considerable overlap of the different relapse groups (Fig 1). For example, among the 289 patients who met at least one criterion for relapse, only 12 (4.2%) restarted TNFi treatment without also having a physician-reported flare, while 44 (15.2%) patients had a physician-reported flare but did not restart TNFi treatment; 207 out of 289 (71.6%) of the patients who relapsed met all three criteria. One hundred fifty (34.2%) patients in the overall cohort (N = 439) completed one year without meeting any of the three criteria for relapse.
Fig 1. Venn diagram of patients meeting criteria for disease relapse.

Red = TNFi restart; green = medication escalation; blue = physician-reported flare; yellow = overlap medication escalation / physician-reported flare; grey = overlap medication escalation / TNFi restart. Percentages are for the 289 patients who met at least one of the three criteria of disease relapse.
High MBDA scores (>44) at baseline were univariately associated with significantly (Bonferroni adjusted P < 0.017) greater proportions of patients meeting the criteria for disease relapse (Table 2). At least one criterion of relapse was met within 12 months of TNFi discontinuation by 59.5%, 68.4% and 81.3% of patients with low, moderate, or high baseline MBDA scores, respectively (P = 0.004). Differences in the cumulative 12-month proportions of patients with relapse and the times to event were relatively small between patients with low or moderate MBDA scores, but patients with high MBDA scores were clearly at increased risk.
Table 2. Disease relapse by three criteria at 12 months for patients classified by baseline MBDA score.
| Criterion for relapse | Total | Low (<30); n = 220 | Moderate (30–44); n = 155 | High (>44); n = 64 | P |
|---|---|---|---|---|---|
| TNFi restart | 219 | 102 (46.4%) | 74 (47.7%) | 43 (67.2%) | 0.011 |
| Medication escalation | 259 | 117 (53.2%) | 92 (59.4%) | 50 (78.1%) | 0.002 |
| Physician-reported flare | 251 | 116 (52.7%) | 87 (56.1%) | 48 (75.0%) | 0.006 |
| Any criterion | 289 | 131 (59.5%) | 106 (68.4%) | 52 (81.3%) | 0.004 |
Any criterion = TNFi re-initation, medication escalation, or physician-reported flare. P-value by Pearson χ2 test. Total N = 439.
Differences in disease relapse were very similar when limited to those patients that were included based on two available DAS28 scores <3.2 in the six months prior to inclusion (S1 Table), although no longer significant for TNFi restart after Bonferroni adjustment. Similar results were also obtained in a sensitivity analysis that excluded the 26 patients who met the inclusion criteria for stable LDA but had moderate DAS28-ESR at baseline (S2 Table). Among patients in remission at baseline (DAS28-ESR <2.6), differences between MBDA categories were slightly less pronounced and not significant after Bonferroni correction (S3 Table).
Univariate Kaplan-Meier survival analyses confirmed the significantly decreased one-year relapse-free survival in patients with high baseline MBDA scores (Fig 2). Pairwise differences in one-year relapse-free survival were not significant between patients with low or moderate MBDA scores, but patients with high MBDA scores had significantly (Bonferroni adjusted P < 0.017) worse relapse-free survival than patients with either moderate or low MBDA scores for all three criteria of relapse. The HRs of high vs. moderate-to-low baseline MBDA scores were 1.61 (95% CI 1.15–2.25, P = 0.005) for TNFi restart, 1.66 (95% CI 1.22–2.26, P = 0.001) for medication escalation, and 1.64 (95% CI 1.20–2.25, P = 0.002) for physician-reported flare.
Fig 2. Kaplan-Meier survival curves.
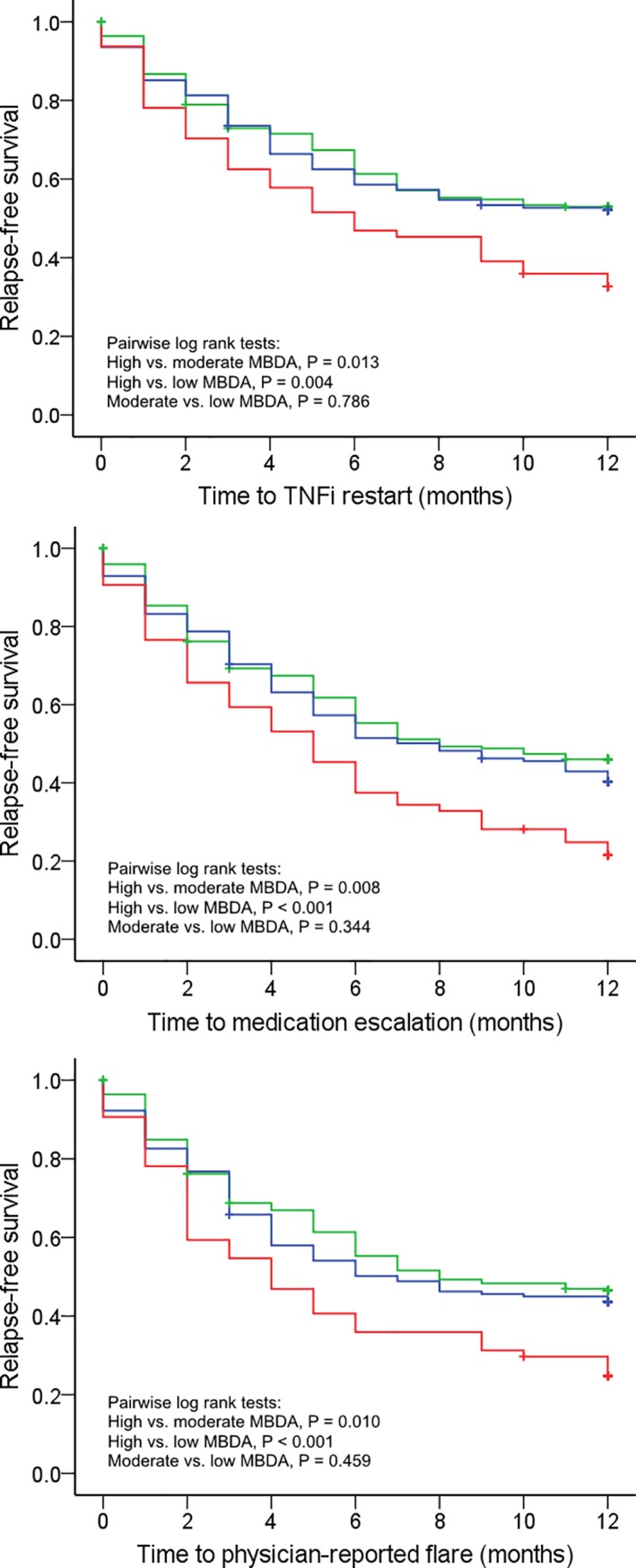
Low MBDA scores (<30, green; n = 220), moderate MBDA scores (30–44, blue; n = 155), high MBDA scores (>40, red; n = 64) for three definitions of disease relapse. TNFi restart (top), medication escalation (middle) and physician-reported flare (bottom).
Univariate and multivariate analysis of risk factors for disease relapse at 12 months
In univariate logistic regression analyses, greater disease duration was significantly associated with all three criteria for disease relapse at 12 months (ORs 1.04–1.05, 95% CI 1.01–1.08) (S4 Table). Higher BMI was associated with increased odds of restarting TNFi (OR 1.05, 95% CI 1.01–1.10), medication escalation (OR 1.07, 95% CI 1.02–1.13) and any criterion (OR 1.06, 95% CI 1.01–1.12). Erosive disease was significantly associated with restarting TNFi (OR 1.62, 95% CI 1.08–2.44), but not with the other two criteria. Baseline DAS28-ESR was significantly associated with medication escalation (OR 1.57, 95% CI 1.21–2.04), physician-reported flare (OR 1.36, 95% CI 1.06–1.75) and any criterion (OR 1.65, 95% CI 1.26–2.17), but not with restarting TNFi treatment (OR 1.25, 95% CI 0.98–1.60).
In multivariate analysis with adjustment for baseline DAS28-ESR, disease relapse after stopping TNFi treatment was more than twice as likely to occur in patients with a high baseline MBDA score, regardless of the relapse criterion used (Table 3). After adjusting for baseline DAS28-ESR, disease duration, BMI and erosions, high baseline MBDA score remained a significant independent predictor of disease relapse by all three criteria (Table 3). However, it did not remain significantly associated with the composite definition of relapse in the fully adjusted model. In a final sensitivity analysis in which all patients with a missing visit were counted as a flare, high MBDA score remained a significant predictor of clinical flare and a marginally significant predictor of medication escalation and TNFi-restart (S5 Table).
Table 3. Univariate and multivariate analyses of high (>44) versus moderate or low baseline MBDA score as a predictor of disease relapse at 12 months.
| Unadjusted | Adjusted | Fully adjusted | ||||
|---|---|---|---|---|---|---|
| Criterion for relapse | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| TNFi restart | ||||||
| MBDA >44 | 2.32 (1.32–4.05) | 0.003 | 2.17 (1.23–3.83) | 0.008 | 1.85 (1.00–3.40) | 0.049 |
| DAS28-ESR | 1.17 (0.91–1.51) | 0.219 | 1.10 (0.84–1.45) | 0.474 | ||
| Disease duration | 1.05 (1.02–1.08) | <0.001 | ||||
| BMI | 1.06 (1.01–1.11) | 0.031 | ||||
| Erosive | 1.30 (1.00–3.40) | 0.248 | ||||
| Medication escalation | ||||||
| MBDA >44 | 2.84 (1.52–5.31) | 0.001 | 2.44 (1.29–4.62) | 0.006 | 1.99 (1.01–3.94) | 0.047 |
| DAS28-ESR | 1.47 (1.13–1.92) | 0.004 | 1.48 (1.11–1.97) | 0.008 | ||
| Disease duration | 1.04 (1.01–1.06) | 0.011 | ||||
| BMI | 1.07 (1.01–1.13) | 0.014 | ||||
| Erosive | 1.24 (0.79–1.97) | 0.353 | ||||
| Physician-reported flare | ||||||
| MBDA >44 | 2.54 (1.39–4.64) | 0.002 | 2.31 (1.25–4.25) | 0.007 | 2.00 (1.06–3.77) | 0.033 |
| DAS28-ESR | 1.27 (0.98–1.65) | 0.069 | 1.20 (0.92–1.58) | 0.184 | ||
| Disease duration | 1.04 (1.01–1.06) | 0.007 | ||||
| BMI | 1.03 (0.98–1.08) | 0.228 | ||||
| Erosive | 1.08 (0.69–1.68) | 0.784 | ||||
| Any criterion | ||||||
| MBDA >44 | 2.52 (1.30–4.89) | 0.006 | 2.12 (1.08–4.16) | 0.029 | 1.68 (0.83–3.40) | 0.147 |
| DAS28-ESR | 1.56 (1.18–2.07) | 0.002 | 1.54 (1.14–2.07) | 0.005 | ||
| Disease duration | 1.04 (1.01–1.07) | 0.010 | ||||
| BMI | 1.06 (1.00–1.12) | 0.037 | ||||
| Erosive | 1.25 (0.78–3.40) | 0.347 | ||||
DAS28-ESR, disease duration and BMI were analyzed as continuous variables; MBDA score (>44) and erosive (yes/no) were analyzed as categorical variables. Adjusted = adjusted for DAS28. Fully adjusted = adjusted for DAS28, disease duration, BMI and erosions. Any criterion includes patients with TNFi re-initation, medication escalation or physician-reported flare. Total N = 439. See Table 2 for n-values by relapse criterion.
Discussion
Our analyses of the POET study show that, for RA patients in remission or stable LDA, a high MBDA score at the time of TNFi discontinuation was significantly associated with disease relapse during the next 12 months. Over 80% of patients with a high baseline MBDA score relapsed according to at least one of the three criteria we used. This rate of relapse was substantially higher for patients with low or moderate MBDA scores, suggesting that patients with low clinical disease activity and high MBDA scores may have inflammation that is partly or entirely subclinical. Several other studies have found the MBDA score to be frequently elevated when conventional clinical measures indicate remission or LDA [19,20,22,23]. Moreover, such patients were at increased risk for progressive joint damage [19,20,22]. Consequently, discontinuation of TNFi treatment in POET may have allowed a resurgence of residual inflammation and subsequent clinical relapse [23]. Our finding that the MBDA score was a predictor of relapse independently of DAS28-ESR suggests that it may be a clinically useful tool for identifying patients who are at increased risk of unsuccessful TNFi discontinuation.
Although high MBDA was an independent predictor of three predefined criteria for relapse (TNFi restart, medication escalation, and physician-reported flare), it should be noted that it did not remain significantly associated with meeting any criterion of flare when adjusting for all other potential predictors, including DAS28 score. Previous studies have explored predictors of disease relapse after TNFi discontinuation and, although results varied considerably, greater clinical disease activity at the time of discontinuation has been identified as a predictor [9–11]. Likewise, our study found that higher baseline DAS28-ESR scores were associated with two criteria for disease relapse, but not with the criterion of restarting TNFi treatment.
In other studies, RF positivity, shorter disease duration, non-smoking and normal body mass index (BMI) have been associated with successful TNFi discontinuation [10,12]. In the current study, higher BMI scores were univariately associated with increased odds of meeting two criteria of disease relapse but not physician-reported flare, and longer disease duration was a strong predictor for all three definitions of disease relapse. Erosive disease was univariately associated with TNFi restart. Neither positivity for RF nor ACPA was associated with disease relapse.
We previously demonstrated that RA patients in remission or stable low disease activity, as defined by the DAS28-ESR, had a relapse risk of approximately 50% within 12 months of discontinuing TNFi treatment in the POET study [24]. With such a high likelihood of relapsing, it may be helpful to have an effective tool to predict the outcome of treatment withdrawal. The MBDA score has been shown to correlate significantly with the DAS28-ESR, DAS28-CRP, simplified disease activity index (SDAI) and clinical disease activity index (CDAI), both overall and in seronegative and seropositive RA patients [16,27,28]. The MBDA score was an independent predictor of disease relapse in a study of RA patients in clinical remission who tapered, and in one arm of the study stopped, all RA therapy, including csDMARDs and bDMARDs [18]. The present study is the first to demonstrate the utility of the MBDA score as a predictor for risk of disease relapse in RA patients who discontinued TNFi treatment at baseline.
Patients with high MBDA had an odds ratio of approximately 2 for experiencing a relapse as defined by the three criteria. However, the proportion of patients with low or moderate MBDA scores who still relapsed within 12 months was also quite high, which may limit the utility of the MBDA to guide TNFi discontinuation in clinical practice. Additionally, since odds ratios tend to overestimate the probability of frequent events (the overall prevalence of relapse ranged from approximately 50%–60% on the different criteria), the hazard ratios or relative risks of around 1.6 found in the one-year survival analyses may be a more appropriate and precise estimate of risk for relapse associated with high MBDA.
Our study has several strengths. The data we analyzed were collected in POET, the largest randomized controlled trial on stopping TNFi in RA patients in remission or stable low disease activity to date. The MBDA score was measured in an unbiased selection of 439 patients in the stop group, comprising 83% of those who stopped TNFi treatment in POET. Most patients in our study had long disease duration (i.e., established RA) and an average age of 60 years, which is representative of the TNFi-using RA population in The Netherlands. Our finding that MBDA score was a predictor of relapse risk is strengthened by our having used 3 different definitions of disease relapse: 1) restarting TNFi treatment, 2) escalation of any DMARD therapy and 3) physician-reported flare, which identified more relapses than with any one criterion alone. MBDA scores at baseline were predictive of each definition of disease relapse.
A limitation of the study is that MBDA score was measured only at baseline. Longitudinal measurements may have provided insight into the effect of TNFi discontinuation on MBDA scores over time, and the potential for early change in MBDA score to predict relapse. Although only the MBDA score has been validated for measuring disease activity, it remains to be explored if any of the 12 biomarkers in the MBDA score might individually have ability to predict disease relapse.
In conclusion, for RA patients in remission or stable LDA, a high baseline MBDA score was frequently observed and was found to be an independent predictor of disease relapse within 12 months of TNFi discontinuation. These results suggest that the MBDA score may be a clinically useful tool for identifying subgroups of patients who have an increased risk of relapse when stopping TNFi treatment. These data should be confirmed in other studies.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
Sensitivity analysis with all patients with a missing visit (missing DAS28 score at 3, 6, 9 or 12 months) counted as a flare on all flare criteria.
(DOC)
Acknowledgments
The authors thank all patients, rheumatology nurses and rheumatologists of the participating centers, the members of the Steering Committee consisting of Renée Allaart (University Medical Center Leiden), Annelies Boonen (Maastricht University Medical Center), Reinhard Bos (Medical Center Leeuwarden), Liesbeth Brouwer (University Medical Center Groningen), Alfons den Broeder (Sint Maartens clinic), Danielle Gerlag (Amsterdam Medical Center), Mieke Hazes (Erasmus University Medical Center), Willem Lems (VU Medical Center), Dirkjan van Schaardenburg (Reade), Janneke Tekstra (University Medical Center Utrecht), and Harald Vonkeman (Arthritis Center Twente MST & University of Twente), and Gerardine Willemsen (patients association), Huib Kooiman (Dutch Ministry of Health, Welfare & Sports (VWS)), and Benien Vingerhoeds (Netherlands Organisation for Health Research and Development (ZonMW)).
Data Availability
Data are from the POET study. The datasets generated and analyzed during the current study are not publicly available due to legal restrictions related to data privacy protection. However, the data are available upon reasonable request to all interested researchers after authorization of the POET steering committee. Researchers interested in data access may contact the PI of the POET study Tim L. Jansen (tjansen@viecuri.nl) or the Arthritis Center Twente, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands at a.h.oudevoshaar@utwente.nl to apply for access.
Funding Statement
ZonMw/VWS had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Crescendo Bioscience funded the shipping of serum samples to its laboratory and the generation of biomarker data, and provided support in the form of salary for one author [EHS] and consulting fee for another [DC], but did not have any additional role in the study design, data collection or decision to publish the manuscript. Authors EHS and DC participated in designing analyses and preparing the manuscript for this study. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22: 1–12. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69: 1898–906. doi: 10.1136/ard.2010.134684 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10: iii–iv, xi–xiii, 1–229. [DOI] [PubMed] [Google Scholar]
- 4.Wiens A, Venson R, Correr CJ, Otuki MF, Pontarolo R. Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy. 2010;30: 339–53. doi: 10.1592/phco.30.4.339 [DOI] [PubMed] [Google Scholar]
- 5.Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73: 516–28. doi: 10.1136/annrheumdis-2013-204577 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y. Intensive treatment and treatment holiday of TNF-inhibitors in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24: 319–26. doi: 10.1097/BOR.0b013e3283524e4c [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal? Ann Rheum Dis. 2013;72 Suppl 2: ii124–7. doi: 10.1136/annrheumdis-2012-202350 [DOI] [PubMed] [Google Scholar]
- 8.van Ingen ILA, Lamers-Karnebeek F, Jansen TL. Optimizing the expediency of TNFi in rheumatoid arthritis: offering a TNFi holiday in patients having reached low-disease activity in the maintenance phase. Expert Opin Biol Ther. 2014;14: 1761–7. doi: 10.1517/14712598.2014.955009 [DOI] [PubMed] [Google Scholar]
- 9.Saleem B, Keen H, Goeb V, Parmar R, Nizam S, Hensor EMA, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis. 2010;69: 1636–42. doi: 10.1136/ard.2009.117341 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Takeuchi T, Mimori T, Saito K, Nawata M, Kameda H, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis. 2010;69: 1286–91. doi: 10.1136/ard.2009.121491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harigai M, Takeuchi T, Tanaka Y, Matsubara T, Yamanaka H, Miyasaka N. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Mod Rheumatol. 2012;22: 814–22. doi: 10.1007/s10165-011-0586-5 [DOI] [PubMed] [Google Scholar]
- 12.Kavanaugh A, Lee SJ, Curtis JR, Greenberg JD, Kremer JM, Soto L, et al. Discontinuation of tumour necrosis factor inhibitors in patients with rheumatoid arthritis in low-disease activity: persistent benefits. Data from the Corrona registry. Ann Rheum Dis. 2014;0: 1–6. doi: 10.1136/annrheumdis-2014-206435 [DOI] [PubMed] [Google Scholar]
- 13.van den Broek M, Visser K, Allaart CF, Huizinga TWJ. Personalized medicine: predicting responses to therapy in patients with RA. Curr Opin Pharmacol. 2013;13: 463–9. doi: 10.1016/j.coph.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 14.van den Broek M, Klarenbeek NB, Dirven L, van Schaardenburg D, Hulsmans HMJ, Kerstens PJSM, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Ann Rheum Dis. 2011;70: 1389–94. doi: 10.1136/ard.2010.147751 [DOI] [PubMed] [Google Scholar]
- 15.Centola M, Cavet G, Shen Y, Ramanujan S, Knowlton N, Swan KA, et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS One. 2013;8: e60635 doi: 10.1371/journal.pone.0060635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis JR, van der Helm-van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2012;64: 1794–803. doi: 10.1002/acr.21767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eastman PS, Manning WC, Qureshi F, Haney D, Cavet G, Alexander C, et al. Characterization of a multiplex, 12-biomarker test for rheumatoid arthritis. J Pharm Biomed Anal. 2012;70: 415–24. doi: 10.1016/j.jpba.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Hirata S, Li W, Defranoux N, Cavet G, Bolce R, Yamaoka K, et al. A multi-biomarker disease activity score tracks clinical response consistently in patients with rheumatoid arthritis treated with different anti-tumor necrosis factor therapies: A retrospective observational study. Mod Rheumatol. 2015;25: 344–9. doi: 10.3109/14397595.2014.958893 [DOI] [PubMed] [Google Scholar]
- 19.van der Helm-van Mil AHM, Knevel R, Cavet G, Huizinga TWJ, Haney DJ. An evaluation of molecular and clinical remission in rheumatoid arthritis by assessing radiographic progression. Rheumatology (Oxford). 2013;52: 839–46. doi: 10.1093/rheumatology/kes378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hambardzumyan K, Bolce R, Saevarsdottir S, Cruickshank SE, Sasso EH, Chernoff D, et al. Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: results from the SWEFOT trial. Ann Rheum Dis. 2015;74: 1102–1109. doi: 10.1136/annrheumdis-2013-204986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YC, Hackett J, Frits M, Iannaccone CK, Shadick NA, Weinblatt ME, et al. Multibiomarker disease activity score and C-reactive protein in a cross-sectional observational study of patients with rheumatoid arthritis with and without concomitant fibromyalgia. Rheumatology (Oxford). 2016;55: 640–8. doi: 10.1093/rheumatology/kev388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Sasso EH, van der Helm-van Mil AHM, Huizinga TWJ. Relationship of multi-biomarker disease activity score and other risk factors with radiographic progression in an observational study of patients with rheumatoid arthritis. Rheumatology (Oxford). 2016;55: 357–66. doi: 10.1093/rheumatology/kev341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rech J, Hueber AJ, Finzel S, Englbrecht M, Haschka J, Manger B, et al. Prediction of disease relapses by multibiomarker disease activity and autoantibody status in patients with rheumatoid arthritis on tapering DMARD treatment. Ann Rheum Dis. 2015;75: 1637–44. doi: 10.1136/annrheumdis-2015-207900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, Tekstra J, van Schaardenburg D, Starmans-Kool M, et al. Stopping Tumor Necrosis Factor Inhibitor Treatment in Patients With Established Rheumatoid Arthritis in Remission or With Stable Low Disease Activity: A Pragmatic Multicenter, Open-Label Randomized Controlled Trial. Arthritis Rheumatol (Hoboken, NJ). 2016;68: 1810–7. doi: 10.1002/art.39626 [DOI] [PubMed] [Google Scholar]
- 25.van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis. 2013;72: 1800–5. doi: 10.1136/annrheumdis-2012-202281 [DOI] [PubMed] [Google Scholar]
- 26.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified Disease Activity Scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38: 44–48. [DOI] [PubMed] [Google Scholar]
- 27.Bakker MF, Cavet G, Jacobs JW, Bijlsma JWJ, Haney DJ, Shen Y, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis. 2012;71: 1692–7. doi: 10.1136/annrheumdis-2011-200963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata S, Dirven L, Shen Y, Centola M, Cavet G, Lems WF, et al. A multi-biomarker score measures rheumatoid arthritis disease activity in the BeSt study. Rheumatology (Oxford). 2013;52: 1202–7. doi: 10.1093/rheumatology/kes362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Sensitivity analysis with all patients with a missing visit (missing DAS28 score at 3, 6, 9 or 12 months) counted as a flare on all flare criteria.
(DOC)
Data Availability Statement
Data are from the POET study. The datasets generated and analyzed during the current study are not publicly available due to legal restrictions related to data privacy protection. However, the data are available upon reasonable request to all interested researchers after authorization of the POET steering committee. Researchers interested in data access may contact the PI of the POET study Tim L. Jansen (tjansen@viecuri.nl) or the Arthritis Center Twente, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands at a.h.oudevoshaar@utwente.nl to apply for access.


